Abstract
Research undertaken over the past few years has brought attention to the role of oxidative stress in the development of neoplasms by damaging nucleic acids, lipids, and proteins, thereby altering their normal function. In general, the levels of antioxidant enzymes are low in patients with neoplasms, and the biomarkers used to quantify oxidative stress have increased levels. Elevated levels of 8-hydroxy-deoxyguanosine (8-OHdG) and malondialdehyde (MDA), as well as decreased levels of antioxidant enzymes, have been observed in patients diagnosed with colorectal cancer (CRC) at various stages of evolution, but further research is needed on the correlation between these biomarkers and disease progression. Inflammation enhances the production of reactive oxygen species and plays an important role in CRC development. Studies in the field of metabolomics have suggested that changes in serum metabolites might be indicators of the progression from adenoma to colorectal carcinoma, particularly those resulting from lipid metabolism. The role of lipidomics in the pathogenesis of CRC warrants further investigation, as these combinations of metabolites (metabolic fingerprints) may have the potential to become clinically useful markers. In this article, we review our current understanding of the interplay between oxidative stress, inflammatory markers and lipidomic products in the pathogenesis of CRC.
1. Introduction
Colon cancer (CRC) is ranked as the third most prevalent form of cancer, resulting in an estimated 0.6 million fatalities annually, with incidence and mortality higher in males than in females (according to the World Health Organization) [1]. In addition, the mortality rate for this disease has been observed to progressively rise over time. Screening colonoscopy is widely regarded as the gold standard for detecting colorectal cancer, as it offers substantial benefits in terms of enhancing survival rates by the early identification and endoscopic removal of adenomatous polyps, which are considered precursors to malignancy. Biopsy specimens obtained from the colorectal mucosa and colonic lesions are valuable diagnostic procedures. However, it is important to note that all these techniques are invasive procedures.
The incidence of right-sided colon cancer has increased in recent years, which may be related to worse right-sided bowel preparation, incomplete colonoscopy, or anatomical obstructions to visibility [2]. Most CRCs develop from an adenomatous polyp originating from the proliferation of the colorectal mucosa [3], and they can be diagnosed through colonoscopy screening. The term “advanced adenoma” refers to a lesion which has at least one of the following characteristics: size of at least 10 mm, villous architecture for at least 25% or high-grade dysplasia [4]. There is a high variability for CRC development from these lesions, depending especially on the age and sex of the patient, but the overall annual progression rate is up to 5% [5].
CRC screening is recommended in adults aged 45–75 years with a life expectancy of more than 10 years, and the decision to screen individuals aged 76–85 years should be personalized and involve a discussion of the associated risks and benefits, considering comorbidity status and projected life expectancy. Patients aged less than 45 years with alarm symptoms (rectal bleeding, iron deficiency anemia, diarrhea, abdominal pain, or weight loss) and patients that are susceptible for hereditary CRC syndromes must also be evaluated [6].
The procedures used for CRC screening include colonoscopy, stool-based tests (Guaiac-based testing, fecal immunochemical testing or multitargeted stool DNA-based testing) or CT colonography [6].
Colonoscopy is the most frequently used screening method given the accessibility, reliability and possibility of treatment, but the discomfort that patients feel during the procedure may be the main reason for reducing the uptake of screening colonoscopy. For this reason, in recent years, research has focused on developing new biomarkers useful for the diagnosis and follow-up of colorectal adenomas.
The appearance of adenomas signifies a disruption in the homeostasis of the colonic epithelium, and the initial process is characterized by abnormal cell growth and differentiation, which is called dysplasia. Dysplasia marks a critical juncture where cells acquire more malignant characteristics but have not yet breached the basement membrane, which is a hallmark of true carcinoma. The transformation to carcinoma is defined by the invasion of the basement membrane of the dysplastic cells.
The surveillance of colonic polyps through colonoscopy is performed at different intervals, depending on the number of polyps, size, and histology. If the baseline finding includes one to two tubular adenomas <10 mm, the recommended interval for first surveillance is 7 to 10 years; for three to four tubular adenomas <10 mm, the recommended interval for first surveillance is 3 to 5 years, and for an advanced adenoma or more than five tubular adenomas <10 mm, the recommended interval for first surveillance is 3 years [7].
Scientists are increasingly inclined toward the utilization of non-invasive methodologies that possess favorable prediction capabilities and a heightened level of sensitivity. Carcinoembryonic antigen (CEA), which was identified more than 50 years ago, is widely regarded as the laboratory indicator most frequently assessed for CRC evaluation. While the CEA showed a rise in approximately 60% to 85% of individuals with colorectal cancer, its specificity was found to be 90%, whereas its sensitivity ranged from 40% to 75% [8]. Furthermore, it was shown that there was a limited increase in the levels of CEA in stage I colorectal cancer, and it was found to be ineffective in distinguishing between benign lesions and malignant polyps [9]. Hence, it is concluded that CEA is not suitable as a universally accepted biomarker for the purpose of diagnosing colorectal cancer and is not advised for utilization in screening examinations.
Oxidative stress has been proven to be implicated in the development of neoplasia, including CRC, through the injury of nucleic acids, proteins and lipids. Elevated levels of oxidative stress within the intestinal lumen, coupled with prolonged exposure of the intestinal mucosa to oxidative stress, have the potential to induce substantial DNA damage, hence initiating genetic mutations [1]. Tumor growth is contingent upon multiple elements within the microenvironment, including diverse oxidants, pro-inflammatory cytokines, and extracellular matrix constituents [1]. The observation of elevated levels of 8-OHdG and MDA in CRC patients has been consistently reported across numerous investigations, as these biomarkers are widely utilized indicators of oxidative stress. However, there are very few studies that assessed the oxidative stress in patients with colonic polyps in order to evaluate the diagnostic capacity of these molecules. While researchers established a positive correlation between the levels of oxidative stress and tumor presence [8,10], no data are provided so far for the utility of these biomarkers in the screening of CRC and detection of colonic adenomas (CAs).
Comparing oxidative stress markers and CEA levels for CRC diagnosis, Zińczuk et al. discovered a positive correlation between the level of MDA and CEA as well as a negative correlation between GPx level and CEA in CRC patients [8]. However, all the assessed redox biomarkers are not exclusive to CRC; therefore, they can only be utilized following the exclusion of other oxidative stress-related conditions.
Probably one of the most useful aspects of discovering new oxidative stress biomarkers besides screening would be the therapeutic implications. Initially, researchers believed that antioxidants could inhibit tumor growth by neutralizing ROS. However, most clinical trials have demonstrated that dietary antioxidants do not successfully prevent cancer [11]. They may really facilitate tumor proliferation by safeguarding cancer cells from apoptosis mediated by ROS [12]. Lately, research has transitioned to pro-oxidant medicines, which seek to elevate ROS levels in cancer cells to trigger apoptosis. Chemotherapy, radiation, and certain pharmaceuticals function by elevating ROS levels. Cancer cells have significant adaptability, frequently acquiring resistance through the upregulation of antioxidant defenses, and this enables them to endure increased ROS while preventing cellular apoptosis [11].
In addition, pro-inflammatory cytokines released in the TME play an important role in the development of CRC from the beginning of carcinogenesis. IL-6 has been identified as the most reliable indicator of inflammation, exhibiting a significant correlation with levels of oxidative stress in CRC as demonstrated in recent research investigations [1,8,10]. Enhancing our understanding of the interplay among these factors in inducing tumor-initiating inflammation or facilitating tumor growth through the secretion of growth-stimulatory cytokines or the mediation of T cell suppression will contribute to the development of targeted strategies aimed at specific inflammatory pathways in the context of colorectal cancer.
Lipidomics, as an important subfield within metabolomics, serves to investigate the alterations in lipid profiles and associated metabolic pathways occurring within organisms under various physiological or pathological conditions. Lipids, which have numerous physiological functions, have been proved to be involved in the development of CRC. Nevertheless, a comprehensive investigation of lipidomic patterns in a substantial number of blood samples for the purpose of identifying diagnostic biomarkers for CA and CRC has not yet been conducted.
Although lipidomics has yielded a substantial amount of information on CRC, there are still obstacles that need to be addressed. These issues mostly revolve around the establishment of standardized techniques for lipid extraction, detection, and data analysis. The diagnostic performance of these lipids has not yet been established, as the main focus was on the association between lipid levels and CRC risk. There are limited data regarding the sensitivity and specificity of these biomarkers in CRC diagnosis. One study that explored the potential lipid biomarkers in relation to CRC progression yielded a sensitivity of 85% and specificity of 80% for the separation of early-stage CRC patients from advanced-stage CRC patients [13].
Additionally, it is crucial to bear in mind that lipidomics represents but a single component of the larger picture. By employing integrative methodologies that incorporate lipidomics alongside genomes, proteomics, and oxidative stress and inflammatory status, a more thorough comprehension of the development of CA and CRC can be attained with promising potential to diagnose CRC based on blood samples.
This review aims to evaluate the evidence linking oxidative stress, inflammation, and lipidomic changes with the adenoma-to-carcinoma sequence in CRC. By synthesizing the current literature, we aim to identify key mechanisms that contribute to CRC progression.
2. Discussion
Generally, malignancy is considered a multifactorial condition involving local, systemic and environmental factors. Unmodifiable risk factors for developing CRC include age, a personal or family history of CRC or adenomatous polyps, a personal history of inflammatory bowel disease, or genetic inheritance (Lynch syndrome, familial adenomatous polyposis, Peutz–Jeghers syndrome). Also, CRC development is closely linked to the overproduction of reactive oxygen species (ROS), which are related to variable risk factors such as smoking, stress, alcohol consumption, the exposure to toxins, inflammatory processes associated with metabolic diseases, lifestyle choices, diet, and dysbiosis [8].
Oxidative stress represents the imbalance between ROS production and the biological processes that try to repair the systemic damage or to detoxify these metabolites [14]. The latest research describes the role of oxidative stress in the development of cancer through the injury of nucleic acids, proteins and lipids [15]. In the case of oncological patients, there is an imbalance regarding antioxidant enzymes and oxidative stress products markers with the latter being consistently higher compared to healthy individuals [16]. The most frequently utilized biomarkers for quantifying oxidative stress are 8-hidroxy-2-deoxyguanosine (8-OHdG), which is a marker of DNA damage and malondialdehyde (MDA), which is the product of lipid peroxidation. The key genes with antioxidant activity include catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione reductase, heme oxygenase-1 and peroxiredoxins [15,16].
In addition to the oxidative stress biomarkers used for lipid peroxidation, in the last few years, researchers have focused on the role of different classes of lipids in colorectal cancer development. Lipidomics is the study of small lipidic molecules (metabolites) that can play different roles, acting as the substrate, intermediate, or final product in various molecular pathways along the cell life cycle. The major application of lipidomics in the field of oncology could be identifying certain biomarkers for the early diagnosis of CRC as well as for monitoring the disease and treatment response. A recent systematic review by Takanashi et al. [17] investigated the lipidomic profile in different types of cancers and its prognostic potential including 38 studies performed on 16 cancer types. Most research focused on patients with CRC, evaluating serum or tissue samples while considering prognostic markers such as advanced stage, reduced disease-free survival and overall survival. The majority classes of lipids reported were the ones that form the cellular membrane, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), sphingomyelin (SM), and phosphatidylinositol (PI). These identified lipids could be promising prognostic indicators in the development of CRC, but further research is needed in order to assess the clinical feasibility of monitoring these lipid biomarkers.
The oxidative stress and lipidomic profile in CRC patients are influenced by the confounding variables listed before, such as obesity, metabolic syndrome, dietary patterns, smoking, medication use, and the inherent heterogeneity among CRC patients based on disease stage, genetic predisposition, and the tumor microenvironment. Obesity promotes the mitochondrial β-oxidation of free fatty acids, consequently increasing electron flow and the accumulation of ROS, ultimately leading to lipid peroxidation [18]. Furthermore, in obese patients, the lysophosphatidylcholines (LPCs), which are known to be pro-inflammatory and proatherogenic, are increased, while ether phospholipids, which possess antioxidant properties, are decreased [19]. Dietary patterns act as modulators of oxidative stress and lipidomic profiles, and the Mediterranean diet, in particular, has been shown to reduce levels of lipid peroxidation and oxidative DNA damage [20]. Smoking also influences oxidative stress not only through the direct introduction of reactive oxygen radicals present in smoke but also by impairing the body’s natural antioxidant defense mechanisms [21]. It alters the lipid profiles systemically, like raising serum MDA [22] and causing changes in the composition of plasma sphingolipids [23]. Given the notable impact of these modifiable risk factors on both oxidative stress and lipidomic profiles, it is essential to consider them in studies investigating these parameters in the context of CRC.
2.1. Carcinogenesis and Oxidative Stress
Oxidative stress and inflammation play an important role in the development of CRC, and without proper regulation of these processes, genetic and epigenetic alterations may arise, initiating carcinogenesis [24]. High doses of ROS or reduced neutralization can lead to severe cell damage and metabolic malfunction [10].
The literature describes three distinct molecular pathways involved in the adenoma to carcinoma transition: the chromosomal instability (CIN) pathway, microsatellite instability (MSI) pathway, and CpG island methylator phenotype (CIMP) pathway, which is also known as the serrated pathway. Based on the current findings, colorectal tumorigenesis appears to have a significant impact on the CIN pathway, particularly through its interaction with the Wnt/β-catenin signaling and base excision repair (BER) pathways [25]. The dysregulation of the Wnt/β-catenin signaling is a hallmark of CIN, driving chromosomal instability and cancer progression [10]. Additionally, the BER pathway is vital for repairing oxidative DNA damage, and defects in this pathway could contribute to the accumulation of mutations and chromosomal instability in colorectal cancer [25].
The genetic impact that ROS generation has is represented by the alterations in the expression of many genes, most prominently on the TP53 gene, which is a key factor in apoptosis, APC and K-Ras [26]. Mutations in the APC gene lead to an aberrant accumulation of β-catenin and subsequent activation of oncogenic transcription programs. APC gene mutation appears early in colon carcinogenesis, and about 70 to 80% of early adenomas suffer APC inactivation [27]. K-Ras is another oncogene which is affected by ROS also in the early adenoma stage and through the PI3K-Akt and MAPK signaling pathways suppresses apoptosis and stimulates proliferation. Transforming growth factor (TGF)-β and TP53 signaling pathway mutations appear in the late adenoma stage, and this results in the loss of growth inhibitory effects of TGF-β and, respectively, uncontrolled proliferation and genomic instability.
ROS affects cellular membranes, initiating lipid peroxidation (LPO). They act as mediators of specific physiological processes when present in low or moderate concentrations, but higher concentrations can be detrimental to all cellular components, drastically altering their function. The most studied biomarkers that reflect LPO are MDA and 4-hydroxy-2-nonenal (4-HNE). These aldehydes either react directly with the DNA or undergo further oxidation through cyclooxygenase-2 (COX-2) and lipooxygenases (LOX), resulting in heptanone-etheno(H-ε)-DNA adducts [27]. These are known to be highly mutagenic and may act as initiators in the adenoma development process and lastly in carcinogenesis [28].
8-OHdG, one of the most studied biomarkers for oxidative stress [1,27], can quantify DNA lesions caused by ROS production. If 8-OHdG residues are not successfully excised from the DNA, guanine:cytosine (GC) → timidine:adenine (TA) transversions can occur. This type of mutation is commonly found affecting potential oncogenes [16].
The link between inflammation and CRC development is established by many epidemiological studies, especially the association of inflammatory bowel disease and colorectal cancer [29]. The presence of chronic inflammation alters the gut microbiota, favoring the overpopulation of pathogenic bacteria inducing a potential protumorigenic microenvironment [30]. The immune cells implicated in the carcinogenesis of CRC release pro-inflammatory cytokines, promoting tumor cell proliferation, invasion, and metastasis. On the other hand, tumor cells themselves produce immunosuppressive cytokines such as interleukin (IL)-6, vascular endothelial growth factor (VEGF) and TGF-β [31]. When genotoxic metabolites act on the epithelial gastrointestinal cell, this leads to an increased production of IL-17 and tumor necrosis factor (TNF)-α [30]. Subsequently, an excess of IL-6, IL-22, IL-23 and IL-4 is produced, facilitating the tumorigenesis process [30,31]. The main mechanisms of action are depicted in Figure 1.
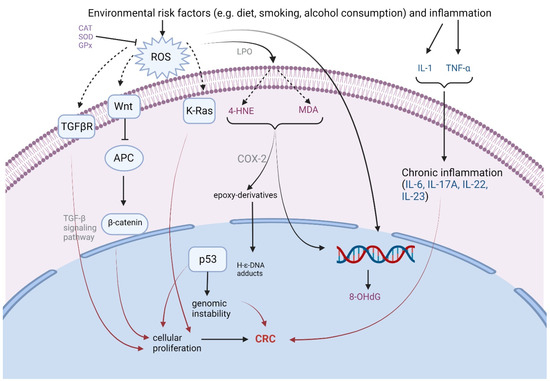
Figure 1.
Mechanisms of carcinogenesis caused by reactive oxygen species and inflammation. ROS—reactive oxygen species; LPO—lipid peroxidation; TGFβR—transforming growth factor-β receptor; APC—adenomatosis polyposis coli protein; 4-HNE—4-hydroxy-2-nonenal; MDA—malondialdehyde; 8-OHdG—8-hidroxy-2-deoxyguanosine; COX-2—cyclooxygenase-2; TNF-α—tumor necrosis factor; H-ε-DNA adducts-heptanone-etheno-DNA adducts; CAT—catalase; GPx—glutathione peroxidase; SOD—superoxide dismutase (created with BioRender.com).
2.1.1. Reactive Oxygen Species Damage to DNA
8-OHdG is a notable biomarker used to measure DNA damage, and it can be induced by hydroxyl radicals, singlet oxygen, or photodynamic action [32]. The 8-OHdG level is usually measured in serum or urine to assess the overall oxidative stress levels. Evaluating 8-OHdG levels in specific cellular populations (ex. leucocytes) may also serve in this matter. Higher amounts of 8-OHdG have been found in patients suffering from diabetes, inflammatory diseases, neoplasia, and in smokers [33,34]. In patients suffering from CRC, two potential sources of increased 8-OHdG have been proposed. The first one is represented by an increased production of ROS, especially in obese patients or those with metabolic syndrome, which are both conditions that are known risk factors for gastrointestinal neoplasia [35]. Another possible source of 8-OHdG is due to increased production in tumor tissue. Kondo et al. [36] and Park et al. [37] reported high levels of 8-OHdG in colorectal tumor cells obtained by surgical resection. Todorovic et al. conducted a study in which they assessed the pro-inflammatory microenvironment in patients that had undergone surgical treatment for CRC and discovered that the frequency of 8-OHdG was twice as high in the cancer tissue of the colon compared to adjacent healthy tissue [38].
Chang et al. reported a four-fold increase in 8-OHdG levels in patients diagnosed with CRC compared to the control group [39]. Recent research confirms this hypothesis. There are a few available studies that assessed the level of oxidative stress biomarkers in patients with colonic polyps in comparison with healthy subjects. Among them, Sato et al. conducted a study on 151 patients investigating the association between 8-OHdG levels and the risk of colorectal tumors [40]. They collected plasma samples from patients that were grouped as follows: adenoma, early cancer and advanced cancer. They found that patients with early-stage cancer had the highest levels of 8-OHdG compared to controls, and the difference was also significant for adenoma patients compared to controls. There were no significant differences in the plasma levels of 8-OHdG in patients with advanced cancer compared to early-stage cancer.
A study conducted on patients diagnosed with CRC at different stages of the disease measured 8-OHdG levels in cancerous and normal tissue and found no significant difference in terms of tumor stage (II vs. III) [41]. Researchers also studied the impact of the 8-OHdG ratios in DNA or the cytoplasm based on the fact that small base modifications are repaired by the base excision repair mechanism, employing the 8-oxoguanine DNA glycosylase (OGG1) pathway [3]. OGG1 is an enzyme that facilitates 8-OHdG transport from the nucleus to the cytoplasm by binding to the 8-oxoG base. The complex OGG1-8-oxoG interacts with the RAS GTPases family in order to facilitate the substitution of GDP with GTP, thereby functioning as a guanine nuclear exchange factor [3,41]. It was found that a loss of function in the OGG1-mediated DNA base excision repair system correlated with increased cancer aggressiveness, whereas normal function of the enzyme led to more stable tumors in terms of proliferation rate and invasion capacity [41].
Wiczkowski et al. investigated the level of 8-OHdG in CRC tissue compared to normal adjacent mucosa, and they also confirmed higher oxidative stress/inadequate DNA repair systems in cancerous tissue with a subsequent higher concentration of 8-OHdG [42]. Additionally, they concluded that these differences were significant only in men and in patients over 65 years old compared to females and younger patients. One explanation might be that the effectiveness of the DNA repair system is a parameter that varies with age. Additionally, Shiota et al. reported that androgens may increase oxidative stress levels [43].
2.1.2. Lipid Peroxidation and Antioxidant Defense
ROS formation leads to lipid peroxidation (LPO). As a result, one of the first consequences of increased ROS levels is the loss of cell membrane function. The LPO products that are most extensively studied are MDA and 4-HNE. They have the capacity to directly bind to the DNA, or they can undergo further oxidation to epoxy derivatives [44]. Antioxidant defense mechanisms consist of enzymes such as SOD, CAT and GPx and non-enzymatic molecules including vitamin C and E. The majority of studies [1,8,39] have found that the antioxidant defense is less active in CRC patients, although other investigations [45,46] have found the opposite regarding the levels of these enzymes. Recent research focused on measuring the levels of lipid peroxidation markers and antioxidant defense in patients diagnosed with different stages of CRC and, to our knowledge, there is only one study evaluating the antioxidant status in patients with colonic polyps [47].
Skrzydlewska et al. measured the level of oxidative stress and antioxidant defense in 81 patients diagnosed with CRC, determining the levels of MDA, 4-HNE, CAT, SOD and GPx [47]. They concluded that MDA and 4-HNE were found in high concentrations in the plasma and tissue of CRC patients, along with the antioxidant defense enzymes, apart from CAT. They also correlated the levels of these markers with the stage of the disease, reporting the highest level in clinical stage IV.
A study that analyzed MDA serum levels in patients diagnosed with different stages of CRC and its association with tumor size also reported higher levels of this biomarker according to pTNM stage [48].
Rasool et al. studied the role of oxidative stress and inflammatory biomarkers in the development and progression of CRC [1]. They assessed the blood samples from patients diagnosed with CRC in comparison with age–sex matched controls, and a significantly increased level of MDA was observed in neoplastic patients, and it was associated with a consistent decrease in the levels of antioxidant markers. In accordance with the previous study, they reported decreased activity of CAT in tumor tissues and an inverse correlation between MDA and SOD.
A recent study [8] that assessed the utility of redox biomarkers in patients with CRC reported that CAT and MDA might be potential useful biomarkers for tumor invasion depth. Apart from other studies, in addition to the measurement of antioxidant and oxidative products levels, they calculated the total antioxidant capacity (TAC), total oxidant status (TOS) and oxidative stress index (OSI) by dividing the TOS level by the TAC level (TOS/TAC ratio). Overall, increased levels of TOS and OSI with accordingly decreased TAC levels were observed in CRC patients. Interestingly, a positive correlation between MDA and carcinoembryonic antigen (CEA) was observed.
2.2. Inflammation and Colorectal Cancer Development
During tumor initiation and progression from adenoma to carcinoma, immune cells located in the tumor microenvironment (TME) release pro-inflammatory cytokines and growth factors that play an important role in carcinogenesis, also enhancing the production of reactive oxygen species and promoting epigenetic changes. DNA damage occurs through an interplay between high levels of oxidative stress and various unregulated immune responses, which are all stimulated by chronic inflammation [30].
Inflammation exerts its influence on cytokine receptor-mediated signaling pathways that govern essential tumor-initiating and tumor-promoting processes in CRC. These processes include the activation of nuclear factor-κB downstream of TNF and IL-1 receptor signaling [49,50].
Although inflammation can increase the risk of developing CRC, most tumors do not have an inflammatory background [31]. In sporadic tumors, their growth relies on the interactions between cells within the tumor microenvironment, which could induce inflammation [51]. When the activation of the Wnt signaling pathway occurs, for example after a mutation in the APC gene, microbial products penetrate the intestinal mucosa with further activation of the myeloid cell and IL-17A release. IL-17A is considered a promoter of early CRC, and it was associated with an increased production of vascular endothelial growth factor (VEGF). Czajka-Francuz et al. investigated the systemic interleukins’ profile in different stages of CRC and concluded that the level of IL-17A was elevated in most of the studies [31].
Another crucial mediator is IL-1, which is secreted by monocytes, stromal cells and tumor epithelial cells. Regardless of tumor-induced inflammation, IL-1 activates T cells to release IL-23 and facilitates inflammation, thereby promoting tumorigenesis [30]. Based on published evidence, increased levels of IL-23 were observed in CRC patients, and its level corresponded with the stage of the disease [31]. There have been reports of the dual functionality of IL-23, exhibiting both pro-tumor and anticancer effects. Experimental studies have shown that IL-23 exhibits various anticancer characteristics [52]. Interleukin-23 stimulation led to the activation of memory T cells, resulting in their proliferation and the production of IFN-gamma, which has been shown to have anti-tumor effects [53]. Conversely, the pro-tumor effect of IL-23 was also noted, as evidenced by the heightened synthesis of IL-17 by Th-17 cells triggered by IL-23 [54].
IL-6 is considered one of the most important molecules released in the tumor microenvironment because of its immunosuppressive properties [31], and it is also considered a CRC growth factor [55]. Macrophages influence the tumor microenvironment and stimulate the differentiation of tumor cells by secreting IL-6 [56]. The findings of the analyzed studies indicate that there was a significant elevation in serum IL-6 levels across all stages of colorectal cancer compared to the control group. A statistically significant difference in IL-6 levels was seen just when comparing stage I to other stages [31]. This observation implies a significant involvement of IL-6 in the initial phases of tumor formation, which persists throughout subsequent tumor growth and invasion.
A study that measured the levels of multiple biochemical markers in patients diagnosed with CRC revealed increased levels of IL-6 and TFG-β compared with the control group as well as increased oxidative stress biomarkers and decreased antioxidants levels [1]. In the context of elevated TGFβ levels, the presence of hypoxic stress leads to the recruitment of regulatory T cells and a concurrent inhibition of the differentiation process of effector T cells hampering the effectiveness of the immune system’s anti-tumor responses in CRC cases [57].
The mechanisms described above are depicted in Figure 2.
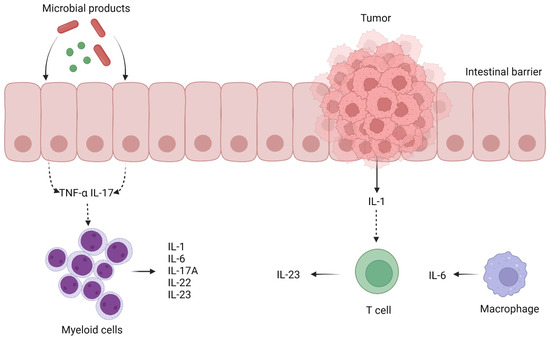
Figure 2.
Immune cells located in the tumor microenvironment (TME) release pro-inflammatory cytokines and growth factors that play an important role in carcinogenesis (created with BioRender.com). TNF-α—tumor necrosis factor-α; IL—interleukin.
2.3. The Lipidomic Profile in Colorectal Cancer Development
Metabolomics represents the omics technology which focuses on the identification and quantification of small molecules (metabolites) in various specimens, as metabolites are present in all biological structures and metabolic processes [58,59]. Lipids have become significant molecules that play a variety of roles in cell death, proliferation, signal transduction, and energy metabolism in human physiological processes [60,61].
High-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) represents the most applied technique for the detection of small metabolites (with molecular weight less than 1000 Da) related to the metabolic signature and pathway alterations. Metabolomics provides a means for qualitative untargeted profiling or a targeted approach for quantitatively assessing potential biomarkers [62].
Growing data point to a direct connection between lipid metabolic disturbance and the development of CRC illness [63]. The lipidomic study revealed that LPCs and PCs exhibit a significant correlation with the formation of colorectal cancer [64]. Ethanolamine plasmalogens and fatty acids (FAs) in plasma are regarded as potential early diagnostic biomarkers for CRC [65], while triacylglycerols (TAGs) are the primary lipid indicators that are affected during the advancement of this disease [66]. Carcinogenesis entails an enhanced metabolic process of lipid membranes, which contributes to the generation of adenosine triphosphate (ATP). This phenomenon may account for the elevated concentrations of choline-related metabolites observed in tumors. Cancer cell proliferation relies upon fatty acid β-oxidation needed as energy support with increases in 3-hydroxybutyrate [67]. In addition to the need for lipid products for the generation of cell membranes (phospholipids, sphingolipids), lipids also act as energy substrates or sources for signaling molecules [68]. To date, very few studies have investigated the lipidomic profile in patients diagnosed with CA compared to patients suffering from CRC.
A recent systematic review investigated the utility of lipidomics in CRC diagnosis and prognosis and included 35 studies [69]. Membrane lipids, particularly PEs and PCs, are prevalent in the reviewed studies. PEs typically exhibit a trend of decline in the bloodstream of CRC patients, while PCs levels variated depending on each subtype with decreased levels of PC 36:2, 36:1 and 38:4 and increased concentrations of PC 32:3, 37:7, 18:1/16:0, 18:1/16:1, 18:0/20:5, 18:0/22:6, and 16:0/22:6 [70,71]. LPCs exhibit more consistency than PCs. Reports indicate a general reduction in LPC levels in the blood samples of the tested groups compared to healthy controls [70]. Data indicates that most TAGs are downregulated in CRC relative to healthy controls. The same observation was noted in patients with advanced adenomas [69].
Chen et al. conducted a study in which they performed an untargeted lipidomic study utilizing serum samples from patients with CRC and CA in order to identify possible screening lipid biomarkers to discriminate between CA and CRC [70]. Overall, 85 lipid species were discovered, and the dominant component was PCs, which was followed by FAs and TAGs. This observation implies that the disruption of PCs, FAs, and TAGs metabolism could potentially play a crucial role in the malignant progression from CA to CRC. In addition, while comparing the CA group to the CRC group, it was observed that most of the distinct lipid species exhibited a substantial downregulation in the serum of the CRC group. Conversely, only 11 distinct lipid species were found to be significantly elevated in the CRC group. The findings from the receiver operating curve (ROC) analysis revealed the presence of seven lipid biomarkers (three PCs, two FAs, and two SMs), which exhibited strong discriminatory ability in distinguishing between the two groups. In the CA group docosanamide, PC 37:7, PC 32:3, and triheptanoin were significantly elevated, whereas PC 36:1e, SM d36:1, and SM d36:0 were downregulated compared to the CRC group.
In accordance with these findings, Zhou et al. [72] performed a serum untargeted lipidomics study for the discovery of colorectal adenomas, and significant disparities in serum lipid profiles were seen between the CA and control groups. The predominant lipids exhibiting a ratio greater than 60% were TAGs and PCs, suggesting a significant imbalance in these two lipid metabolic pathways. Among the various lipids examined, a total of 12 differential lipids exhibited promising diagnostic potential as potential biomarkers for the detection of CA, and these include PC 30:1, PC 44:5 and 4-dodecylbenzenesulfonic acid.
Some studies have documented decreased levels of LPCs in the blood samples of CRC patients [69,71,72]. On the other hand, elevated levels of PC (16:0–18:1), LPC (16:0), and LPC (18:1) have been found in CRC tissue [73]. These contradictory findings might be attributed to the sample type analyzed (tissue vs. blood), the stage of the disease, or the specific lipid species utilized. LPCs are known to be involved in various signaling processes and inflammatory response [68,74,75]. The contrasting findings observed in PC and LPC levels in tissue versus blood suggest a complex systemic reaction in response to the tumor [69]. The tumor’s increased demand for PC might lead to its accumulation within the tumor microenvironment, thus altering the systemic reserve and processing capability of lipids. Further research is needed to fully elucidate the interplay between the tumor microenvironment and the rest of the organism.
One study was conducted to analyze the serum lipidomic profile of patients diagnosed with stage III or IV colorectal cancer who were scheduled for surgical resection compared to a control group without any pathological findings during colonoscopy [73]. The study identified a total of 25 molecules that exhibit potential as biomarkers for the detection of CRC, which were mainly choline-dependent phospholipids, ceramides and different esters (of fatty acids or cholesterol).
Other studies have examined the lipidomic profile of CRC patients using various sources such as tumor tissue, fecal samples, and cell cultures. The results also aligned with those observed in serum samples. The lipidomic profile from human cell lines revealed an increase in PC 34:1, PE 36:2, SM d18:1/16:0, hexosylceramide (HexCer) d18:1/24:0 and HexCer d18:1/24:1 [76]. Significant metabolites obtained by lipidomic analysis from fecal samples in CRC patients compared to controls included SM and Cer [77]. The lipidomic profile from tumor tissue matched with adjacent non-diseased mucosa showed increases in SM, Cer and triacylglycerol (TG), and these molecules could be used to differentiate between tumor tissue and nonaffected mucosa [78].
The alterations in SM levels in CRC appear to be more complex and context dependent. Some studies have indicated an increase in SM species with shorter acyl chains (32–34 carbons) and a decrease in SM species with longer acyl chains (more than 34 carbons) in cancerous tissues [78,79]. Lower levels of some SM species including SM (d18:1/14:0) and SM (d18:1/16:0) were also observed in APC mutant organoids [80]. The content of sphingosine, a key metabolite in sphingolipid metabolism, is often found to be higher in CRC tissues compared to adjacent healthy tissue [81], suggesting a shift toward a more permissive signaling environment [82]. As sphingosine in its phosphorylated form, sphingosine-1-phosphate (S1P) acts as a potent a pro-oncogenic signaling molecule, promoting cell survival and proliferation while inhibiting apoptosis [83]. The observed changes in SM levels likely reflect a dysregulation in sphingolipid metabolism that favors tumor growth and survival. The variability in SM alterations in different studies might be due to the inherent heterogeneity of CRC or the particular methods employed for lipid analysis [84]. This highlights the need for more standardized and comprehensive studies to identify consistent patterns of SM alteration in CRC.
Triglycerides, the primary form of energy storage, also exhibit altered levels in the context of CRC tissue. Depleted TGs in benign polyps in an FAP model suggested their possible function as an energy source during polyp development and proliferation [84]. In adenocarcinoma samples, there can be a decrease in TG species with shorter chain lengths (<53 carbons) and an increase in polyunsaturated TGs (>56 carbons) [78]. Notably, lipid droplets, which are cellular organelles composed of TGs, are found to be more abundant in CRC compared to normal adjacent colonic tissues [85,86]. Their presence in CRC stem cells could raise the question of whether high concentrations of intracellular TG storage could facilitate treatment resistance and disease recurrence [87]. This highlights the metabolic dependency of CRC cells on lipids as an energy source, possibly as a consequence of altered glycolytic metabolism, that could have possible therapeutic implications.
The current evidence points to a dynamic interplay where the tumor consequentially alters systemic and local lipid compositions, depending on the stage of development. On the other hand, these altered lipid levels, particularly those involving various signaling molecules like S1P and energy storage lipids like TGs, can facilitate tumor growth, stemness, and potentially contribute to treatment resistance, driving the progression of CRC. Further research is needed to fully understand the intricate mechanisms and therapeutic implications of these lipid alterations in CRC.
3. Conclusions
The interaction between oxidative stress and inflammation is believed to play a crucial role in the development of CRC. The oxidative stress in malignant cells, affecting both proteins and lipids, leads to significant DNA damage and impairing also nearby nonmalignant cells. The antioxidant defense system plays an important role in the prevention of neoplasia for scavenging oxygen free radicals. On the other hand, the adaptability of cancer cells by upregulating antioxidant defenses enables them to prevent apoptosis caused by increased ROS production [11]. While several oxidative stress markers like 8-OHdG and MDA are found to be increased in CRC patients, there are other modifiable risk factors such as obesity, smoking or diet that could alter both oxidative stress and lipidomic profiles.
Recent research focused on metabolomics techniques to identify serum fingerprints for diagnosing this disease. Multiple lipid species have been proposed as biomarkers for CRC screening, but for the moment, there are many limitations for application in daily clinical practice, and the sensitivity and specificity data for lipid biomarkers in CRC diagnosis are also limited. Furthermore, standardized analytical methods are necessary in order to obtain reliable results.
Further research is needed to assess the diagnostic accuracy of lipid biomarkers in CRC detection.
Author Contributions
The authors confirm contribution to the paper as follows: manuscript conception and design: B.M.B., S.V.C. and O.H.O.; draft manuscript preparation: B.M.B. and S.V.C.; revision and editing: I.P., I.C.C., A.C., A.V.S.-T., N.D.S. and V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| 8-OHdG | 8-hydroxy-deoxyguanosine |
| CA | colonic adenoma |
| CAT | catalase |
| CEA | carcinoembryonic antigen |
| CRC | colorectal cancer |
| FAs | fatty acids |
| GPx | glutathione peroxidase |
| HPLC-MS | high-performance liquid chromatography coupled with mass spectrometry |
| LPC | lysophosphatidylcholine |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| OGG1 | 8-oxoguanine DNA glycosylase 1 |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| SOD | superoxide dismutase |
| SM | sphingomyelin |
| TAG | triacylglycerol |
| TG | triglyceride |
| TGF-β | transforming growth factor β |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor-α |
References
- Rasool, M.; Malik, A.; Waquar, S.; Ain, Q.T.; Rasool, R.; Asif, M.; Hamid Hamdard, M. Assessment of clinical variables as predictive markers in the development and progression of colorectal cancer. Bioengineered 2021, 12, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Bonnington, S.N. Surveillance of colonic polyps: Are we getting it right? World J. Gastroenterol. 2016, 22, 1925. [Google Scholar] [CrossRef]
- Aceto, G.M.; Catalano, T.; Curia, M.C. Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition. BioMed. Res. Int. 2020, 2020, 1726309. [Google Scholar] [CrossRef]
- Brenner, H.; Hoffmeister, M.; Stegmaier, C.; Brenner, G.; Altenhofen, L.; Haug, U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007, 56, 1585–1589. [Google Scholar] [CrossRef]
- Eide, T.J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986, 38, 173–176. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Colorectal Cancer Screening Guideline. 2024. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf (accessed on 17 December 2024).
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Kaltenbach, T.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020, 91, 463–485.e5. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef]
- Acevedo-León, D.; Monzó-Beltrán, L.; Pérez-Sánchez, L.; Naranjo-Morillo, E.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Sáez, G. Oxidative Stress and DNA Damage Markers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 11664. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Yu, J.; Tan, Z.; Rao, T.; Chen, Y.; Peng, J. LC-MS-based lipid profile in colorectal cancer patients: TAGs are the main disturbed lipid markers of colorectal cancer progression. Anal. Bioanal. Chem. 2019, 411, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Marciano, F.; Vajro, P. Oxidative Stress and Gut Microbiota. In Gastrointestinal Tissue; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–123. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, Y.; Kahyo, T.; Sekihara, K.; Kawase, A.; Setou, M.; Funai, K. Prognostic potential of lipid profiling in cancer patients: A systematic review of mass spectrometry-based studies. Lipids Health Dis. 2024, 23, 154. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar]
- Pietiläinen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppänen-Laakso, T.; Yki-Järvinen, H.; Kaprio, J.; Oresic, M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PLoS ONE 2007, 2, e218. [Google Scholar]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar]
- Isik, B.; Ceylan, A.; Isik, R. Oxidative Stress in Smokers and Non-smokers. Inhal. Toxicol. 2007, 19, 767–769. [Google Scholar]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar]
- Zięba, S.; Błachnio-Zabielska, A.; Maciejczyk, M.; Pogodzińska, K.; Szuta, M.; Giudice, G.L.; Giudice, R.L.; Zalewska, A. Impact of Smoking on Salivary Lipid Profile and Oxidative Stress in Young Adults: A Comparative Analysis between Traditional Cigarettes, E-Cigarettes, and Heat-Not-Burn Products. Med. Sci. Monit. 2024, 30, e942507. [Google Scholar] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of Nitrative and Oxidative DNA Damage in Inflammation-Related Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef]
- Pierantoni, C.; Cosentino, L.; Ricciardiello, L. Molecular Pathways of Colorectal Cancer Development: Mechanisms of Action and Evolution of Main Systemic Therapy Compunds. Dig. Dis. 2024, 42, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-E.; Kim, J.-H.; Che, Y.-H.; Kim, Y.-J.; Sung, J.-Y.; Kim, Y.-W.; Choe, B.-G.; Lee, S.; Park, J.-H. Role of the WNT/β-catenin/ZKSCAN3 Pathway in Regulating Chromosomal Instability in Colon Cancer Cell lines and Tissues. Int. J. Mol. Sci. 2022, 23, 9302. [Google Scholar] [CrossRef]
- Tudek, B.; Speina, E. Oxidatively damaged DNA and its repair in colon carcinogenesis. Mutat. Res. Mol. Mech. Mutagen. 2012, 736, 82–92. [Google Scholar] [CrossRef]
- Speed, N.; Blair, I.A. Cyclooxygenase- and lipoxygenase-mediated DNA damage. Cancer Metastasis Rev. 2011, 30, 437–447. [Google Scholar] [CrossRef]
- Potack, J.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008, 2, 61–73. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Czajka-Francuz, P.; Cisoń-Jurek, S.; Czajka, A.; Kozaczka, M.; Wojnar, J.; Chudek, J.; Francuz, T. Systemic Interleukins’ Profile in Early and Advanced Colorectal Cancer. Int. J. Mol. Sci. 2021, 23, 124. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef]
- Bolajoko, E.B.; Mossanda, K.S.; Adeniyi, F.; Akinosun, O.; Fasanmade, A.; Moropane, M. Antioxidant and oxidative stress status in type 2 diabetes and diabetic foot ulcer. South Afr. Med. J. 2008, 98, 614–617. [Google Scholar]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Angelico, F.; Loffredo, L.; Del Ben, M.; Pignatelli, P.; Martini, A.; Violi, F. Oxidative stress-mediated arterial dysfunction in patients with metabolic syndrome: Effect of ascorbic acid. Free Radic. Biol. Med. 2007, 43, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Toyokuni, S.; Tanaka, T.; Hiai, H.; Onodera, H.; Kasai, H.; Imamura, M. Overexpression of the hOGG1 gene and high 8-hydroxy-2’-deoxyguanosine (8-OHdG) lyase activity in human colorectal carcinoma: Regulation mechanism of the 8-OHdG level in DNA. Clin. Cancer Res. 2000, 6, 1394–1400. [Google Scholar]
- Park, Y.J.; Choi, E.Y.; Choi, J.Y.; Park, J.G.; You, H.J.; Chung, M.H. Genetic changes of hOGG1 and the activity of oh8Gua glycosylase in colon cancer. Eur. J. Cancer 2001, 37, 340–346. [Google Scholar] [CrossRef]
- Todorović, S.; Ćeranić, M.S.; Tošković, B.; Diklić, M.; Ajtić, O.M.; Subotički, T.; Vukotić, M.; Dragojević, T.; Živković, E.; Oprić, S.; et al. Proinflammatory Microenvironment in Adenocarcinoma Tissue of Colorectal Carcinoma. Int. J. Mol. Sci. 2024, 25, 10062. [Google Scholar] [CrossRef]
- Chang, D.; Wang, F.; Zhao, Y.S.; Pan, H.Z. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ. Sci. BES. 2008, 21, 286–289. [Google Scholar] [CrossRef]
- Sato, T.; Takeda, H.; Otake, S.; Yokozawa, J.; Nishise, S.; Fujishima, S.; Orii, T.; Fukui, T.; Takano, J.; Sasaki, Y.; et al. Increased plasma levels of 8-hydroxydeoxyguanosine are associated with development of colorectal tumors. J. Clin. Biochem. Nutr. 2010, 47, 59–63. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kitajima, Y.; Kai, K.; Komukai, S.; Tanaka, T.; Koga, Y.; Manabe, T.; Noshirο, H. Predictive value of the ratio of 8-hydroxydeoxyguanosine levels between cancerous and normal tissues in patients with stage II/III colorectal cancer. Oncol. Rep. 2019, 41, 3041–3050. [Google Scholar] [CrossRef]
- Wiczkowski, A. 8-hydroxy-2’-deoxyguanosine in colorectal adenocarcinoma–is it a result of oxidative stress? Med. Sci. Monit. 2013, 19, 690–695. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-L.; Pan, J.; Choudhury, S.; Roy, R.; Hu, W.; Tang, M.-S. Formation of trans-4-hydroxy-2-nonenal-and other enal-derived cyclic DNA adducts from omega-3 and omega-6 polyunsaturated fatty acids and their roles in DNA repair and human p53 gene mutation. Mutat. Res. 2003, 531, 25–36. [Google Scholar] [CrossRef]
- Skrzydlewska, E. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Rainis, T.; Maor, I.; Lanir, A.; Shnizer, S.; Lavy, A. Enhanced oxidative stress and leucocyte activation in neoplastic tissues of the colon. Dig. Dis. Sci. 2007, 52, 526–530. [Google Scholar] [CrossRef]
- Beno, I.; Staruchová, M.; Volkovová, K.; Bátovský, M. Increased antioxidant enzyme activities in the colorectal adenoma and carcinoma. Neoplasma 1995, 42, 265–269. [Google Scholar]
- Rašić, I. The Relationship Between Serum Level of Malondialdehyde and Progression of Colorectal Cancer. Acta. Clin. Croat. 2018, 57, 411–416. [Google Scholar] [CrossRef]
- Eckmann, L.; Nebelsiek, T.; Fingerle, A.A.; Dann, S.M.; Mages, J.; Lang, R.; Robine, S.; Kagnoff, M.F.; Schmid, R.M.; Karin, M.; et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 15058–15063. [Google Scholar] [CrossRef]
- Shaked, H.; Hofseth, L.J.; Chumanevich, A.A.; Wang, J.; Wang, Y.; Taniguchi, K.; Guma, M.; Shenouda, S.; Clevers, H.; Harris, C.C.; et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 14007–14012. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Shan, B.E.; Hao, J.S.; Li, Q.X.; Tagawa, M. Antitumor activity and immune enhancement of murine interleukin-23 expressed in murine colon carcinoma cells. Cell Mol. Immunol. 2006, 3, 47–52. [Google Scholar]
- Ma, X.; Shou, P.; Smith, C.; Chen, Y.; Du, H.; Sun, C.; Kren, N.P.; Michaud, D.; Ahn, S.; Vincent, B.; et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 2020, 38, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Lankford, C.S.R.; Frucht, D.M. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 2003, 73, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef]
- Kasprzak, A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Skibbe, K.; Adamczyk, A.; Buer, J.; Geffers, R.; Hansen, W.; Pastille, E.; Jendrossek, V. Hypoxia Enhances Immunosuppression by Inhibiting CD4+ Effector T Cell Function and Promoting Treg Activity. Cell Physiol. Biochem. 2017, 41, 1271–1284. [Google Scholar] [CrossRef]
- Ni, Y.; Xie, G.; Jia, W. Metabonomics of human colorectal cancer: New approaches for early diagnosis and biomarker discovery. J. Proteome Res. 2014, 13, 3857–3870. [Google Scholar] [CrossRef]
- Bathe, O.F.; Farshidfar, F. From genotype to functional phenotype: Unraveling the metabolomic features of colorectal cancer. Genes 2014, 5, 536–560. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Muro, E.; Relat-Goberna, J.; Sasse, S.; Bedigian, A.; Coughlin, M.L.; Garcia-Manyes, S.; Eggert, U.S. Dividing cells regulate their lipid composition and localization. Cell 2014, 156, 428–439. [Google Scholar] [CrossRef]
- Silvente-Poirot, S.; Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 2014, 343, 1445–1446. [Google Scholar] [CrossRef]
- Zhu, J.; Djukovic, D.; Deng, L.; Gu, H.; Himmati, F.; Chiorean, E.G.; Raftery, D. Colorectal cancer detection using targeted serum metabolic profiling. J. Proteome Res. 2014, 13, 4120–4130. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. Lond Engl. 2018, 38, 27. [Google Scholar] [CrossRef]
- Shen, S.; Yang, L.; Li, L.; Bai, Y.; Cai, C.; Liu, H. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 2017, 1068–1069, 41–48. [Google Scholar] [CrossRef]
- Liu, T.; Tan, Z.; Yu, J.; Peng, F.; Guo, J.; Meng, W.; Chen, Y.; Rao, T.; Liu, Z.; Peng, J. A conjunctive lipidomic approach reveals plasma ethanolamine plasmalogens and fatty acids as early diagnostic biomarkers for colorectal cancer patients. Expert Rev. Proteom. 2020, 17, 233–242. [Google Scholar] [CrossRef]
- Li, N.; Sancak, Y.; Frasor, J.; Atilla-Gokcumen, G.E. A Protective Role for Triacylglycerols during Apoptosis. Biochemistry 2018, 57, 72–80. [Google Scholar] [PubMed]
- Jung, J.H.; Taniguchi, K.; Lee, H.M.; Lee, M.Y.; Bandu, R.; Komura, K.; Lee, K.Y.; Akao, Y.; Kim, K.P. Comparative lipidomics of 5-Fluorouracil–sensitive and–resistant colorectal cancer cells reveals altered sphingomyelin and ceramide controlled by acid sphingomyelinase (SMPD1). Sci. Rep. 2020, 10, 6124. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Klekowski, J.; Chabowski, M.; Krzystek-Korpacka, M.; Fleszar, M. The Utility of Lipidomic Analysis in Colorectal Cancer Diagnosis and Prognosis—A Systematic Review of Recent Literature. Int. J. Mol. Sci. 2024, 25, 7722. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Zhou, H.; Zhu, Y.; Liang, Y.; Zhu, P.; Zhang, Q. UHPLC-HRMS–based serum lipisdomics reveals novel biomarkers to assist in the discrimination between colorectal adenoma and cancer. Front. Oncol. 2022, 12, 934145. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, S.; Zhu, J.; Sheng, H.; Mao, W.; Fu, Z.; Chen, Z. Plasma lipid-based machine learning models provides a potential diagnostic tool for colorectal cancer patients. Clin. Chim. Acta. 2022, 536, 191–199. [Google Scholar] [CrossRef]
- Zhou, H.; Nong, Y.; Zhu, Y.; Liang, Y.; Zhang, J.; Chen, H.; Zhu, P.; Zhang, Q. Serum untargeted lipidomics by UHPLC-ESI-HRMS aids the biomarker discovery of colorectal adenoma. BMC Cancer 2022, 22, 314. [Google Scholar] [CrossRef]
- Răchieriu, C.; Eniu, D.T.; Moiş, E.; Graur, F.; Socaciu, C.; Socaciu, M.A.; Al Hajjar, N. Lipidomic Signatures for Colorectal Cancer Diagnosis and Progression Using UPLC-QTOF-ESI+MS. Biomolecules 2021, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, R.; Spagou, K.; Vorkas, P.; Lewis, M.; Kinross, J.; Want, E.; Shion, H.; Goldin, R.; Darzi, A.; Takats, Z.; et al. Chemical mapping of the colorectal cancer microenvironment via MALDI imaging mass spectrometry (MALDI-MSI) reveals novel cancer-associated field effects. Mol. Oncol. 2014, 8, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, J.; Zhang, X.; Feng, S.; DI, W.; Wang, Y.; Zhu, H. The remodeling roles of lipid metabolism in colorectal cancer cells and immune microenvironment. Oncol. Res. 2023, 30, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, M.I.Y.; Ortega-Deballon, P.; Hermite, L.; Pais-De-Barros, J.; Gobbo, J.; Garrido, C. Lipidomic profiling of exosomes from colorectal cancer cells and patients reveals potential biomarkers. Mol. Oncol. 2022, 16, 2710–2718. [Google Scholar] [CrossRef]
- Telleria, O.; Alboniga, O.E.; Clos-Garcia, M.; Nafría-Jimenez, B.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. A Comprehensive Metabolomics Analysis of Fecal Samples from Advanced Adenoma and Colorectal Cancer Patients. Metabolites 2022, 12, 550. [Google Scholar] [CrossRef]
- Ecker, J.; Benedetti, E.; Kindt, A.S.; Höring, M.; Perl, M.; Machmüller, A.C.; Sichler, A.; Plagge, J.; Wang, Y.; Zeissig, S.; et al. The Colorectal Cancer Lipidome: Identification of a Robust Tumor-Specific Lipid Species Signature. Gastroenterology 2021, 161, 910–923.e19. [Google Scholar] [CrossRef]
- Kelson, C.O.; Zaytseva, Y.Y. Altered lipid metabolism in APC-driven colorectal cancer: The potential for therapeutic intervention. Front. Oncol. 2024, 14, 1343061. [Google Scholar] [CrossRef]
- Jukes, Z.; Freier, A.; Glymenaki, M.; Brown, R.; Parry, L.; Want, E.; Vorkas, P.A.; Li, J.V. Lipid profiling of mouse intestinal organoids for studying APC mutations. Biosci. Rep. 2021, 41, BSR20202915. [Google Scholar] [CrossRef]
- Markowski, A.R.; Błachnio-Zabielska, A.U.; Pogodzińska, K.; Markowska, A.J.; Zabielski, P. Diverse Sphingolipid Profiles in Rectal and Colon Cancer. Int. J. Mol. Sci. 2023, 24, 10867. [Google Scholar] [CrossRef]
- Li, Y.; Nicholson, R.J.; Summers, S.A. Ceramide signaling in the gut. Mol. Cell Endocrinol. 2022, 544, 111554. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef]
- Esplin, E.D.; Hanson, C.; Wu, S.; Horning, A.M.; Barapour, N.; Nevins, S.A.; Jiang, L.; Contrepois, K.; Lee, H.; Guha, T.K.; et al. Multiomic analysis of familial adenomatous polyposis reveals molecular pathways associated with early tumorigenesis. Nat. Cancer 2024, 5, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.d.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Zaytseva, Y. Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).