Introduction
Consciousness generally refers to the feeling of 'self- awareness' [
1]. However, the true scope of consciousness is broader than just self-awareness, as conscious control can guide thoughts and sensations towards appropriate responses [
2]. All forms of voluntary motor responses require conscious control for achieving their desired effects. Similarly, we assimilate the meaning of sensory information when we are conscious of it. Consciousness is also actively involved in the processing of higher cognitive functions, which include thinking, reasoning, memory, and generating appropriate motor responses. These cognitive functions lack aptitude and meaning if we are consciously inattentive and distracted. Meticulous conscious control is necessary to explore memory for relevant images, perform calculations, and choose words for an appropriate response. Such processes require the prompt coordination of signals from different brain lobes in a specific order [
3]. Since we can carry out these functions consciously, they would share an intimate relationship with conscious processes. Hence, an awareness of the 'whole-self' would be a more comprehensive term for consciousness than just 'self-awareness'. It seems that consciousness interacts with the cortical areas of cognition, and creates awareness and meaning in their functions. This interaction may occur through both short and long axonal extensions of cortical neurons, forming an intricate network of interwoven fibers within the deep white matter of the brain, connecting specific brain regions to facilitate coordination [
4]. It can access and retrieve information from any lobe and connect it to the functions of other lobes to configure a productive response [
5]. The network wired with different lobes and engendering symphony to the brain’s processes could also be a potential venue for the genesis of consciousness. However, to date, there has not been a satisfactory explanation for either the nature or the mechanism of consciousness.
The brain possesses an inherent proclivity for the lateralization of its functions, wherein each hemisphere primarily governs specific functions [
6]. For instance, comprehension and motor expression of language are well- developed in the left hemisphere as opposed to the right side of the brain [
7]. Likewise, analysis and calculations, recognition of letters and numbers and whole word processing can be carried out by the left hemisphere [
8]. Conversely, the right hemisphere is involved in interpreting creativity, perception, and recognition of faces, objects, and places [
9]. It remains unclear whether conscious processes are more prevalent in one hemisphere of the brain as compared to the other. The concept is intriguing as recurring damage to focal areas of the brain in the setting of ischemic infarctions does not affect consciousness, and signifies that consciousness is a distributed function of the brain [
10]. In this paper, we present our findings regarding consciousness disorders in cases of large segment occlusion of the middle cerebral artery. These findings support the modular distribution of conscious processes.
Materials and Methods
A prevalent distribution of conscious processes within one hemisphere could lead to frequent episodes of unconsciousness from recurrent dysfunction in that hemisphere. Therefore, we employed the pathological event of ischemic infarction which was large enough and could dysfunction the whole hemisphere with certainty [
11]. The middle cerebral artery (MCA) in each hemisphere was particularly suitable for our study, given its extensive territory covering the central two-thirds of a hemisphere [
12]. More proximal occlusion of the MCA leads to a nearly complete dysfunction of the hemisphere due to pronounced ischemia, effectively interrupting signaling within the hemisphere [
13]. We obtained sample from two different centers as well- developed infarctions of proximal MCA were infrequent, likely due to patients arriving promptly for better treatment.
Ethical approval, study design and statistics
This is a prospective, cross-sectional observational study in patients with acute ischemic stroke due to proximal segment occlusion of MCA. Ethical approval for the study was granted by the review board of the District Headquarter Teaching Hospital Kohat (registration No. 2965/K-17). Prior to their participation in the study, a written informed consent was obtained from a close relative of each patient. Data analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 20. We assessed levels of consciousness using the Glasgow Coma Scale and determined the severity of stroke using the National Institute of Health Stroke Scale (NIHSS) [
14]. Tables were used to compare the severity of stroke and levels of unconsciousness between the two hemispheres. We employed Fisher’s exact test to evaluate the significance of consciousness disorder in a specific hemisphere.
Neuroimaging criteria for the large segment’s occlusion
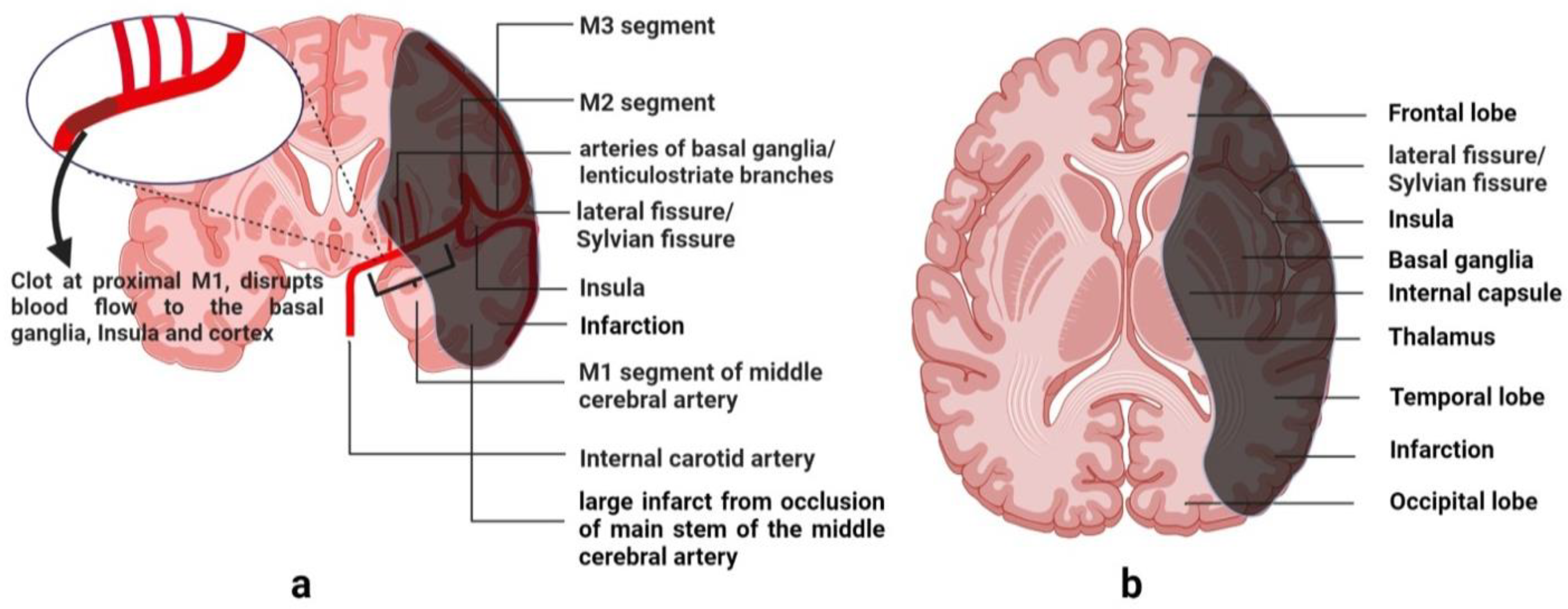
The neurovascular territory of the middle cerebral artery (MCA) is the central two-thirds of a hemisphere. Middle cerebral artery is a continuation of the intracranial part of the internal carotid artery. It is divided into five segments, M1 to M5, where M1 and M2 are denoted as large segments of MCA [
12]. These large segments give penetrating branches to the ipsilateral basal ganglia, internal capsule and the deep white matter of the hemisphere. The anatomical course and the territory of MCA are shown in
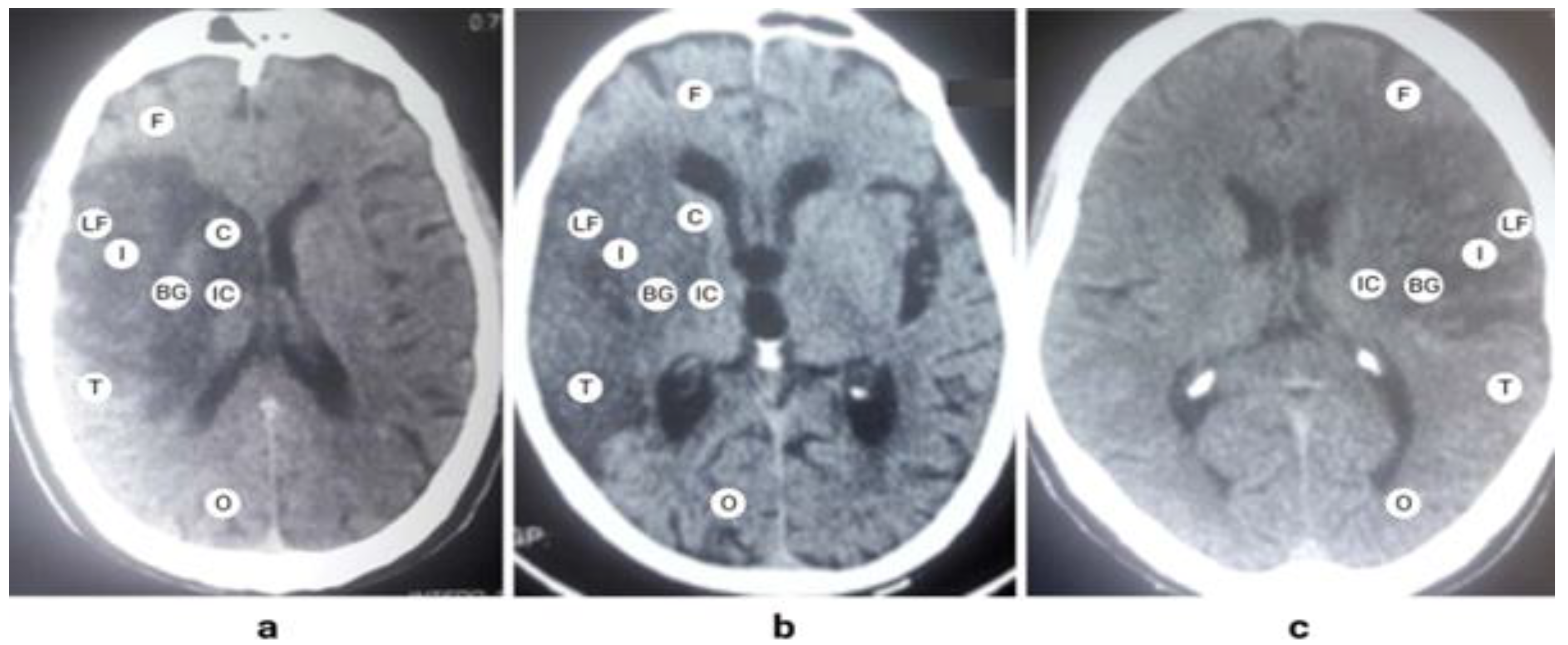
Figure 1. Being the main part, M1 segment’s occlusion produces a major infarction by limiting blood supply to a large part of the hemisphere. A characteristic pattern formed by the proximal occlusion of the MCA is a confluent signal of infarction involving the internal capsule, basal ganglia, and the insula, as shown in
Figure 2. It extends to all lobes of the brain except the occipital lobe.
Significance of MCA’s territory for consciousness
The middle cerebral artery covers a substantial volume of the brain, which approximates to two-thirds of a hemisphere. It supplies specialized groups of cells within important lobes, including the frontal, parietal, and temporal lobes. These cells are located in the sensory association areas of the brain, where they interpret conscious meanings of sensory information, facilitate thinking, and engage in reasoning [
15]. They assist in language comprehension and analysis of the three-dimensional position of an object. Occluding the main stem of the MCA turns the whole territory into a dead tissue. This not only disrupts signaling within the same hemisphere but also hinders communication with the contralateral hemisphere. It was likely to produce unconsciousness if conscious processes were more prevalent in the affected hemisphere.
Clinimetric assessment of consciousness
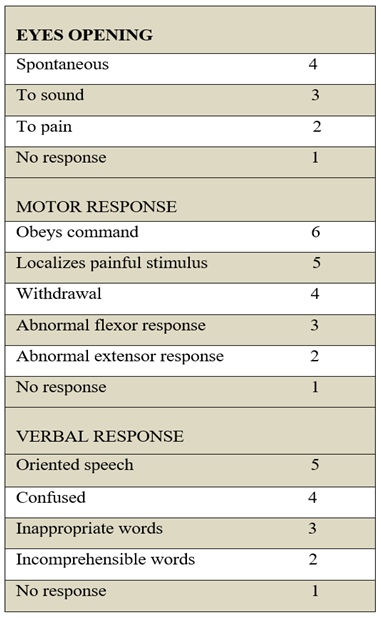
We employed Glasgow Coma Scale (GCS) for the assessment of conscious level. This clinimetric scale has numerical scoring for three motor responses that include eyes opening, speech and the limbs’ movement. The cumulative score of all three components represents the conscious level of a patient [
16]. The detail of the numerical scoring is given in
Table 1. Aphasia due to stroke is a clinical limitation to the GCS score as it produces a false-positive decrement of 5 verbal points to the conscious level despite the normal scores for eyes and limbs. For this reason, verbal response scoring was not included and categorization of conscious level was entirely based on the eyes and limbs’ responses. Hence, the cumulative scores of seven or less for the eyes and limbs represented an altered conscious level, and scores of eight to ten represented a normal conscious level in our patients. We evaluated the symptoms of a major stroke using the National Institutes of Health Stroke Scale (NIHSS) and confirmed the presence of a whole-territory infarction of the MCA with brain computed tomography (CT) imaging performed in the emergency department.
Inclusion criteria
The main determinants of patients’ selection in our study were the following,
Patient of any age with a normal behavior and orientation about self before the onset of the stroke. This information was obtained through general observations made by immediate family members of the patient prior to the stroke event.
Well-developed infarction of proximal MCA on the brain CT at presentation.
Patients arriving within 48 hours of symptoms’ onset at the hospital with no significant edema of ischemic core on brain CT that could contribute to unconsciousness.
Exclusion criteria
The following comorbid conditions and risk factors that could contribute to unconsciousness were carefully excluded.
Patients with metabolic derangements like liver cirrhosis, renal failure, and hypoglycemia and electrolyte imbalance.
Concurrent fits with stroke.
Previous documentation of a major stroke that caused significant loss of brain volume in the territory of MCA.
Raised intracranial pressure due to marked hemorrhagic transformation of the ischemic core.
Data availability
Anonymized data will be shared with any researcher upon request.
Results
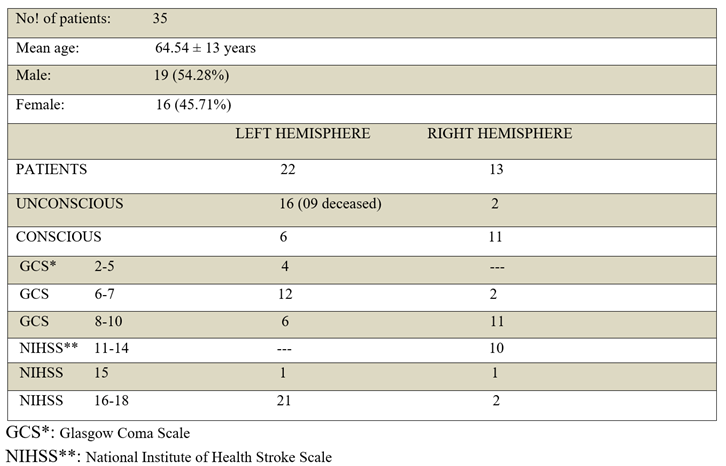
Patients’ demographics and clinical presentation
A fully resolved infarction of the proximal M1 segment was a rare clinical presentation due to earlier arrival of patients for better treatment. A total of 35 patients were enrolled in the study from September 24, 2020 till May 07, 2022. Of these, twenty-two patients had involvement of the left hemisphere (22 versus 13, frequency: 62.85%, odds: 1.69). Male and female participants constituted 19 (54.28%) and 16 (45.71%) respectively, with a mean age of 64.54 ± 13 years (range: 32 to 95). Severe contralateral hemiplegia and facial weakness were common in the proximal occlusion of MCA, leading to a baseline NIHSS score of 11 for stroke in either hemisphere. Aphasia and cognitive deficits in comprehension were commonly seen in the left hemisphere’s stroke that contributed to a higher severity of NIHSS (range: 15 to 18). This was attributed to patients' inability to respond to consciousness-related questions within the NIHSS. Contralateral hemisensory neglect was prevalent in right hemisphere strokes, with no impact on comprehension, resulting in a reduced NIHSS severity (range: 11 to 17).
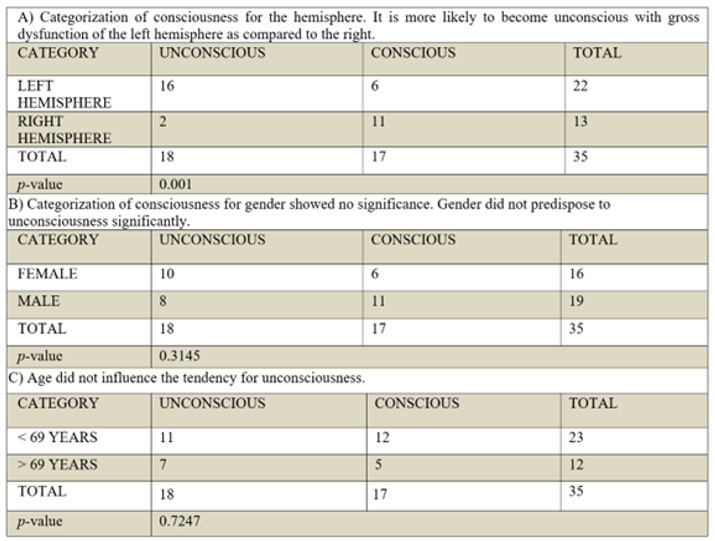
The hemisphere prone to unconsciousness
A striking difference was observed in the GCS scores of the two hemispheres. Patients with left hemisphere involvement were more prone to unconsciousness as compared to the right hemisphere (16 versus only 2 unconscious patients of the right hemisphere, OR: 14.63, 95% CI: 2.47-86.05). Their level of consciousness significantly declined to the lowest GCS scores (Frequency: 72%, GCS: 2/10=3/22 cases, GCS: 5/10=1/22 cases, GCS: 6/10=7/22 cases, GCS: 7/10=5/22 cases). In comparison, only 2 patients with right hemisphere involvement attained the upper cutoff mark of 7/10 GCS for unconsciousness (Frequency: 15%, GCS: 7/10=2/13 cases). The raised intracranial pressure from cerebral edema was probably not contributing to the current difference of unconsciousness, given that the volume of infarctions and the time elapsed before the documentation of unconsciousness—within 48 hours—were similar for both hemispheres. The observed difference in unconsciousness as interpreted with the Fisher’s exact test, proved significant and was unlikely due to chance (p- value= 0.001). It signified a left predominant distribution of conscious processes in most individuals. Among the eleven conscious patients of the right hemisphere with normal comprehension, as well as six conscious patients of the left hemisphere with no comprehension for NIHSS questions, it was highly likely that a prevailing left-sided distribution of conscious processes existed. Hence, a total of 33 patients exhibited a left-prevalent distribution of conscious processes (94%), aligning with the left hemisphere's dominance for right-handedness, speech, and language comprehension in the general population (90%). The tendency for unconsciousness was independent of age (p-value=0.7247), and gender (p-value= 0.3145), as shown in
Table 2.
Severity and outcome of stroke
Unconscious patients in either hemisphere had NIHSS scores 6 points higher than conscious patients of the right hemisphere. Nine of sixteen unconscious patients of the left hemisphere deceased within 7 to 10 days of hospital stay (16 Unconscious; NIHSS: 18/42; 56.25% deceased, 6 Conscious; NIHSS: 15-18/42, all alive within the hospital). The occurrence of severe aspiration pneumonia subsequent to unconsciousness probably contributed to their high mortality rate. A few conscious patients of the left hemisphere also attained higher NIHSS scores of 18 due to the Wernicke’s aphasia. In contrast, conscious patients of the right hemisphere had a suboptimum severity of NIHSS due to normal cognition, which helped them respond correctly to the consciousness-related questions within the NIHSS (2 Unconscious; NIHSS: 17/42, 11 Conscious; NIHSS: 11-15/42).
Table 3 depicts individual scores of patients in the two hemispheres.
Discussions
We observed the catastrophic event of proximal occlusion of the middle cerebral artery (MCA) and examined its effects on consciousness, sensory-motor functions, and in-hospital mortality within 48 hours of symptom onset, prior to the development of cerebral edema. The MCA is a major and central vessel in the anterior circulation, sustaining vital structures of the brain covering the lateral convexity of the central two-thirds of a hemisphere. In addition to the basal nuclei in the deep white matter, the frontal, parietal, and temporal lobes fall within the territory of MCA. These structures have a significant contribution to the conscious interpretation of comprehension, orientation, reasoning and thinking. In the wake of proximal occlusion of MCA with a gross and rapid loss of function in these structures, we were able to ascertain quantitative differences in the distribution of conscious processes between the two hemispheres. A fully developed infarct of the whole territory of MCA was an uncommon observation due to awareness in the general population about better treatment outcomes within the first few hours of stroke. The M1-segment of the left MCA was more susceptible to occlusion than the right MCA. 62.85% of the patients had involvement of the left MCA. Hedna et al had a similar observation in their study [
17]. A plausible explanation is that the left common carotid artery originates directly from the aortic arch in the superior mediastinum, whereas the right common carotid artery arises from the brachiocephalic trunk. This makes the left MCA more prone to the embolic events. It is also possible that the left hemisphere might have greater metabolic demands than the right. The baseline severity of motor weakness, resulting in contralateral hemiplegia and facial weakness was similar for both hemispheres. These deficits led to a baseline NIHSS score of 11 points. However, left hemisphere involvement added 3 to 6 points of severity on NIHSS compared to the right side. Factors contributing to increased severity in these patients included unconsciousness, aphasia, and lack of comprehension to verbal questions within the NIHSS. Out of 22 left hemisphere cases, 21 patients scored 16 to 18 points, while 1 patient scored 15 points on the NIHSS. In comparison, patients with right hemisphere involvement often exhibited lower severity on the NIHSS. They usually had intact cognition and speech, and were able to respond to the verbal questions. Hemisensory neglect on the contralateral side was a common deficit in these patients. Out of 13 patients of the right hemisphere, 10 patients scored between 11 to 14 points, while 2 unconscious patients attained 16 to 18 points. Other studies also corroborate lower functional outcomes and higher NIHSS scores in left hemisphere lesions [
17,
18].
The left hemisphere regulates critical functions of the brain. Of these, the comprehension of written and spoken language and its motor expression are distinctive functions. In addition, it determines self-orientation of normal behavior and right-handedness in 90% of the general population [
19,
20]. The self-orientation of sound behavior is what represents our normal cognition. The thinking, reasoning, memory and appropriate motor response were severely affected in the left hemisphere’s stroke. Hence, the left hemisphere has greater significance in facilitating day-to-day activities. The contiguity of many specialized functions within the left hemisphere would definitely give it leverage in decision-making over the right hemisphere. For instance, comprehension and motor expression of language are well-developed in the left hemisphere, and it would need less assistance from the right hemisphere to generate appropriate verbal responses. Our results clearly show predominant distribution of conscious processes in the left hemisphere. Its dysfunction frequently produced unconsciousness, indicating that conscious processes were more concentrated in the left hemisphere. Out of 22 patients of the left hemisphere, 16 became unconscious in the acute phase of stroke within the first 24 hours. There was neither any significant edema of the ischemic core nor a midline shifts on the initial brain CT that could suggest the possibility of raised intracranial pressure. Hence, unconsciousness in these patients was purely related to the loss of function in the left hemisphere and not due to global rise in intracranial pressure. Their level of consciousness dropped significantly to the lower limits of the 10-point Glasgow coma scale. Out of 16 unconscious patients of the left hemisphere, 4 patients attained 2-5 points, 7 patients scored 6 points, while 5 patients scored 7 points. Only two cases of the right hemisphere developed unconsciousness, and each scored 7 points. Variation in the severity of unconsciousness was only observed in the left hemisphere stroke, suggesting that conscious processes were predominantly skewed to the left. This characterization of a quantitative difference in the content distribution of consciousness between the two hemispheres has been documented for the first time and is testable. The left- biased distribution of conscious processes has several scientific implications. Notably, consciousness works similar to other ordinary functions of the brain in that its processes are also more predominant on one side. Our finding also provides insights into the basic questions about the nature and existence of consciousness within the brain. If it is testable, it is unequivocally a brain- derived function that stems from the ordinary activity of cortical neurons. The manifestation of unconsciousness due to dysfunction in two-thirds of the left hemisphere signifies that the neurons and their connections determining consciousness span over a widespread area of the brain. Consequently, consciousness is a substantial functional component within the consciousness- cognition pair.
Unconsciousness was also related to the increased mortality. Nine of sixteen unconscious patients of the left hemisphere died during their hospital stay. The increased severity of aspiration pneumonia in the unconscious patients probably contributed to their high mortality. It would have not been related to the possibility of brain herniation as conscious patients of both the hemispheres also had similar infarctions and there was no incidence of sudden death to suggest brain herniation. They developed aspiration pneumonia subsequent to unconsciousness. While sixteen of twenty-two cases of the left hemisphere were unconscious, the remaining six patients also had no conscious control for an appropriate response and behavior. They were unable to interpret and respond to simple questions such as ‘close your eyes’, ‘protrude your tongue’, and ‘raise the unaffected left upper limb’. It was indicating a strong link between the processes of consciousness and cognition in the ipsilateral cerebral cortex. It appears that consciousness and cognition are strongly linked and do not dissociate. Disrupting cognition will impair conscious control for self-orientation of normal behavior and vice versa. In other words, there is an abundance of cortically assigned areas of consciousness in the left hemisphere that link different areas of cognition with subcortical neuronal fibers of coordination. We have illustrated this interaction with a simplified scheme in
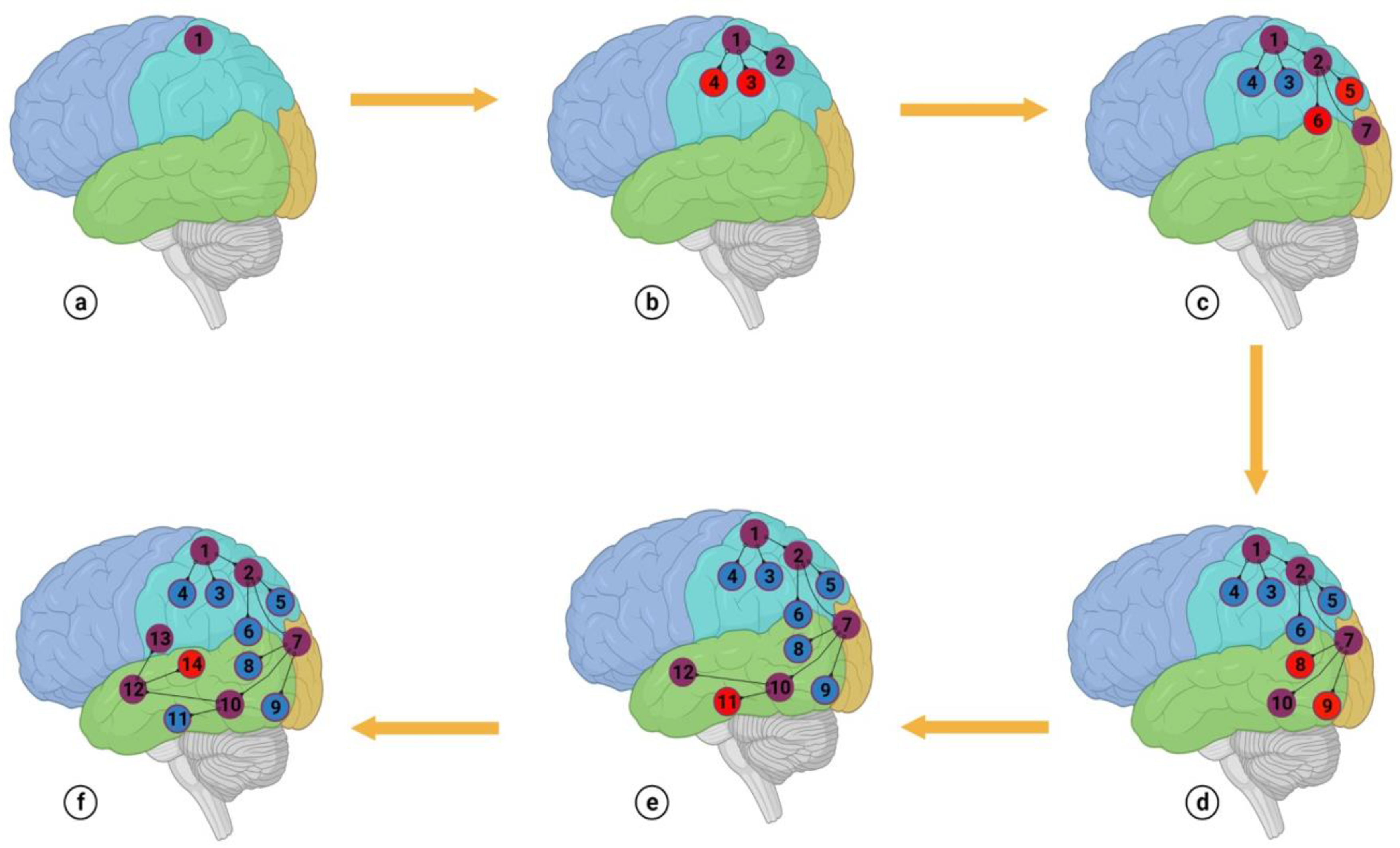
Figure 3.
In this model, we identify two distinct types of cortical attributes—one for consciousness and the other for cognition. Attributes specific to consciousness stimulate the cortical attributes of cognition for a particular function in a sequence and add conscious meaning to the intended function. For instance, developing a conscious thought about a cup of coffee in our mind requires stimulation of several different aspects of cognition for the intended thought. Most of these aspects pertain to the cup's shape, color, and the taste, warmth, and color of the coffee. If these aspects are considered individually, and are not coordinated, the thought of a cup of coffee would not be generated in our mind. A complete understanding of the intended thought only forms when all the relevant aspects are connected and stimulated sequentially. Hence, coordinating the cortical aspects of cognition gives meaning and awareness to the intended thought. If we imagine the shape of a 'glass' without activating other aspects like its name, texture, use, and how it's held, we won't be able to create a meaningful mental image. We propose that cortical attributes of consciousness primarily govern this coordination.
Conscious-control can dominate cognition if most of the subcortical fibers belong to the cortical areas of consciousness [
3,
5]. The fibers can stimulate different areas of cognition in a sequence to develop thinking, reasoning and appropriate motor response. The predominance of conscious processes throughout an entire hemisphere also carries a clinical implication; the presence of unconsciousness should be indicative of a profound brain insult rather than a localized injury. The idea of an extensive area of the brain governing consciousness is further substantiated by the observation of unconsciousness in disorders that result in whole-brain dysfunction. These conditions include generalized seizures, hypoglycemia, subarachnoid hemorrhage, midbrain infarction and systemic shock [21-23]. Preserved consciousness in split-brain syndrome and altered consciousness in unilateral temporal lobe epilepsy are further clinical observations that support prevalent distribution of consciousness in either hemisphere [
24,
25]. The limitations of our study are a limited sample size and observing a time-lapse of 24 to 48 hours before the assessment of unconsciousness. In a future study, we recommend observing unconsciousness by confirming the proximal occlusion of MCA with CT angiography and major infarct core with CT perfusion scan in the first few hours of stroke. Subsequent results would be more persuasive as the possibility of elevated pressure due to a major stroke will be alleviated.