Abstract
The global rise in type 2 diabetes mellitus (T2DM) calls for an urgent focus on lifestyle interventions, particularly physical activity, as a critical factor in its management and prevention. This systematic review evaluates the impact of physical activity and sedentary behavior on glycemic control in individuals with T2DM. Studies included in this review were selected based on specific criteria: randomized controlled trials involving adults aged 18 and older, published in English between January 2018 and May 2024, with full-text availability and quantifiable outcome results. Exclusion criteria included non-peer-reviewed research, small sample sizes, and studies limited to abstracts, posters, or editorials. The analysis of the selected studies revealed that regular physical activity, including aerobic exercises and resistance training, significantly improves glycemic control as measured by fasting blood glucose (FBG) and glycated hemoglobin (HbA1C) levels. Reductions in sedentary behavior were also associated with better metabolic outcomes, highlighting the importance of integrating physical activity into daily routines for individuals with T2DM. These findings feature the need for continued research to refine and optimize lifestyle interventions to mitigate the global burden of T2DM.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a significant global health issue, affecting millions worldwide. According to the International Diabetes Federation (I.D.F.), approximately 463 million adults aged 20–79 had diabetes in 2019, a number expected to rise to 700 million by 2045 if current trends persist [1].
While T2DM affects populations worldwide, its prevalence varies among regions and countries. Developed countries, including the United States, Canada, and Europe, tend to have higher prevalence rates due to urbanization, sedentary lifestyles, and dietary habits [2]. However, developing countries are catching up rapidly due to rapid urbanization, adoption of Western dietary patterns, and lifestyle changes [3].
The American Diabetes Association recommends engaging in at least 150 min of moderate-intensity aerobic activity per week, spread over a minimum of three days, with no more than two consecutive days without exercising [4]. Moreover, resistance training is recommended at least twice weekly [4].
Various factors contribute to the increasing prevalence of this condition, including unhealthy diets high in processed foods, physical inactivity, obesity, genetics, ageing populations, and socioeconomic factors [5]. Urbanization and globalization have also led to changes in dietary habits and lifestyles, contributing to the rise in obesity and diabetes. T2DM significantly increases the risk of various complications, including cardiovascular diseases, kidney failure, nerve damage, blindness, and lower limb amputations [6]. It also imposes a substantial economic burden on healthcare systems and societies due to increased healthcare costs, productivity lost, and reduced quality of life. Efforts to address its rising prevalence include public health campaigns promoting healthy lifestyles, initiatives to improve access to nutritious foods, strategies to increase physical activity, and early detection and management programs [7].
Lifestyle factors are crucial in managing type 2 diabetes [8]. Diet, exercise, and weight management are essential to control blood sugar levels [6]. Embracing a balanced, healthy diet that includes whole grains, fruits, vegetables, lean proteins, and beneficial fats can improve blood sugar levels and prevent sudden spikes. Additionally, regular physical activity can enhance insulin sensitivity, allowing the body to use it more effectively and lowering blood sugar levels [9,10]. Excess body weight, especially obesity, is a major risk factor [11,12]. Losing weight can improve insulin sensitivity, reduce blood sugar levels, and decrease the need for diabetes medication [10]. Lifestyle interventions that control calorie intake, portion sizes, and physical activity are well-recognized strategies for managing diabetes-related weight issues [13].
Managing lifestyle factors can effectively help control or slow the onset of complications related to diabetes, such as cardiovascular disease, kidney disease, nerve damage, and eye issues [14]. A healthy lifestyle not only improves diabetes management, but also enriches overall health and wellbeing. Regular exercise strengthens the heart, improves circulation, reduces stress, and boosts mood. A balanced diet provides essential nutrients, supports immune function, and helps maintain a healthy weight. These lifestyle factors contribute to better energy levels, quality of life, and longevity for these individuals [15,16].
Physical activity is also considered essential for maintaining cardiovascular health, which is particularly important for individuals with T2DM with an increased risk of heart disease and stroke [17,18]. Exercise helps lower blood pressure, improve lipid profiles, and reduce the risk of cardiovascular events. On the other hand, sedentary behavior is correlated with an increased risk of cardiovascular disease independent of physical activity levels [19]. Monitoring activity and sedentary patterns can help identify individuals at higher risk and guide interventions to improve cardiovascular health. Physical activity is key in weight management strategies and is essential for controlling this disease [20]. Regular exercise helps burn calories, build lean muscle mass, and maintain a healthy weight. Sedentary behavior, such as excessive screen time or inactive leisure activities, is associated with weight gain and obesity, contributing to insulin resistance and poor glycemic control [21]. Assessing activity levels and sedentary behaviors can help alongside strategies for weight management and obesity prevention in individuals with T2DM [22,23,24]. This holistic approach can improve adherence to lifestyle recommendations, enhance glycemic control, and reduce the risk of complications in individuals with T2DM [25]. Aerobic, resistance, and mixed exercise training can improve HRPF components. Resistance training is a specialized conditioning strategy that uses body weight, barbells, and various training modalities to build muscle [11]. Aerobic training reduces body fat and improves cardiometabolic and mental health in teenagers. The typical aerobic exercise strategy is moderate-intensity continuous training (MICT) with extended sessions [16].
Despite the significant body of research on the impact of physical activity on T2DM management, there is still a need to perform comprehensive reviews that consolidate findings from recent studies, particularly those focusing on comparing different types of physical activity and their specific effects on glycemic control [26]. Additionally, while the detrimental effects of sedentary behavior on T2DM are recognized, there is a need to evaluate how systematically reducing sedentary time can independently contribute to better health outcomes in this population [27]. This systematic review aims to fill these gaps by synthesizing recent evidence on the role of physical activity and sedentary behavior in managing T2DM, providing a clearer understanding of their respective impacts and guiding future research and clinical practice.
2. Materials and Methods
We conducted a comprehensive literature search in PubMed using the keywords “physical activity and type 2 diabetes” and “sedentary behavior and type 2 diabetes”. We then manually searched all eligible papers using references from the first search results, reviews, and other relevant publications. As this study is a literature review, ethical approval was not needed.
The selection criteria were limited to free complete texts in English, randomized clinical trials, and prospective cohort studies involving people aged 18 and older. To make this study up to date, only articles published from January 2018 to May 2024 were considered, and this review excluded articles restricted to abstracts, posters, editorials, and comments. The exclusion criteria included studies with a small sample (10 or less) and non-peer-reviewed research. Case studies were also excluded. Studies without sufficient data and those without quantifiable outcome results were likewise removed.
For the statistical analysis, we used IBM SPSS Statistics 29. The review is outlined here using the Preferred Reporting Items for Systematic Review and Meta-Analysis (P.R.I.S.M.A.) guidelines (Figure 1).
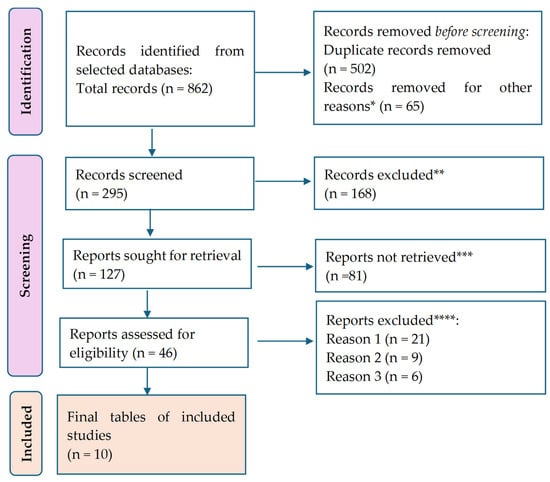
Figure 1.
P.R.I.S.M.A. flow diagram for the results. * Studies are not relevant to the present review. ** Studies do not help us to provide an answer to the research question. *** Unable to find the full text of the study. **** Reason 1—wrong setting (non-T2DM populations, non-free-living conditions); reason 2—wrong patient population; reason 3—research question not relevant.
A total of 888 citations were retrieved after scanning the database above. After eliminating duplicate entries and excluding 65 items that did not satisfy the search parameters, the list was reduced to 295 remaining articles. A total of 168 studies were excluded from consideration as they did not meet our research requirements based on their abstracts. Additionally, 81 papers were further eliminated because they needed to address the specific question of this study. Furthermore, 21 studies were excluded due to the unavailability of the full text. Another nine studies were omitted because they focused on the wrong age group. Lastly, 11 articles were disregarded as they were written in a language other than English. Thus, we based our final analysis on ten search results that met the criteria for our investigation.
The remaining studies met the inclusion standards, and the data from these publications are shown in Table 1, in the Results section.
The search resulted in a total of 797 citations for “physical activity and type 2 diabetes”.
The search for “sedentary behavior and type 2 diabetes” led to 65 articles.
Our search filters in PubMed included the following criteria: free full text, randomized controlled trial, human subjects, English, from January 2018 to May 2024.
3. Results
In Table 1 we present the characteristics of each study. We included the list of studies analysed in this review, and their conclusions.

Table 1.
Characteristics of each study.
Table 1.
Characteristics of each study.
| Study (Year) | Participants | Mean Age (±S.D.) | FBG */HbA1C/HOMA-IR ** Means at Baseline (±SD ***) | Intervention | FBG/HbA1C/HOMA-IR Means After Intervention (±SD) | Differences in FBG/HbA1C/HOMA-IR (±SD) | p Value | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Whitaker et al. [28] (2019) | 1922 | 45.3 years (±3.5) | FBG: 95.0 mg/dL (±12.6) | The activity recorded by the accelerometer during the 20-year examination, including sedentary behavior, light physical activity (L.P.A.), and moderate-to-vigorous physical activity (M.V.P.A.), was linked to cardiometabolic risk factors after a decade, specifically during the year 30 examination. Subsequently, we replicated this procedure by analyzing the impact of alterations in accelerometer-measured activity during the two examinations (e.g., sedentary = year 30 sedentary − year 20 sedentary) on modifications in the dependent variable, specifically the change in cardiometabolic risk factors over the same 10-year span (e.g., risk score = year 30 risk score − year 20 risk score). Sedentary time at year 20 − 490.1 ± 102.4, sedentary time at year 30 − 532.3 ± 102.7 | FBG: 94.6 mg/dL (±14.0) | FBG: −0.4 mg/dL | 0.034 | Substituting 30 min of inactive time with 30 min of low-intensity physical activity led to a decrease of 0.15 cm in waist circumference, a decrease of 0.20 μU/mL in insulin levels, an increase of 0.20 mg/dL in HDL-C levels, and a decrease of 0.01 standard deviation in the composite risk score. Substituting an equivalent amount of inactive time with moderate-to-vigorous physical activity led to a decrease of 0.73 μU/mL in insulin levels and a decrease of 4.57 mg/dL in lipid levels. Substituting L.P.A. with M.V.P.A. led to a decrease of 0.54 μU/mL in insulin levels and a decrease of 4.51 mg/dL in triglyceride levels. |
| Zheng et al. [29] (2020) | 94 | 21.7 years (±3.38) | FBG: 106 mg/dL (±5.4) | At their initial visit, participants were directed to wear the activPAL device for a continuous period of seven days while adhering to their usual daily routine. The activPAL device was removed on the morning of the seventh day. On the ninth day, participants returned to the laboratory after a 10 h period of not eating to collect blood samples and return the activPAL device. | FBG: 86.3 mg/dL (±2.1) | FBG: −9.7 mg/dL | 0.001 | Essentially, inactive behaviors such as prolonged periods of sitting, extended sedentary episodes, and sedentary bouts can significantly contribute to the development of cardiovascular and metabolic disorders in physically active young boys. |
| Debache et al. [30] (2019) | 131 | 50.55 years (±9.57) | FBG: 96.9 mg/dL (±11.4) | Participants were instructed to wear accelerometers on their trunk and right upper leg for seven consecutive days while engaging in their normal daily activities, excluding water-based activities, throughout their waking hours under unrestricted living conditions. Furthermore, they donned a compact G.P.S. gadget and meticulously recorded the locations they frequented along with the duration of device usage. | FBG: 74.84 mg/dL (±37.74) | FBG: −21.16 mg/dL | 0.001 | The glucose level was shown to be greater when both short and long sedentary sequences contributed equally to the overall inactive duration. For instance, a 0.1 increase in the Gini index corresponded to a 3.0 mg/dL fall in glucose levels. The counter-intuitive finding was validated by the inverse relationship between the ratio of sedentary time spent in bouts to total sedentary time and the glucose level. As the proportion of sedentary time spent in bouts fell, the glucose level climbed. The transition from a ratio of 0.9707 (1st quartile) to 0.9892 (3rd quartile) corresponded to a 1.3 mg/dL reduction in glucose level. |
| Honda et al. [31] (2019) | 1758 | 63.6 years (±0.8) | HOMA-IR: 1.49 (±1.35) | Participants were equipped with an accelerometer for a minimum of seven days and had a thorough health check-in in 2012. A 75 g oral glucose tolerance test confirmed the diagnosis of diabetes mellitus. Logistic and linear regression models were used to estimate the relationships among sedentary time, the occurrence of diabetes mellitus, the levels of the homeostasis model assessment of insulin resistance. | H.O.M.A.-I.R.: 1.15 (±0.97) | HOMA-IR: 0.34 | 0.102 | After accounting for demographic and lifestyle factors, such as moderate-to-vigorous physical activity, individuals who spent ten or more hours engaging in sedentary time had a significantly greater likelihood of developing diabetes than those who spent less than 6 h engaging in sedentary time. This notable correlation persisted even after accounting for general and abdominal obesity (as assessed by body mass index and waist circumference). However, it diminished after accounting for dietary energy consumption or the homeostasis model assessment of insulin resistance. There was a positive correlation between sedentary time and degrees of insulin resistance, as measured by the homeostasis model evaluation, among non-diabetic subjects. This correlation remained significant even after adjusting for factors such as obesity or calorie intake. |
| Mossavar-Rahmani et al. [32] (2020) | Not provided | Not provided | Not provided | The measured activity was quantified as 150 min per week of moderate-intensity physical activity, 75 min per week of high-intensity activity, or an equivalent combination of both when scaled to 7 days of accelerometer wear. Model 1 was adjusted for age, sex, and use of medications; model 2 adjusted model 1 for income, education, employment status, smoking, alcohol consumption, and health insurance status; model 3 adjusted model 2 for time and covariates. We considered HbA1C from model 3 only from each quartile as it considered most variables. | HbA1C: 0.17% (±0.15–0.19) | There was a tendency for a decrease in the average increase in HbA1c when comparing the groups | 0.001 | There was a tendency for a decrease in the average increase in HbA1c when comparing the group with the least amount of sedentary behavior to the group with the highest amount. In general, independent of the glycemic level or presence of cardiometabolic disease, persons who followed the recommendations for moderate-to-vigorous physical activity during visit 1 experienced considerably smaller average increases in fasting glucose levels compared to adults who did not follow the recommendations, after accounting for all relevant factors. |
| Banitalebi et al. [33] (2019) | 14 SIT, 14 A + R | 55.36 years (±5.94) SIT; 54.14 years (±5.43) A + R | FBG: 210.07 mg/dL (±32.91) SIT; 214.64 mg/dL (±27.67) A + R | 3 days a week, 50 min, 10 weeks, S.I.T.: treadmill, cycle ergometer | FBG: 137.36 mg/dL (±32.95) SIT; 163.86 mg/dL (±71.47) A + R | FBG: −72.71 mg/dL (SIT group); −50.78 mg/dL (A + R group) | 0.001 | Both exercise groups showed significant and equivalent improvements in aerobic capacity, fasting glucose, and HbA1c compared to the control group. The insulin, weight, and body mass index levels, regardless of the group, decreased dramatically over time. There was no correlation between alterations in myokines and changes in body composition or metabolic profile. |
| A + R: bilateral leg press, lateral pull-down, bench press, bilateral biceps curl, and bilateral triceps push-down | Both S.I.T. and A + R training did not affect myokines tested 48 h after exercise in individuals with type 2 diabetes, even though they improved aerobic capacity and glucose homeostasis compared to the control group. | |||||||
| Gallardo-Gómez et al. [34] (2024) | 1446 T2DM patients from 126 studies | Not provided | HbA1C: 9% (±0.62) | To investigate the correlation between varying amounts of physical activity (both overall and specific to interventions) and glycemic control in individuals diagnosed with type 2 diabetes. | HbA1C: 8.18% (±0.16) | HbA1C: 0.82% (±0.11) | 0.001 | When individuals accumulated 1100 MET min/week (metabolic equivalent task), which is considered the ideal dose with multicomponent therapies, they experienced clinically significant reductions in HbA1c levels for severe uncontrolled diabetes (ranging from −1.06% to −0.67%) and uncontrolled diabetes (ranging from −0.65% to −0.50%). |
| Mendes et al. [35] (2019) | 15 | 60.25 years (±3.14) | FBG: 114.25 mg/dL (±24.65) control session of rest (CON); 112.67 mg/dL (±21.98) HIIT; 115.75 mg/dL (± 21.84) moderate-intensity continuous training (MICN) | While in the postprandial stage, the participants experienced three different experimental conditions in a random order. These conditions included a treadmill walking H.I.I.T. session consisting of five sets of 3 min at 70% of heart rate reserve (H.R.R.) followed by 3 min at 30% H.R.R., a treadmill walking M.I.C.T. session lasting 30 min at 50% H.R.R., and CON. FBG measurements were obtained before, throughout, and up to 50 min following the experimental circumstances. | FBG: 114.08 mg/dL (±24.27) CON; 110.50 mg/dL (±17.73) HIIT; 114.00 mg/dL (±22.31) MICN | FBG: 0.17 mg/dL CON; 2.17 mg/dL HIIT; 1.75 mg/dL MICN | 0.001 | Both high-intensity interval training and moderate-intensity continuous training treadmill walking sessions resulted in decreased blood glucose levels during exercise and a 50 min recovery period in the laboratory compared to the control group. This effect was observed due to the interaction between time and condition. The impact of H.I.I.T. was more significant than that of M.I.C.T. |
| Liu et al. [36] (2019) | 163 participants with H.I.I.T. out of 345 from 13 studies | 15.3 years (±2.2) and 70.1 years (±2.4) (across the 13 studies) | Not provided | The clinical parameters and social cognitive factors of the intervention and control groups were compared after 16 weeks of physical activity (P.A.) intervention among patients with prediabetes. Participants in the intervention group were instructed to do at least 150 min of moderate-intensity P.A. per week in the morning (6:00 a.m. to 11:59 a.m.) or afternoon (12:00 p.m. to 5:59 p.m.). | Not provided | HbA1C: −0.29% (±0.55) FBG: −0.41 mg/dL (±0.91) | 0.001 | HIIT resulted in a noteworthy decrease in body mass index, body fat percentage, HbA1c levels, fasting insulin levels, and VO2peak (maximum oxygen consumption) in individuals diagnosed with T2M. Concerning alterations in the body composition of patients, H.I.I.T. demonstrated a significant improvement in body weight (mean difference: −1.22 kg) and body mass index (mean difference: −0.40 kg/m2) compared to M.I.C.T. |
| Shamizadeh et al. [37] (2020) | 136 prediabetes participants in the intervention group | 51.3 years (±11.2) | FBG: 108.4 mg/dL (±6.1) | Over 16 weeks, the participants were educated about the risk of diabetes and motivated to engage in physical activity as a straightforward method to lower their chances of developing diabetes. Each participant was instructed to abstain from eating for a period of 10–12 h before the blood tests were taken at baseline and after 16 weeks. | FBG: 99.4 mg/dL (±8.1) | FBG: 9 mg/dL | 0.002 | The general linear mixed model analysis revealed substantial decreases in FBG, body mass index (B.M.I.), weight, and diastolic blood pressure (B.P.) in the intervention group compared to the control group. The primary physical activity intervention resulted in a medium-sized drop in FBG levels compared to the control group after 16 weeks. Thus, physical activity intervention positively decreases the likelihood of prediabetes progressing to diabetes in rural patients. |
FBG *—fasting blood glucose; ** HOMA-IR: homeostatic model assessment of insulin resistance; *** S.D.—standard deviation.
Statistical Analysis of the Results
To analyze the studies above, we extracted those that measured fasting blood glucose and added the relevant information in Table 2 below. We then used these statistical data of FBG to determine whether physical activity interventions are effective in reducing fasting glucose levels. By comparing glucose levels before and after the intervention, we could quantify the impact and assess the significance of the changes observed.

Table 2.
Data needed for statistical purposes.
The results table provides a detailed summary of fasting blood glucose levels before and after various interventions across studies from 2018 to 2024. For instance, Whitaker et al. [28] analyzed a large cohort of 1922 participants, reporting a mean FBG of 95 mg/dL at baseline with an SD of 12.6. Post intervention, the mean FBG slightly decreased to 94.6 mg/dL, accompanied by an increased SD of 14, indicating a modest improvement in glucose control, though with greater variability. In contrast, Zheng et al. [29] studied 94 participants and reported a significant reduction in mean FBG from 106 mg/dL (SD = 5.4) to 86.3 mg/dL (SD = 2.1). This substantial decrease indicates a highly effective intervention for lowering blood glucose levels, evidenced by the smaller post-intervention standard deviation, implying more consistent outcomes among the participants.
In some studies, baseline values [28,29,30,35,37] were below the diagnostic threshold for type 2 diabetes mellitus (T2DM) (>125 mg/dL). This discrepancy can be explained by several factors:
- Diversity of study populations: some studies included participants with prediabetes or impaired glucose tolerance, rather than exclusively focusing on individuals diagnosed with T2DM. These participants may have had baseline FBG levels below the diabetic threshold but were still at risk of developing T2DM. Some studies also included mixed cohorts, where participants with both prediabetes and T2DM were analyzed together, resulting in an overall lower baseline FBG mean.
- Variability in inclusion criteria: the inclusion criteria for studies varied, with some trials assessing the impact of physical activity on glucose metabolism in individuals at different stages of metabolic dysregulation (e.g., those with insulin resistance or early-stage T2DM). Some studies focused on lifestyle interventions in high-risk populations rather than focusing exclusively on patients with established T2DM, a fact which may explain why their baseline FBG values were lower than expected for a diabetic cohort.
- Impact of medication use: although pharmacological treatment details were not available for all studies, it is possible that participants in certain trials were already receiving a glucose-lowering therapy at baseline, a fact which could have resulted in lower FBG values before the intervention.
- Variations in measurement techniques and study design: differences in laboratory standards, sample timing, and glucose measurement protocols among studies could have influenced baseline FBG values. Some studies reported mean FBG levels, which may not fully capture individual variations or the presence of outliers.
Debache et al. [30] also demonstrated notable results with their intervention, where 131 participants experienced a drop in mean FBG from 96.9 mg/dL (SD = 11.4) to 74.84 mg/dL (SD = 37.74). While the mean FBG decreased significantly, the high post-intervention S.D. indicates considerable variability in how participants responded to the intervention. Banitalebi et al. [33] investigated two different training methods: sprint interval training (S.I.T.) and aerobic plus resistance (A + R) training. The S.I.T. group showed a remarkable reduction in mean FBG from 210.07 mg/dL (SD = 32.91) to 137.36 mg/dL (SD = 32.95), whereas the A + R group experienced a decrease from 214.64 mg/dL (SD = 27.67) to 163.86 mg/dL (SD = 71.47). Both interventions were effective, particularly S.I.T., but the variability in the A + R group post intervention suggests diverse responses. Mendes et al. [35] focused on high-intensity interval training (H.I.I.T.) and found a slight reduction in mean FBG from 112.67 mg/dL (SD = 21.98) to 110.5 mg/dL (SD = 17.73) among 15 participants, indicating a less pronounced but consistent effect. Lastly, Shamizadeh et al. [37] reported a decrease in mean FBG from 108.4 mg/dL (SD = 6.1) to 99.4 mg/dL (SD = 8.1) in 136 participants, highlighting the intervention’s effectiveness in lowering blood glucose levels with moderate variability post intervention.
Based on the above table, we generated a forest plot, a graphical representation commonly used in meta-analyses to display the results of multiple studies examining the same research question [38].
Figure 2 presents a forest plot of the six studies that analyzed FBG, such a plot being another graphical tool commonly used in meta-analyses to assess publication bias or the presence of small-study effects [39].
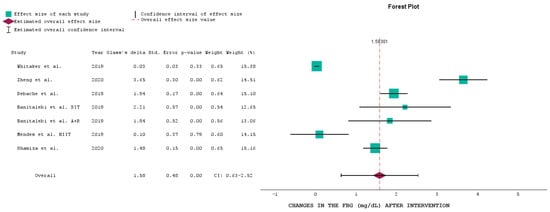
Figure 2.
Forest plot of the selected studies analyzing FBG at baseline and after the intervention [28,29,30,33,35,37].
It plots the effect size of individual studies against a measure of study precision, usually the standard error or sample size [40].
The overall effect size of the interventions in relation to FBG levels was 1.58 mg/dL (CI: 0.63 to 2.52), indicating a significant reduction in FBG levels across the studies. The p-value for the overall effect size was 0.00, highlighting the statistical significance of the combined interventions. This suggests that, on average, the interventions effectively reduced FBG levels among participants.
Whitaker et al. [28] showed a minimal effect size of 0.03 with a CI crossing zero (0.03 ± 0.03), indicating no significant change in FBG levels post intervention. This result is reflected in the p-value of 0.33, suggesting a lack of statistical significance.
Zheng et al. [29] demonstrated a substantial effect size of 3.65 (CI: 3.35 to 3.95), indicating a significant reduction in FBG levels. The p-value was 0.00, confirming the statistical significance of this intervention.
Debache et al. [30] reported an effect size of 1.94 (CI: 1.77 to 2.11), showing a significant reduction in FBG levels with a p-value of 0.00. Banitalebi et al. [33] included two interventions: S.I.T. (effect size of 2.21, CI: 1.64 to 2.78) and A + R (effect size of 1.84, CI: 1.32 to 2.36), both indicating significant reductions in FBG levels with p-values of 0.00. Mendes et al. [35], for the H.I.I.T. intervention, showed a smaller effect size of 0.10 (CI: −0.27 to 0.47), with a p-value of 0.79, indicating no significant change in FBG levels. Shamizadeh et al. [37] reported an effect size of 1.48 (CI: 1.33 to 1.63) with a p-value of 0.00, indicating a significant reduction in FBG levels.
Most studies showed significant reductions in FBG levels, with p-values of 0.00, except for Whitaker et al. [28] and Mendes et al. [35]. This suggests that most interventions were effective in lowering FBG levels, although the degree of effectiveness varied across studies.
The confidence intervals for Zheng et al. [29], Debache et al. [30], and both interventions by Banitalebi et al. [33] are relatively narrow, indicating consistent results within these studies. In contrast, the wider confidence intervals for Whitaker et al. [28] and Mendes et al. [35] suggest more variability in the outcomes, a phenomenon which may explain the need for significant findings in these studies.
The forest plot presented here highlights the overall effectiveness of various physical activity and lifestyle interventions in reducing FBG levels, with significant reductions observed in most studies. However, the effectiveness varies, emphasizing the need for tailored approaches to optimize glucose management in different populations. The overall effect size reinforces the importance of such interventions in managing blood glucose levels and reducing the risk of diabetes-related complications.
An effect size of 1.58 using Glass’s delta is considered large [41], indicating a substantial difference between the baseline and the end of the trial. In the context of the impact of physical activity and lower sedentarism on fasting glucose levels, this would mean that engaging in more physical activity and reducing sedentary behavior leads to a significantly lower fasting glucose level compared to the baseline (less physical activity and more sedentary behavior).
The funnel plot in Figure 3 illustrates the distribution of studies based on their effect sizes and standard errors, providing insights into potential publication bias and the overall consistency of the studies [42].
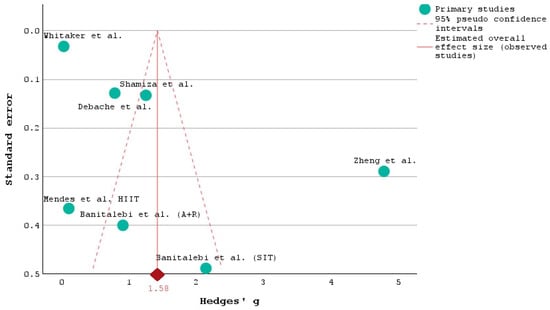
Figure 3.
Funnel plot of the selected studies analyzing the FBG at baseline and after the intervention [28,29,30,33,35,37].
The plot appears to be relatively symmetrical, suggesting that there is no significant publication bias [43]. Studies with both small and large sample sizes are represented on either side of the average effect size (Hedges’ g), indicating a balanced distribution of effect sizes [44,45].
Most studies cluster around the overall effect size estimate of 1.58, indicated by the red diamond at the bottom of the plot. This central tendency reinforces the reliability of the overall effect size derived from the meta-analysis. Studies with larger sample sizes, such as those by Whitaker et al. [28], Shamizadeh et al. [37], and Debache et al. [30], are positioned higher on the plot with lower standard errors, reflecting greater precision in their effect size estimates. Conversely, studies with smaller sample sizes, like those by Banitalebi et al. [33] (S.I.T. and A + R) and Mendes et al. [35], have larger standard errors and are positioned lower on the plot, indicating less precision.
The study by Zheng et al. [29] stands out with a particularly large effect size (Hedges’ g > 5) and a relatively low standard error, indicating a very strong and precise intervention effect. This study’s deviation from the rest suggests that it implemented a uniquely effective intervention or had a specific population that responded exceptionally well.
The consistency in the studies’ effect sizes included in our analysis around the overall estimate suggests that the interventions generally have a positive and significant impact on the outcomes measured (likely FBG levels, as discussed in previous conclusions). The funnel plot included in Figure 3 supports the conclusion that physical activity and related interventions effectively reduce FBG levels across diverse populations and study designs.
Furthermore, we looked at the studies that examined the changes in HbA1c before and after the intervention. Thus, we found that Mossavar-Rahmani et al. [32], Gallardo-Gómez et al. [34], and Liu et al. [36] analyzed the changes in the glycation of hemoglobin, and we present the results below. The study conducted by Mossavar-Rahmani et al. [32] analyzed three models; therefore, we included all three in our analysis. Model 1 was adjusted for age at V1, sex, and use of medications that affect the dependent variable, model 2 added income, education, employment status, smoking, alcohol consumption, and health insurance status to model 1, and model 3 adjusted model 2 for other variables and time spent engaging in sedentary behavior.
Table 3 below shows the analysis conducted by Mossavar-Rahmani et al. [32] which provides a detailed view of HbA1c levels across different quartiles for three models, reflecting the variations and statistical significance in these measurements.

Table 3.
Changes in cardiovascular disease risk factors based on sedentary time as presented by Mossavar-Rahmani et al. [32].
In quartile 1, HbA1c levels were consistently at 0.13 across all models, indicating similar baseline glycemic control among the lowest quartile participants. This consistency suggests that the initial glycemic status is relatively stable and not influenced by different model adjustments. In quartile 2, HbA1c levels remained steady at 0.14 across all models, reinforcing the trend observed in quartile 1. Quartile 3 showed an increase in HbA1c levels to 0.16 across all models, highlighting a consistent rise in glycemic levels as we move to higher quartiles. This increase suggests that the factors influencing HbA1c in quartile 3 are similarly accounted for across different models, pointing to a reliable pattern of glycemic deterioration in this quartile. In quartile 4, HbA1c levels reached 0.17 in model 1 while remaining at 0.16 in models 2 and 3. The slight difference in model 1’s higher HbA1c level could indicate that additional variables accounted for in model 1 contribute to a marginally higher glycemic impact. However, the consistent HbA1c levels in models 2 and 3 suggest a plateau effect at higher quartiles, where further adjustments do not significantly alter the glycemic outcome.
Figure 4 shows the percentage change in HbA1C levels across different studies and models.
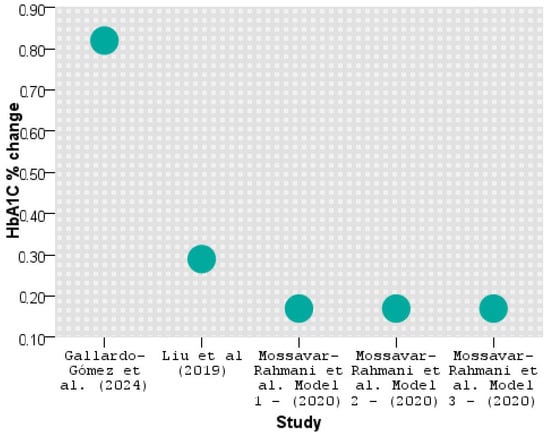
Figure 4.
HbA1C reduction across studies [32,34,36].
As seen above, Gallardo-Gómez et al. [34] significantly improved HbA1C by lowering the hemoglobin glycation by 0.82%, followed by Liu et al. [36] with 0.29%. The results are similar in all three models applied by Mossavar-Rahmani et al. [32].
Finally, one study by Honda et al. [31] measured the HOMA-IR. The HOMA-IR is used to estimate insulin resistance, a critical factor in developing type 2 diabetes and other metabolic conditions. A decrease in HOMA-IR indicates an improvement in insulin sensitivity. Honda et al. [31] reported a decrease in HOMA-IR of 0.34 following physical activity and reduced sedentary behavior intervention, suggesting that these lifestyle changes benefit insulin resistance.
4. Discussion
Regular physical activity is crucial for managing diabetes, as it controls glycemic levels, enhances insulin sensitivity, and reduces the risk of cardiovascular complications [46].
Improved glucose control is linked to enhanced self-management of diabetes. To boost self-management, physicians should prioritize enhancing patients’ understanding and confidence in areas such as nutrition, glucose management, and sports. The Diabetes Self-Management Questionnaire tool addresses these aspects [47].
Research studies have demonstrated that people with diabetes who participate in regular aerobic exercises and strength training have better glycemic control and overall health [48]. However, barriers such as time constraints, fear of hypoglycemia during exercise, and lack of motivation may hinder physical activity participation in this population [49].
Our study aligns with previous studies indicating a strong association between the overall level of physical activity and immediate glycemic management in individuals with diabetes. Mersarwi et al. [50] discovered that engaging in physical activity is linked to early improvements in glucose regulation, such as enhanced insulin sensitivity and improved beta-cell function, and they found a consistent connection between the level of physical activity and later changes in long-term glucose regulation, specifically HbA1c levels. HbA1c levels also appeared to be more closely correlated with a reduction in sedentary behavior. We also found in the literature a more significant impact on glycemic control when sedentary time was minimized, regardless of the total amount of physical activity performed [51,52,53,54].
One exercise program conducted by Pippi et al. [55] which included a combination of different workouts was shown to help improve the health of two groups of children and adolescents who were overweight or obese. Within the children group, the findings revealed enhancements in all anthropometric measurements (except body weight) and physical performance indicators (excluding the H.G. test for the left hand) among both children and boys. The authors noticed a decrease in waist circumference and waist-to-height ratio values in girls, with such parameters being associated with an increased risk of cardiovascular disease. Within the group of adolescents, this exercise intervention resulted in better anthropometric characteristics, including those linked with decreased cardiovascular risk and enhanced strength values for both boys and girls. In the same study, the exercise program reduced weight within the subgroup of girls. Utilizing a multimodal approach that incorporates strength testing and the analysis of body composition using A.D.P. can serve as a valuable method for monitoring health and evaluating the effectiveness of an exercise program in overweight/obese young individuals. Additional research involving bigger sample sizes will be necessary to validate and apply the findings of this preliminary study [55,56].
Physical activity reduces sedentary behavior, and improved sleep quality can complement traditional diabetes management, leading to better health outcomes for individuals with diabetes [57,58,59]. Additionally, personalized approaches that consider individual preferences, barriers, and socio-cultural factors are essential for promoting long-term adherence to lifestyle modifications [60].
Promoting healthy lifestyle habits and addressing behavioral risk factors can be fundamental components of strategies to prevent and manage T2DM at the population level [61]. Implementing systematic screening for lifestyle-related risk factors, such as physical inactivity and unhealthy dietary patterns, during routine health check-ups, especially among individuals with risk factors, is essential [62]. Positive screenings can trigger referrals to nutritionists, exercise specialists, or lifestyle coaches for further evaluation and intervention. Integrating lifestyle education components into diabetes prevention programs can be highly effective [63]. Education on this should be included in school curricula to increase awareness among children and adolescents about the significance of healthy habits for overall wellbeing and long-term health [64]. Additionally, it could also help prevent chronic diseases such as T2DM.
Sedentary behavior has been consistently linked to detrimental effects on metabolic health, particularly in individuals with T2M [65,66]. Research indicates that regular physical activity plays a crucial role in managing and preventing the progression of the disease, as it helps improve glucose control, insulin sensitivity, and overall metabolic function [67]. In contrast, sedentary lifestyles have been associated with increased risk factors for metabolic disorders, including T2M, due to the negative impact on blood glucose levels and insulin response [68]. Individuals who engage in prolonged periods of sitting or inactivity are more likely to experience elevated blood sugar levels and insulin resistance, exacerbating the symptoms and complications of diabetes [69]. These findings feature the importance of promoting physical activity and reducing sedentary behavior as integral components of diabetes management and prevention strategies [70].
The evidence overwhelmingly supports the benefits of physical activity for managing type 2 diabetes [71,72,73]. Engaging in regular exercise has been shown to improve blood glucose control, reduce insulin resistance, and lower the risk of complications associated with the disease [74]. Additionally, physical activity plays a crucial role in weight management, which is a critical factor in diabetes management. However, it is essential not to overlook the detrimental effects of sedentary behavior on individuals with type 2 diabetes. Prolonged sitting has been linked to increased insulin resistance, higher levels of inflammation, and a greater risk of developing cardiovascular diseases [75]. Therefore, incorporating regular physical activity and reducing sedentary behavior are central components of an effective diabetes management plan. Future research should focus on developing personalized strategies to promote physical activity and reduce sedentary time in individuals with type 2 diabetes to optimize their health outcomes and quality of life.
A sedentary lifestyle contributes to the disease’s pathogenesis and hinders effective management strategies [76]. Addressing sedentary behavior through targeted interventions is crucial in preventing and treating type 2 diabetes, as highlighted by the need to promote physical activity and reduce sedentary time to improve metabolic health outcomes [77,78]. Also, lowering HbA1C through physical activity is an effective strategy for managing and preventing type 2 diabetes and lowering blood glucose levels, both of which are vital components of diabetes management and prevention [79,80]. The benefits extend beyond blood glucose control, impacting overall health, mental wellbeing, and quality of life [81].
Some individuals with T2DM may have physical limitations such as neuropathy, peripheral artery disease, or arthritis, making certain types of physical activity challenging or painful [82,83]. At the same time, managing T2DM can be a barrier. Individuals may need to gain knowledge about appropriate exercise types, duration, intensity, and timing concerning their medication and meal schedules [84].
To tackle the various obstacles faced by individuals with type 2 diabetes, a comprehensive approach that includes education, support, and environmental modifications is needed [85].
Healthcare providers can effectively help overcome these obstacles by offering tailored exercise suggestions, addressing issues related to hypoglycemia, and providing access to resources for physical activity programs or support groups [86]. In addition, community-based interventions that prioritize enhancing accessibility to secure and reasonably priced exercise facilities, offering social support, and advocating for lifestyle improvements might assist patients with T.D.M. in adopting and sustaining regular physical activity routines [87,88,89,90,91].
Limitations
An essential constraint of our research is the restricted sample size of participants in most of the studies that we selected for analysis. Moreover, this research is a cross-sectional correlation study, and our results cannot be interpreted as a cause-and-effect link. This is because diabetes, regardless of fat, may independently lead to sleep disturbance. Prospective research might be developed to assess the impact of any modification to sedentary patterns on glycemic management with greater accuracy.
Also, some studies reported nutritional guidelines and dietary habits, which we considered in our interpretation. Since our focus was on physical activity interventions, we prioritized studies that controlled for diet or reported minimal dietary changes during the study period.
We acknowledge the importance of additional patient characteristics such as blood pressure, lipid profile, smoking status, and alcohol consumption for understanding diabetes management. However, these variables were not consistently reported across all the studies included in our analysis. Given this limitation, we prioritized glycemic outcomes (FBG, HbA1c, and insulin resistance), which were more uniformly documented across studies.
Potential inaccuracies and bias in self-reported data on sitting time and screen time must be considered, and these measures may have been underestimated. While previous studies have examined overall sitting time rather than specific activities, we recommend that future studies focus on analyzing distinct forms of sedentary behavior, as these impact cognitive performance assessments significantly.
5. Conclusions
The studies included in this meta-analysis collectively provide strong evidence of the positive effects of physical activity interventions on fasting blood glucose and glycated hemoglobin levels, highlighting the significant impact of reducing sedentary behavior and increasing physical activity. Whitaker et al. demonstrated that substituting 30 min of sedentary time with low-intensity physical activity resulted in a slight decrease in FBG levels, from 95.0 mg/dL to 94.6 mg/dL, and improvements in other cardiometabolic risk factors. These findings suggest that even modest increases in physical activity can contribute to better glycemic control and reduced metabolic risks over time. Zheng et al. further emphasized the importance of reducing sedentary behavior, showing a substantial reduction in FBG levels, from 106 mg/dL to 86.3 mg/dL, among participants who reduced their sedentary time. Debache et al. provided additional support for the benefits of physical activity by documenting a significant reduction in FBG levels after participants engaged in regular physical activity monitored through accelerometers. At the same time, Honda et al., Gallardo-Gómez et al., and Mendes et al. provide evidence that sedentary time is associated with higher levels of insulin resistance. Thus, increased time spent sitting and using screens will likely affect cognition negatively. Furthermore, this behavior is also associated with other health problems, such as markers for heart and metabolic conditions, previously confirmed in this particular group of people. These findings indicate that reducing sedentary time and incorporating regular physical activity is crucial for managing insulin resistance and maintaining healthy FBG levels in older populations, often at higher risk of diabetes and other metabolic conditions.
Author Contributions
Conceptualization, S.I., D.E.T., N.M.M., V.I., A.P., S.D., I.E.I., C.I.B., L.C.P. and A.P.S.; methodology, A.N.T., E.M.H., A.P., M.S.P., S.D., I.E.I., C.I.B., L.C.P., F.G. and A.P.S.; software, S.I., D.E.T., A.N.T. and E.M.H.; validation, S.I., D.E.T., M.S.P., N.M.M., V.I., A.N.T., E.M.H., A.P., S.D., I.E.I., C.I.B., L.C.P. and A.P.S.; formal analysis, S.I., D.E.T., N.M.M., V.I., S.D., I.E.I., A.P.S. and F.G.; investigation, S.I., D.E.T., N.M.M., V.I., A.N.T., E.M.H., A.P., I.E.I., C.I.B., L.C.P. and A.P.S.; resources, S.I., D.E.T., M.S.P., E.M.H., A.P., S.D. and A.P.S.; data curation, S.I., D.E.T., N.M.M., M.S.P., L.C.P., F.G. and A.P.S.; writing—original draft preparation V.I., A.N.T., E.M.H., A.P. and I.E.I.; writing—review and editing, S.I., D.E.T., N.M.M., V.I., A.N.T., M.S.P., E.M.H., F.G., A.P., S.D., I.E.I., C.I.B., L.C.P. and A.P.S.; visualization, S.I., D.E.T., F.G. and A.P.S.; supervision, S.I., N.M.M. and A.P.S.; project administration, S.I., D.E.T., N.M.M., M.S.P., V.I., A.N.T., E.M.H., A.P., S.D., I.E.I., C.I.B., L.C.P., F.G. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casari, S.; Di Paola, M.; Banci, E.; Diallo, S.; Scarallo, L.; Renzo, S.; Gori, A.; Renzi, S.; Paci, M.; de Mast, Q.; et al. Changing Dietary Habits: The Impact of Urbanization and Rising Socio-Economic Status in Families from Burkina Faso in Sub-Saharan Africa. Nutrients 2022, 14, 1782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association Professional Practice Committee. Introduction and Methodology: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S1–S4. [CrossRef]
- Nicoara, A.D.; Alexandrescu, L.; Tofolean, D.E.; Iliescu, M.G.; Condur, L.M.; Tofolean, I.T. The Impact of Cardiac Rehabilitation On Quality Of Life In Elderly Heart Failure Patients-Literature Review. Balneo PRM Res. J. 2024, 15, 723. [Google Scholar] [CrossRef]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care. 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar] [PubMed] [PubMed Central]
- 2aviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2015, 12, 4–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Diabetes and Nutrition Study Group (D.N.S.G.) of the European Association for the Study of Diabetes (E.A.S.D.). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Ormiston, C.K.; Wang, X.; Sui, J.; et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: The Healthy Heroes randomized control trial. Cell Metab. 2022, 34, 1442–1456.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efrem, I.C.; Moța, M.; Vladu, I.M.; Mitrea, A.; Clenciu, D.; Timofticiuc, D.C.P.; Diaconu, I.D.; Turcu, A.; Crișan, A.E.; Geormăneanu, C.; et al. A Study of Biomarkers Associated with Metabolic Dysfunction-Associated Fatty Liver Disease in Patients with Type 2 Diabetes. Diagnostics 2022, 12, 2426. [Google Scholar] [CrossRef] [PubMed]
- Alexescu, T.G.; Bordea, I.R.; Cozma, A.; Rajnoveanu, R.; Buzioanu, A.D.; Nemes, R.M.; Tudorache, S.I.; Boca, B.M.; Todea, D.A. Metabolic Profile and risk of early Atherosclerosis in Patients with Obesity and Overweight. Rev. Chim. 2019, 70, 3627–3633. [Google Scholar]
- Shechter, A.; Foster, G.D.; Lang, W.; Reboussin, D.M.; St-Onge, M.P.; Zammit, G.; Newman, A.B.; Millman, R.P.; Wadden, T.A.; Jakicic, J.M.; et al. Effects of a lifestyle intervention on R.E.M. sleep-related O.S.A. severity in obese individuals with type 2 diabetes. J. Sleep Res. 2017, 26, 747–755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hawkins, M.; Marcus, B.; Pekow, P.; Rosal, M.C.; Tucker, K.L.; Spencer, R.M.C.; Chasan-Taber, L. The Impact of a Randomized Controlled Trial of a Lifestyle Intervention on Sleep Among Latina Postpartum Women. Ann. Behav. Med. 2021, 55, 892–903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicolae, M.; Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Frecus, C.E.; Mihai, L.; Lupu, V.V.; Ion, I.; Pantazi, A.C.; Nelson Twakor, A.; et al. Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children. Nutrients 2023, 15, 3430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Zhang, Y.; Shen, S.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: A multicentre, open-label, randomized controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577, Erratum in: Lancet Diabetes Endocrinol. 2023, 11, e13. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Celli, A.; Barnouin, Y.; Jiang, B.; Blevins, D.; Colleluori, G.; Mediwala, S.; Armamento-Villareal, R.; Qualls, C.; Villareal, D.T. Lifestyle Intervention Strategy to Treat Diabetes in Older Adults: A Randomized Controlled Trial. Diabetes Care. 2022, 45, 1943–1952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Courcoulas, A.P.; Gallagher, J.W.; Neiberg, R.H.; Eagleton, E.B.; DeLany, J.P.; Lang, W.; Punchai, S.; Gourash, W.; Jakicic, J.M. Bariatric Surgery vs Lifestyle Intervention for Diabetes Treatment: 5-Year Outcomes From a Randomized Trial. J. Clin. Endocrinol. Metab. 2020, 105, 866–876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johansen, M.Y.; MacDonald, C.S.; Hansen, K.B.; Karstoft, K.; Christensen, R.; Pedersen, M.; Hansen, L.S.; Zacho, M.; Wedell-Neergaard, A.S.; Nielsen, S.T.; et al. Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 637–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghavami, H.; Radfar, M.; Soheily, S.; Shamsi, S.A.; Khalkhali, H.R. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, the result of a randomized clinical trial. Agri 2018, 30, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (I.D.P.P.). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Khoo, J.; Gani, L.U.; Malakar, R.D.; Tay, T.L.; Tirukonda, P.S.; Kam, J.W.; Tin, A.S.; Tang, T.Y. Effect of multidisciplinary intensive targeted care in improving diabetes mellitus outcomes: A randomized controlled pilot study—The Integrated Diabetes Education, Awareness and Lifestyle modification in Singapore (IDEALS) Program. Trials 2019, 20, 549. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dambha-Miller, H.; Day, A.J.; Strelitz, J.; Irving, G.; Griffin, S.J. Behaviour change, weight loss and remission of Type 2 diabetes: A community-based prospective cohort study. Diabet. Med. 2020, 37, 681–688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Syrjälä, M.B.; Bennet, L.; Dempsey, P.C.; Fharm, E.; Hellgren, M.; Jansson, S.; Nilsson, S.; Nordendahl, M.; Rolandsson, O.; Rådholm, K.; et al. Health effects of reduced occupational sedentary behaviour in type 2 diabetes using a mobile health intervention: A study protocol for a 12-month randomized controlled trial-the ROSEBUD study. Trials 2022, 23, 607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitaker, K.M.; Pettee Gabriel, K.; Buman, M.P.; Pereira, M.A.; Jacobs DRJr Reis, J.P.; Gibbs, B.B.; Carnethon, M.R.; Staudenmayer, J.; Sidney, S.; Sternfeld, B. Associations of Accelerometer-Measured Sedentary Time and Physical Activity With Prospectively Assessed Cardiometabolic Risk Factors: The CARDIA Study. J. Am. Heart Assoc. 2019, 8, e010212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, C.; Tian, X.Y.; Sun, F.H.; Huang, W.Y.; Sheridan, S.; Wu, Y.; Wong, S.H. Associations of Sedentary Patterns with Cardiometabolic Biomarkers in Physically Active Young Males. Med. Sci. Sports Exerc. 2021, 53, 838–844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Debache, I.; Bergouignan, A.; Chaix, B.; Sneekes, E.M.; Thomas, F.; Sueur, C. Associations of Sensor-Derived Physical Behavior with Metabolic Health: A Compositional Analysis in the Record Multisensor Study. Int. J. Environ. Res. Public Health 2019, 16, 741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Honda, T.; Kishimoto, H.; Mukai, N.; Hata, J.; Yoshida, D.; Hirakawa, Y.; Shibata, M.; Ohara, T.; Kumagai, S.; Ninomiya, T. Objectively measured sedentary time and diabetes mellitus in a general Japanese population: The Hisayama Study. J. Diabetes Investig. 2019, 10, 809–816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mossavar-Rahmani, Y.; Hua, S.; Qi, Q.; Strizich, G.; Sotres-Alvarez, D.; Talavera, G.A.; Evenson, K.R.; Gellman, M.D.; Stoutenberg, M.; Castañeda, S.F.; et al. Are sedentary behavior and physical activity independently associated with cardiometabolic benefits? The Hispanic Community Health Study/Study of Latinos. B.M.C. Public Health 2020, 20, 1400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banitalebi, E.; Kazemi, A.; Faramarzi, M.; Nasiri, S.; Haghighi, M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019, 217, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Gómez, D.; Salazar-Martínez, E.; Alfonso-Rosa, R.M.; Ramos-Munell, J.; Del Pozo-Cruz, J.; Del Pozo Cruz, B.; Álvarez-Barbosa, F. Optimal Dose and Type of Physical Activity to Improve Glycemic Control in People Diagnosed With Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2024, 47, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. Int. J. Environ. Res. Public Health 2019, 16, 4163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.X.; Zhu, L.; Li, P.J.; Li, N.; Xu, Y.B. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 575–593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shamizadeh, T.; Jahangiry, L.; Sarbakhsh, P.; Ponnet, K. Social cognitive theory-based intervention to promote physical activity among prediabetic rural people: A cluster randomized controlled trial. Trials 2019, 20, 98. [Google Scholar] [CrossRef]
- Afonso, J.; Ramirez-Campillo, R.; Clemente, F.M.; Büttner, F.C.; Andrade, R. The Perils of Misinterpreting and Misusing “Publication Bias” in Meta-analyses: An Education Review on Funnel Plot-Based Methods. Sports Med. 2024, 54, 257–269. [Google Scholar] [CrossRef]
- Dettori, J.R.; Norvell, D.C.; Chapman, J.R. Seeing the Forest by Looking at the Trees: How to Interpret a Meta-Analysis Forest Plot. Glob. Spine J. 2021, 11, 614–616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marfo, P.; Okyere, G.A. The accuracy of effect-size estimates under normals and contaminated normals in meta-analysis. Heliyon 2019, 5, e01838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panjeh, S.; Nordahl-Hansen, A.; Cogo-Moreira, H. Establishing new cutoffs for Cohen’s d: An application using known effect sizes from trials for improving sleep quality on composite mental health. Int. J. Methods Psychiatr. Res. 2023, 32, e1969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taylor, J.M.; Alanazi, S. Cohen’s and Hedges’ g. J. Nurs. Educ. 2023, 62, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Aloe, A.M. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 2021, 40, 403–426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.L.; Edwards, H.; Do, T.N.D.; Finlayson, K. Effectiveness of a theory-based foot care education program (3STEPFUN) in improving foot self-care behaviours and foot risk factors for ulceration in people with type 2 diabetes. Diabetes Res. Clin. Pract. 2019, 152, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Rizvi, A.A.; Giglio, R.V.; Stoian, A.P.; Ligi, D.; Mannello, F. Impact of Glucose-Lowering Medications on Cardiovascular and Metabolic Risk in Type 2 Diabetes. J. Clin. Med. 2020, 9, 912. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Borba, A.K.O.T.; Arruda, I.K.G.; Marques, A.P.O.; Leal, M.C.C.; Diniz, A.D.S. Knowledge and attitude about diabetes self-care of older adults in primary health care. Cien Saude Colet. 2019, 24, 125–136, (In Portuguese; In English). [Google Scholar] [CrossRef] [PubMed]
- Mesarwi, O.; Polak, J.; Jun, J.; Polotsky, V.Y. Sleep disorders and the development of insulin resistance and obesity. Endocrinol. Metab. Clin. N. Am. 2013, 42, 617–634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munteanu, I.; Marc, M.; Gheorghevici, C.; Diaconu, G.A.; Feraru, N.; Sion, D.; Nemes, R.M.; Mahler, B. Sleep Quality Aspects in Post-COVID-19 Patients. J. Pers. Med. 2023, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Anca, P.S.; Toth, P.P.; Kempler, P.; Rizzo, M. Gender differences in the battle against COVID-19: Impact of genetics, comorbidities, inflammation and lifestyle on differences in outcomes. Int. J. Clin. Pract. 2021, 75, e13666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Jenkins, A.J.; Greenlaw, N.; Dudman, K.; Fernandes, T.; Carty, D.M.; Hughes, A.D.; Januszewski, A.S.; Stehouwer, C.D.; Petrie, J.R. Cardiometabolic risk factors, peripheral arterial tonometry and metformin in adults with type 1 diabetes participating in the REducing with MetfOrmin Vascular Adverse Lesions trial. Diab. Vasc. Dis. Res. 2023, 20, 14791641231183634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crofford, O.B. Diabetes control and complications. Annu. Rev. Med. 1995, 46, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R.; Mascherini, G.; Izzicupo, P.; Bini, V.; Fanelli, C.G. Effects of a Mixed Exercise Program on Overweight and Obese Children and Adolescents: A Pilot, Uncontrolled Study. Int. J. Environ. Res. Public Health 2022, 19, 9258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maranna, H.; Lal, P.; Mishra, A.; Bains, L.; Sawant, G.; Bhatia, R.; Kumar, P.; Beg, M.Y. Negative pressure wound therapy in grade 1 and 2 diabetic foot ulcers: A randomized controlled study. Diabetes Metab. Syndr. 2021, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Atila, C.; Holze, F.; Murugesu, R.; Rommers, N.; Hutter, N.; Varghese, N.; Sailer, C.O.; Eckert, A.; Heinrichs, M.; Liechti, M.E.; et al. Oxytocin in response to MDMA provocation test in patients with arginine vasopressin deficiency (central diabetes insipidus): A single-centre, case-control study with nested, randomized, double-blind, placebo-controlled crossover trial. Lancet Diabetes Endocrinol. 2023, 11, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.; Cucinotta, D. The late complications of diabetes mellitus. Ann. Ital. Med. Int. 1991, 6 Pt 2, 126–136. [Google Scholar] [PubMed]
- Diepersloot, R.J.; Bouter, K.P.; Hoekstra, J.B. Influenza infection and diabetes mellitus. Case for annual vaccination. Diabetes Care 1990, 13, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J. D.C.C.T.: Implications for diabetes care in the UK. Diabet. Med. 1993, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J. Diabetes: The science and the art. Hyperglycemia v Complications. Arch. Intern. Med. 1983, 143, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, E.; Beheshtirouy, S.; Aghamohammadzadeh, N.; Ghaffary, S.; Sarbakhsh, P.; Shaseb, E. Management of diabetic neuropathy with memantine: A randomized clinical trial. Diab Vasc. Dis. Res. 2023, 20, 14791641231191093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mindrescu, N.M.; Guja, C.; Jinga, V.; Ispas, S.; Curici, A.; Nelson Twakor, A.; Pantea Stoian, A.M. Interactions between Gut Microbiota and Oral Antihyperglycemic Drugs: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ispas, S.; Tuta, L.A.; Botnarciuc, M.; Ispas, V.; Staicovici, S.; Ali, S.; Nelson-Twakor, A.; Cojocaru, C.; Herlo, A.; Petcu, A. Metabolic Disorders, the Microbiome as an Endocrine Organ, and Their Relations with Obesity: A Literature Review. J. Pers. Med. 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keen, H. The Diabetes Control and Complications Trial (D.C.C.T.). Health Trends. 1994, 26, 41–43. [Google Scholar] [PubMed]
- Fernando, D.J. Knowledge about diabetes and metabolic control in diabetic patients. Ceylon Med. J. 1993, 38, 18–21. [Google Scholar] [PubMed]
- Furrer, R.; Hawley, J.A.; Handschin, C. The molecular athlete: Exercise physiology from mechanisms to medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leibold, N. The Effect of a School Nurse Led Education Intervention on Blood Pressure and Physical Activity Levels in Adolescents. Ph.D. Thesis, College of Saint Mary, Moraga, CA, USA, March 2009. [Google Scholar]
- Motoc, N.Ș.; Man, M.A.; Tudorache, S.I.; Rusu, E.; Brailescu, C.M.; Mahler-Boca, B.; Campean, A.U.; Pop, C.M. The Importance of Mask Type and Mask Materials in Sleep Apnea Patients. Rev Chim. 2019, 70, 3273–3276. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Stoian, A.P.; Rizzo, M. Metabolic Syndrome: From Molecular Mechanisms to Novel Therapies. Int. J. Mol. Sci. 2021, 22, 10038. [Google Scholar] [CrossRef]
- Sathish, T.; MacMillan, F. Prevention of Type 2 Diabetes with Lifestyle Interventions: Evidence vs. Reality. Diabetology 2023, 4, 427–429. [Google Scholar] [CrossRef]
- Bassin, S.R.; Srinath, R. The Impact of Physical Activity in Patients With Type 2 Diabetes. Am. J. Lifestyle Med. 2023, 19, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Ghahri, S.; Dianatinasab, A.; Fararouei, M.; Dianatinasab, M. Exercise and Type 2 Diabetes. Adv. Exp. Med. Biol. 2020, 1228, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Cannata, F.; Vadalà, G.; Russo, F.; Papalia, R.; Napoli, N.; Pozzilli, P. Beneficial Effects of Physical Activity in Diabetic Patients. J. Funct. Morphol. Kinesiol. 2020, 5, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keadle, S.K.; Conroy, D.E.; Buman, M.P.; Dunstan, D.W.; Matthews, C.E. Targeting Reductions in Sitting Time to Increase Physical Activity and Improve Health. Med. Sci. Sports Exerc. 2017, 49, 1572–1582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamilton, M.T.; Hamilton, D.G.; Zderic, T.W. Sedentary behavior as a mediator of type 2 diabetes. Med. Sport. Sci. 2014, 60, 11–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorenzo, C.; Hartnett, S.; Hanley, A.J.; Rewers, M.J.; Wagenknecht, L.E.; Karter, A.J.; Haffner, S.M. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: The insulin resistance atherosclerosis study. J. Clin. Endocrinol. Metab. 2013, 98, 1622–1630. [Google Scholar] [CrossRef]
- Suceveanu, A.I.; Mazilu, L.; Katsiki, N.; Parepa, I.; Voinea, F.; Pantea-Stoian, A.; Rizzo, M.; Botea, F.; Herlea, V.; Serban, D.; et al. NLRP3 Inflammasome Biomarker—Could Be the New Tool for Improved Cardiometabolic Syndrome Outcome. Metabolites 2020, 10, 448. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin. J. Med. 2017, 84 (Suppl. S1), S15–S21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delevatti, R.S.; Kanitz, A.C.; Alberton, C.L.; Marson, E.C.; Lisboa, S.C.; Pinho, C.D.; Lovatel, G.A.; Korb, A.; Bertoldi, K.; Macedo, R.C.; et al. Glucose control can be similarly improved after aquatic or dry-land aerobic training in patients with type 2 diabetes: A randomized clinical trial. J. Sci. Med. Sport 2016, 19, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Summaries for patients. Effects of aerobic training, resistance training, or both on control of blood sugar in type 2 diabetes. Ann. Intern. Med. 2007, 147, I16. [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S151–S167. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Owen, N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.; Barnett, J.; Mutrie, N. Physical activity consultation for people with Type 2 diabetes: Evidence and guidelines. Diabet. Med. 2007, 24, 857–864. [Google Scholar] [CrossRef]
- Sigal, R.J.; Armstrong, M.J.; Colby, P.; Kenny, G.P.; Plotnikoff, R.C.; Reichert, S.M.; Riddell, M.C. Physical activity and diabetes. Can. J. Diabet. 2018, 42, S54–S63. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.B.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Kathuria, A.; Al Mahmeed, W.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; Cosentino, F.; et al. Post-COVID syndrome, inflammation, and diabetes. J. Diabetes Complicat. 2022, 36, 108336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corina, A.; Abrudan, M.B.; Nikolic, D.; Cătoi, A.F.; Chianetta, R.; Castellino, G.; Citarrella, R.; Stoian, A.P.; Pérez-Martínez, P.; Rizzo, M. Effects of Aging and Diet on Cardioprotection and Cardiometabolic Risk Markers. Curr. Pharm. Des. 2019, 25, 3704–3714. [Google Scholar] [CrossRef] [PubMed]
- Pantea, S.A.; Andronache, L.; Hainarosie, R.; Păduraru, D.; Badiu, C.; Arsene, A.; Mehedintu, C.; Ditu, G.; Pițuru, S.M.; Orlov, C.; et al. Dietary habits and lifestyle in school-aged children from Bucharest, Romania. J. Mind Med. Sciences. 2018, 5, 85–92. [Google Scholar]
- Reurean-Pintilei, D.; Potcovaru, C.-G.; Salmen, T.; Mititelu-Tartau, L.; Cinteză, D.; Lazăr, S.; Pantea Stoian, A.; Timar, R.; Timar, B. Assessment of Cardiovascular Risk Categories and Achievement of Therapeutic Targets in European Patients with Type 2 Diabetes. J. Clin. Med. 2024, 13, 2196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).