From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients
Abstract
1. Introduction
2. Discussion
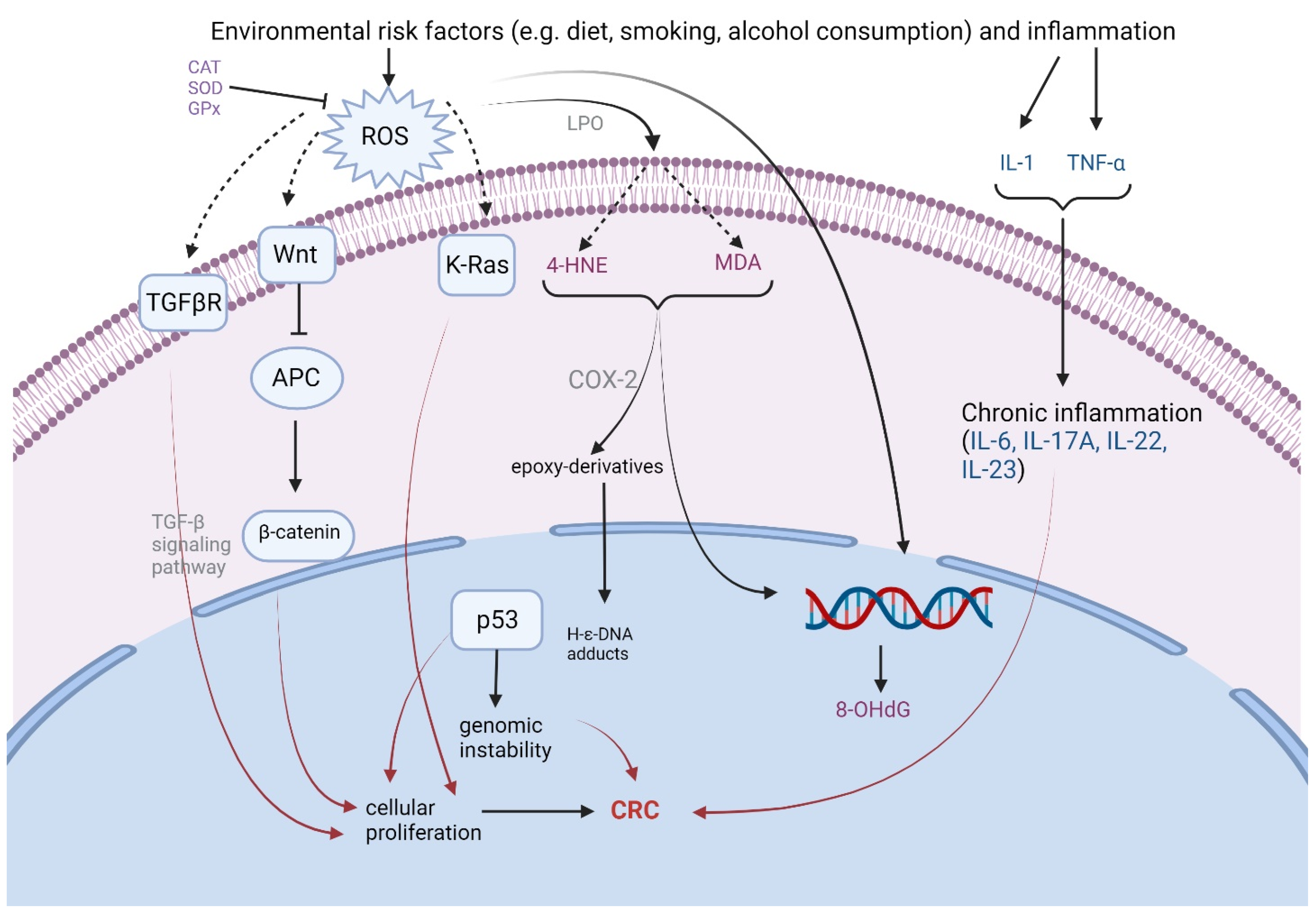
2.1. Carcinogenesis and Oxidative Stress
2.1.1. Reactive Oxygen Species Damage to DNA
2.1.2. Lipid Peroxidation and Antioxidant Defense
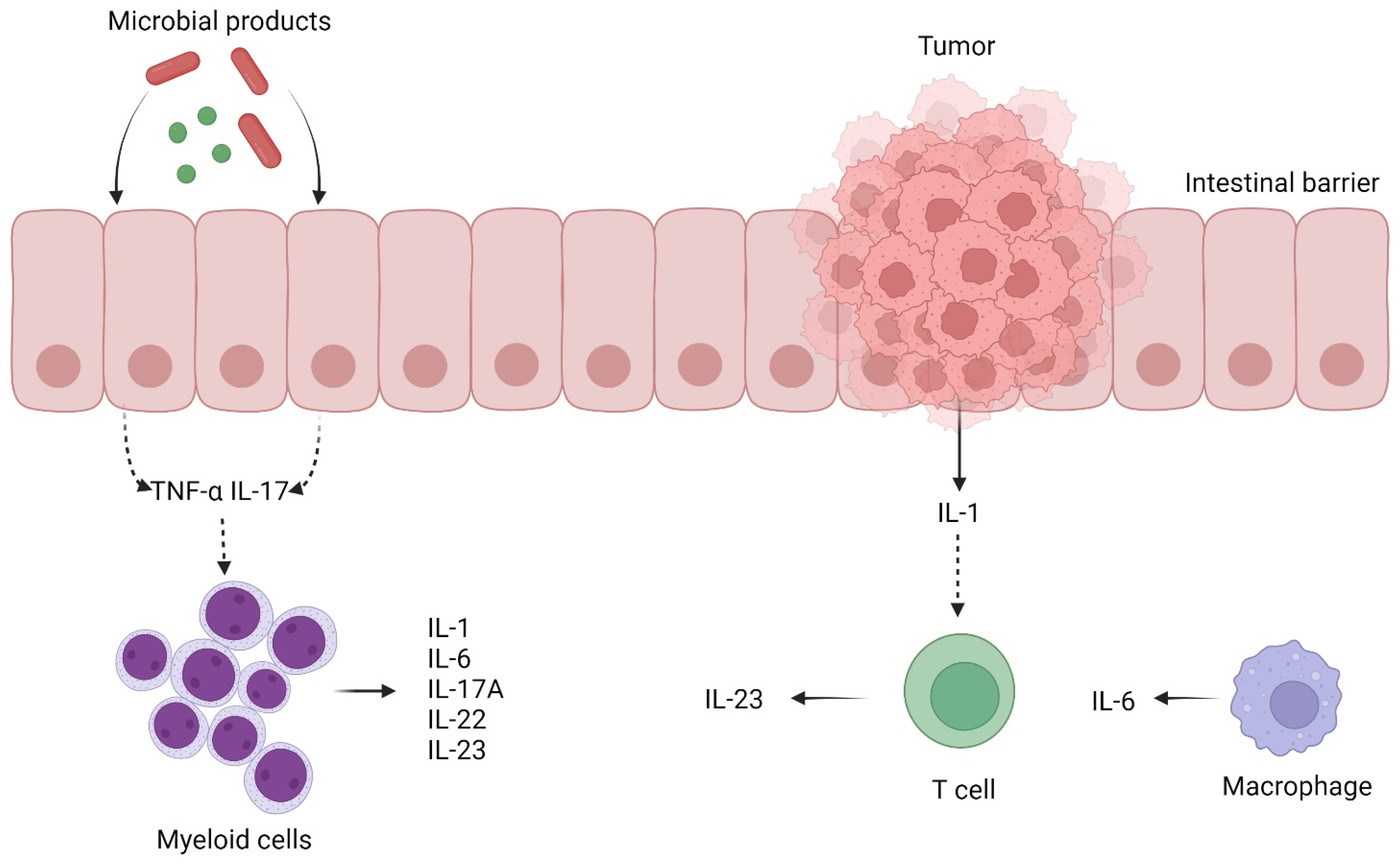
2.2. Inflammation and Colorectal Cancer Development
2.3. The Lipidomic Profile in Colorectal Cancer Development
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| 8-OHdG | 8-hydroxy-deoxyguanosine |
| CA | colonic adenoma |
| CAT | catalase |
| CEA | carcinoembryonic antigen |
| CRC | colorectal cancer |
| FAs | fatty acids |
| GPx | glutathione peroxidase |
| HPLC-MS | high-performance liquid chromatography coupled with mass spectrometry |
| LPC | lysophosphatidylcholine |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| OGG1 | 8-oxoguanine DNA glycosylase 1 |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| SOD | superoxide dismutase |
| SM | sphingomyelin |
| TAG | triacylglycerol |
| TG | triglyceride |
| TGF-β | transforming growth factor β |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor-α |
References
- Rasool, M.; Malik, A.; Waquar, S.; Ain, Q.T.; Rasool, R.; Asif, M.; Hamid Hamdard, M. Assessment of clinical variables as predictive markers in the development and progression of colorectal cancer. Bioengineered 2021, 12, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, S.N. Surveillance of colonic polyps: Are we getting it right? World J. Gastroenterol. 2016, 22, 1925. [Google Scholar] [CrossRef]
- Aceto, G.M.; Catalano, T.; Curia, M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. BioMed. Res. Int. 2020, 2020, 1726309. [Google Scholar] [CrossRef]
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef]
- Eide, T.J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986, 38, 173–176. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Colorectal Cancer Screening Guideline. 2024. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf (accessed on 17 December 2024).
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Kaltenbach, T.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020, 91, 463–485.e5. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef]
- Acevedo-León, D.; Monzó-Beltrán, L.; Pérez-Sánchez, L.; Naranjo-Morillo, E.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Sáez, G. Oxidative Stress and DNA Damage Markers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 11664. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Yu, J.; Tan, Z.; Rao, T.; Chen, Y.; Peng, J. LC-MS-based lipid profile in colorectal cancer patients: TAGs are the main disturbed lipid markers of colorectal cancer progression. Anal. Bioanal. Chem. 2019, 411, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Marciano, F.; Vajro, P. Oxidative Stress and Gut Microbiota. In Gastrointestinal Tissue; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–123. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, Y.; Kahyo, T.; Sekihara, K.; Kawase, A.; Setou, M.; Funai, K. Prognostic potential of lipid profiling in cancer patients: A systematic review of mass spectrometry-based studies. Lipids Health Dis. 2024, 23, 154. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar]
- Pietiläinen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppänen-Laakso, T.; Yki-Järvinen, H.; Kaprio, J.; Oresic, M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS ONE 2007, 2, e218. [Google Scholar]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative Stress in Smokers and Non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar]
- Zięba, S.; Błachnio-Zabielska, A.; Maciejczyk, M.; Pogodzińska, K.; Szuta, M.; Giudice, G.L.; Giudice, R.L.; Zalewska, A. Impact of Smoking on Salivary Lipid Profile and Oxidative Stress in Young Adults: A Comparative Analysis between Traditional Cigarettes, E-Cigarettes, and Heat-Not-Burn Products. Med. Sci. Monit. 2024, 30, e942507. [Google Scholar] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of Nitrative and Oxidative DNA Damage in Inflammation-Related Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef]
- Pierantoni, C.; Cosentino, L.; Ricciardiello, L. Molecular Pathways of Colorectal Cancer Development: Mechanisms of Action and Evolution of Main Systemic Therapy Compunds. Dig. Dis. 2024, 42, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-E.; Kim, J.-H.; Che, Y.-H.; Kim, Y.-J.; Sung, J.-Y.; Kim, Y.-W.; Choe, B.-G.; Lee, S.; Park, J.-H. Role of the WNT/β-catenin/ZKSCAN3 Pathway in Regulating Chromosomal Instability in Colon Cancer Cell lines and Tissues. Int. J. Mol. Sci. 2022, 23, 9302. [Google Scholar] [CrossRef]
- Tudek, B.; Speina, E. Oxidatively damaged DNA and its repair in colon carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2012, 736, 82–92. [Google Scholar] [CrossRef]
- Speed, N.; Blair, I.A. Cyclooxygenase- and lipoxygenase-mediated DNA damage. Cancer Metastasis Rev. 2011, 30, 437–447. [Google Scholar] [CrossRef]
- Potack, J.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008, 2, 61–73. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Czajka-Francuz, P.; Cisoń-Jurek, S.; Czajka, A.; Kozaczka, M.; Wojnar, J.; Chudek, J.; Francuz, T. Systemic Interleukins’ Profile in Early and Advanced Colorectal Cancer. Int. J. Mol. Sci. 2021, 23, 124. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef]
- Bolajoko, E.B.; Mossanda, K.S.; Adeniyi, F.; Akinosun, O.; Fasanmade, A.; Moropane, M. Antioxidant and oxidative stress status in type 2 diabetes and diabetic foot ulcer. South Afr. Med. J. 2008, 98, 614–617. [Google Scholar]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Angelico, F.; Loffredo, L.; Del Ben, M.; Pignatelli, P.; Martini, A.; Violi, F. Oxidative stress-mediated arterial dysfunction in patients with metabolic syndrome: Effect of ascorbic acid. Free Radic. Biol. Med. 2007, 43, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Toyokuni, S.; Tanaka, T.; Hiai, H.; Onodera, H.; Kasai, H.; Imamura, M. Overexpression of the hOGG1 gene and high 8-hydroxy-2’-deoxyguanosine (8-OHdG) lyase activity in human colorectal carcinoma: Regulation mechanism of the 8-OHdG level in DNA. Clin. Cancer Res. 2000, 6, 1394–1400. [Google Scholar]
- Park, Y.J.; Choi, E.Y.; Choi, J.Y.; Park, J.G.; You, H.J.; Chung, M.H. Genetic changes of hOGG1 and the activity of oh8Gua glycosylase in colon cancer. Eur. J. Cancer 2001, 37, 340–346. [Google Scholar] [CrossRef]
- Todorović, S.; Ćeranić, M.S.; Tošković, B.; Diklić, M.; Ajtić, O.M.; Subotički, T.; Vukotić, M.; Dragojević, T.; Živković, E.; Oprić, S.; et al. Proinflammatory Microenvironment in Adenocarcinoma Tissue of Colorectal Carcinoma. Int. J. Mol. Sci. 2024, 25, 10062. [Google Scholar] [CrossRef]
- Chang, D.; Wang, F.; Zhao, Y.S.; Pan, H.Z. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ. Sci. BES. 2008, 21, 286–289. [Google Scholar] [CrossRef]
- Sato, T.; Takeda, H.; Otake, S.; Yokozawa, J.; Nishise, S.; Fujishima, S.; Orii, T.; Fukui, T.; Takano, J.; Sasaki, Y.; et al. Increased plasma levels of 8-hydroxydeoxyguanosine are associated with development of colorectal tumors. J. Clin. Biochem. Nutr. 2010, 47, 59–63. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kitajima, Y.; Kai, K.; Komukai, S.; Tanaka, T.; Koga, Y.; Manabe, T.; Noshirο, H. Predictive value of the ratio of 8-hydroxydeoxyguanosine levels between cancerous and normal tissues in patients with stage II/III colorectal cancer. Oncol. Rep. 2019, 41, 3041–3050. [Google Scholar] [CrossRef]
- Wiczkowski, A. 8-hydroxy-2’-deoxyguanosine in colorectal adenocarcinoma–is it a result of oxidative stress? Med. Sci. Monit. 2013, 19, 690–695. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-L.; Pan, J.; Choudhury, S.; Roy, R.; Hu, W.; Tang, M.-S. Formation of trans-4-hydroxy-2-nonenal-and other enal-derived cyclic DNA adducts from omega-3 and omega-6 polyunsaturated fatty acids and their roles in DNA repair and human p53 gene mutation. Mutat. Res. 2003, 531, 25–36. [Google Scholar] [CrossRef]
- Skrzydlewska, E. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Rainis, T.; Maor, I.; Lanir, A.; Shnizer, S.; Lavy, A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Dig. Dis. Sci. 2007, 52, 526–530. [Google Scholar] [CrossRef]
- Beno, I.; Staruchová, M.; Volkovová, K.; Bátovský, M. Increased antioxidant enzyme activities in the colorectal adenoma and carcinoma. Neoplasma 1995, 42, 265–269. [Google Scholar]
- Rašić, I. The Relationship Between Serum Level of Malondialdehyde and Progression of Colorectal Cancer. Acta. Clin. Croat. 2018, 57, 411–416. [Google Scholar] [CrossRef]
- Eckmann, L.; Nebelsiek, T.; Fingerle, A.A.; Dann, S.M.; Mages, J.; Lang, R.; Robine, S.; Kagnoff, M.F.; Schmid, R.M.; Karin, M.; et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 15058–15063. [Google Scholar] [CrossRef]
- Shaked, H.; Hofseth, L.J.; Chumanevich, A.A.; Wang, J.; Wang, Y.; Taniguchi, K.; Guma, M.; Shenouda, S.; Clevers, H.; Harris, C.C.; et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 14007–14012. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Shan, B.E.; Hao, J.S.; Li, Q.X.; Tagawa, M. Antitumor activity and immune enhancement of murine interleukin-23 expressed in murine colon carcinoma cells. Cell Mol. Immunol. 2006, 3, 47–52. [Google Scholar]
- Ma, X.; Shou, P.; Smith, C.; Chen, Y.; Du, H.; Sun, C.; Kren, N.P.; Michaud, D.; Ahn, S.; Vincent, B.; et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020, 38, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Lankford, C.S.R.; Frucht, D.M. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 2003, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef]
- Kasprzak, A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Skibbe, K.; Adamczyk, A.; Buer, J.; Geffers, R.; Hansen, W.; Pastille, E.; Jendrossek, V. Hypoxia Enhances Immunosuppression by Inhibiting CD4+ Effector T Cell Function and Promoting Treg Activity. Cell Physiol. Biochem. 2017, 41, 1271–1284. [Google Scholar] [CrossRef]
- Ni, Y.; Xie, G.; Jia, W. Metabonomics of human colorectal cancer: New approaches for early diagnosis and biomarker discovery. J. Proteome Res. 2014, 13, 3857–3870. [Google Scholar] [CrossRef]
- Bathe, O.F.; Farshidfar, F. From genotype to functional phenotype: Unraveling the metabolomic features of colorectal cancer. Genes 2014, 5, 536–560. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Muro, E.; Relat-Goberna, J.; Sasse, S.; Bedigian, A.; Coughlin, M.L.; Garcia-Manyes, S.; Eggert, U.S. Dividing cells regulate their lipid composition and localization. Cell 2014, 156, 428–439. [Google Scholar] [CrossRef]
- Silvente-Poirot, S.; Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 2014, 343, 1445–1446. [Google Scholar] [CrossRef]
- Zhu, J.; Djukovic, D.; Deng, L.; Gu, H.; Himmati, F.; Chiorean, E.G.; Raftery, D. Colorectal cancer detection using targeted serum metabolic profiling. J. Proteome Res. 2014, 13, 4120–4130. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. Lond Engl. 2018, 38, 27. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 2017, 1068–1069, 41–48. [Google Scholar] [CrossRef]
- Liu, T.; Tan, Z.; Yu, J.; Peng, F.; Guo, J.; Meng, W.; Chen, Y.; Rao, T.; Liu, Z.; Peng, J. A conjunctive lipidomic approach reveals plasma ethanolamine plasmalogens and fatty acids as early diagnostic biomarkers for colorectal cancer patients. Expert Rev. Proteom. 2020, 17, 233–242. [Google Scholar] [CrossRef]
- Li, N.; Sancak, Y.; Frasor, J.; Atilla-Gokcumen, G.E. A Protective Role for Triacylglycerols during Apoptosis. Biochemistry 2018, 57, 72–80. [Google Scholar] [PubMed]
- Jung, J.H.; Taniguchi, K.; Lee, H.M.; Lee, M.Y.; Bandu, R.; Komura, K.; Lee, K.Y.; Akao, Y.; Kim, K.P. Comparative lipidomics of 5-Fluorouracil–sensitive and–resistant colorectal cancer cells reveals altered sphingomyelin and ceramide controlled by acid sphingomyelinase (SMPD1). Sci. Rep. 2020, 10, 6124. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Klekowski, J.; Chabowski, M.; Krzystek-Korpacka, M.; Fleszar, M. The Utility of Lipidomic Analysis in Colorectal Cancer Diagnosis and Prognosis—A Systematic Review of Recent Literature. Int. J. Mol. Sci. 2024, 25, 7722. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Zhou, H.; Zhu, Y.; Liang, Y.; Zhu, P.; Zhang, Q. UHPLC-HRMS–based serum lipisdomics reveals novel biomarkers to assist in the discrimination between colorectal adenoma and cancer. Front. Oncol. 2022, 12, 934145. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, S.; Zhu, J.; Sheng, H.; Mao, W.; Fu, Z.; Chen, Z. Plasma lipid-based machine learning models provides a potential diagnostic tool for colorectal cancer patients. Clin. Chim. Acta. 2022, 536, 191–199. [Google Scholar] [CrossRef]
- Zhou, H.; Nong, Y.; Zhu, Y.; Liang, Y.; Zhang, J.; Chen, H.; Zhu, P.; Zhang, Q. Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma. BMC Cancer 2022, 22, 314. [Google Scholar] [CrossRef]
- Răchieriu, C.; Eniu, D.T.; Moiş, E.; Graur, F.; Socaciu, C.; Socaciu, M.A.; Al Hajjar, N. Lipidomic Signatures for Colorectal Cancer Diagnosis and Progression Using UPLC-QTOF-ESI+MS. Biomolecules 2021, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, R.; Spagou, K.; Vorkas, P.; Lewis, M.; Kinross, J.; Want, E.; Shion, H.; Goldin, R.; Darzi, A.; Takats, Z.; et al. Chemical mapping of the colorectal cancer microenvironment via MALDI imaging mass spectrometry (MALDI-MSI) reveals novel cancer-associated field effects. Mol. Oncol. 2014, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, J.; Zhang, X.; Feng, S.; DI, W.; Wang, Y.; Zhu, H. The remodeling roles of lipid metabolism in colorectal cancer cells and immune microenvironment. Oncol. Res. 2023, 30, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, M.I.Y.; Ortega-Deballon, P.; Hermite, L.; Pais-De-Barros, J.; Gobbo, J.; Garrido, C. Lipidomic profiling of exosomes from colorectal cancer cells and patients reveals potential biomarkers. Mol. Oncol. 2022, 16, 2710–2718. [Google Scholar] [CrossRef]
- Telleria, O.; Alboniga, O.E.; Clos-Garcia, M.; Nafría-Jimenez, B.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. A Comprehensive Metabolomics Analysis of Fecal Samples from Advanced Adenoma and Colorectal Cancer Patients. Metabolites 2022, 12, 550. [Google Scholar] [CrossRef]
- Ecker, J.; Benedetti, E.; Kindt, A.S.; Höring, M.; Perl, M.; Machmüller, A.C.; Sichler, A.; Plagge, J.; Wang, Y.; Zeissig, S.; et al. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology 2021, 161, 910–923.e19. [Google Scholar] [CrossRef]
- Kelson, C.O.; Zaytseva, Y.Y. Altered lipid metabolism in APC-driven colorectal cancer: The potential for therapeutic intervention. Front. Oncol. 2024, 14, 1343061. [Google Scholar] [CrossRef]
- Jukes, Z.; Freier, A.; Glymenaki, M.; Brown, R.; Parry, L.; Want, E.; Vorkas, P.A.; Li, J.V. Lipid profiling of mouse intestinal organoids for studying APC mutations. Biosci. Rep. 2021, 41, BSR20202915. [Google Scholar] [CrossRef]
- Markowski, A.R.; Błachnio-Zabielska, A.U.; Pogodzińska, K.; Markowska, A.J.; Zabielski, P. Diverse Sphingolipid Profiles in Rectal and Colon Cancer. Int. J. Mol. Sci. 2023, 24, 10867. [Google Scholar] [CrossRef]
- Li, Y.; Nicholson, R.J.; Summers, S.A. Ceramide signaling in the gut. Mol. Cell Endocrinol. 2022, 544, 111554. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef]
- Esplin, E.D.; Hanson, C.; Wu, S.; Horning, A.M.; Barapour, N.; Nevins, S.A.; Jiang, L.; Contrepois, K.; Lee, H.; Guha, T.K.; et al. Multiomic analysis of familial adenomatous polyposis reveals molecular pathways associated with early tumorigenesis. Nat. Cancer 2024, 5, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.d.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Zaytseva, Y. Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berechet, B.M.; Orășan, O.H.; Negrean, V.; Para, I.; Chiș, I.C.; Sporiș, N.D.; Cozma, A.; Sitar-Tăuț, A.V.; Clichici, S.V. From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients. J. Mind Med. Sci. 2025, 12, 16. https://doi.org/10.3390/jmms12010016
Berechet BM, Orășan OH, Negrean V, Para I, Chiș IC, Sporiș ND, Cozma A, Sitar-Tăuț AV, Clichici SV. From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients. Journal of Mind and Medical Sciences. 2025; 12(1):16. https://doi.org/10.3390/jmms12010016
Chicago/Turabian StyleBerechet, Bianca Mihaela, Olga Hilda Orășan, Vasile Negrean, Ioana Para, Irina Camelia Chiș, Nicolae Dan Sporiș, Angela Cozma, Adela Viviana Sitar-Tăuț, and Simona Valeria Clichici. 2025. "From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients" Journal of Mind and Medical Sciences 12, no. 1: 16. https://doi.org/10.3390/jmms12010016
APA StyleBerechet, B. M., Orășan, O. H., Negrean, V., Para, I., Chiș, I. C., Sporiș, N. D., Cozma, A., Sitar-Tăuț, A. V., & Clichici, S. V. (2025). From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients. Journal of Mind and Medical Sciences, 12(1), 16. https://doi.org/10.3390/jmms12010016






