The Role of Cone-Beam Computed Tomography CT Extremity Arthrography in the Preoperative Assessment of Osteoarthritis
Abstract
1. Introduction
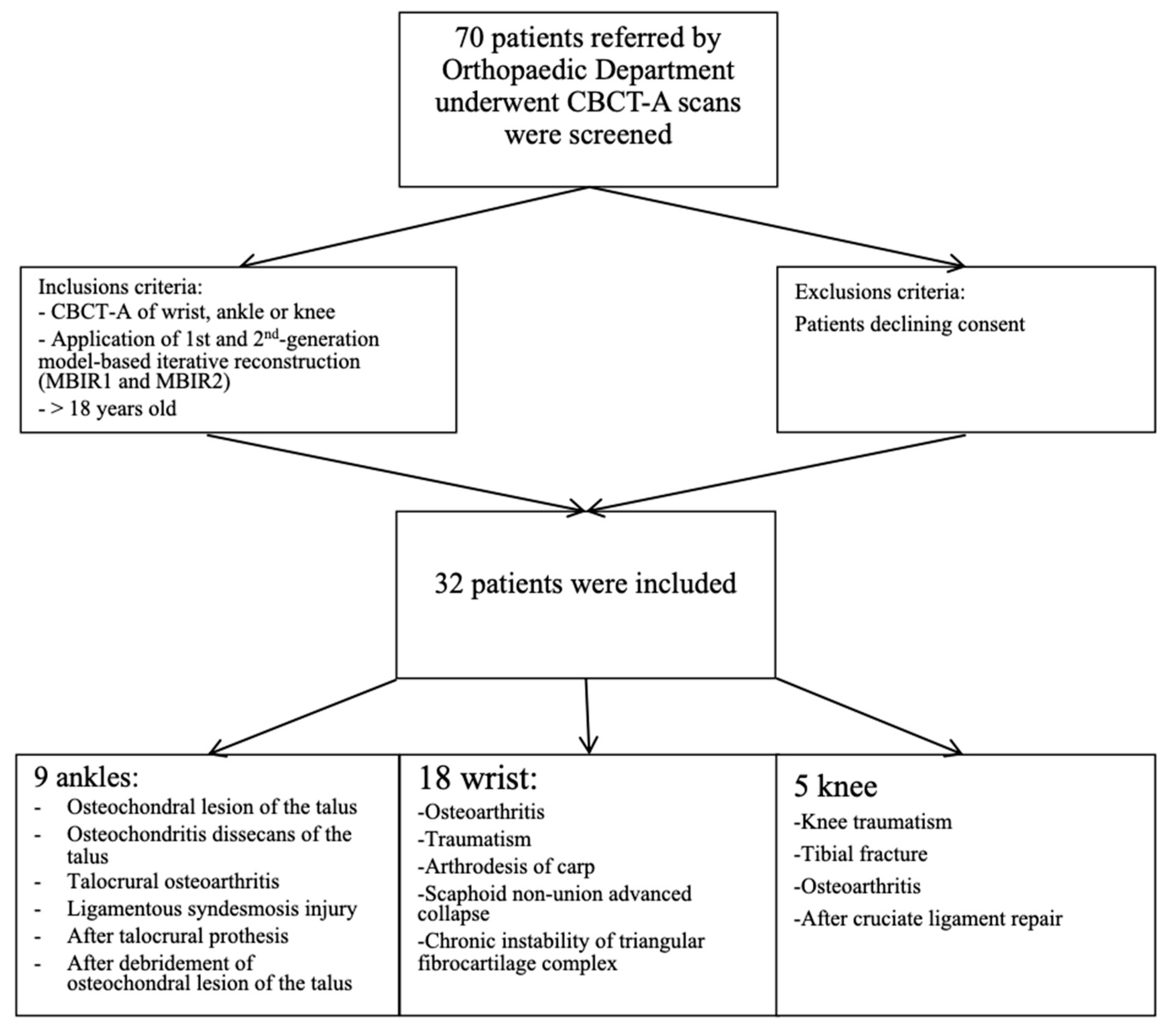
2. Materials and Methods
2.1. Patient Population
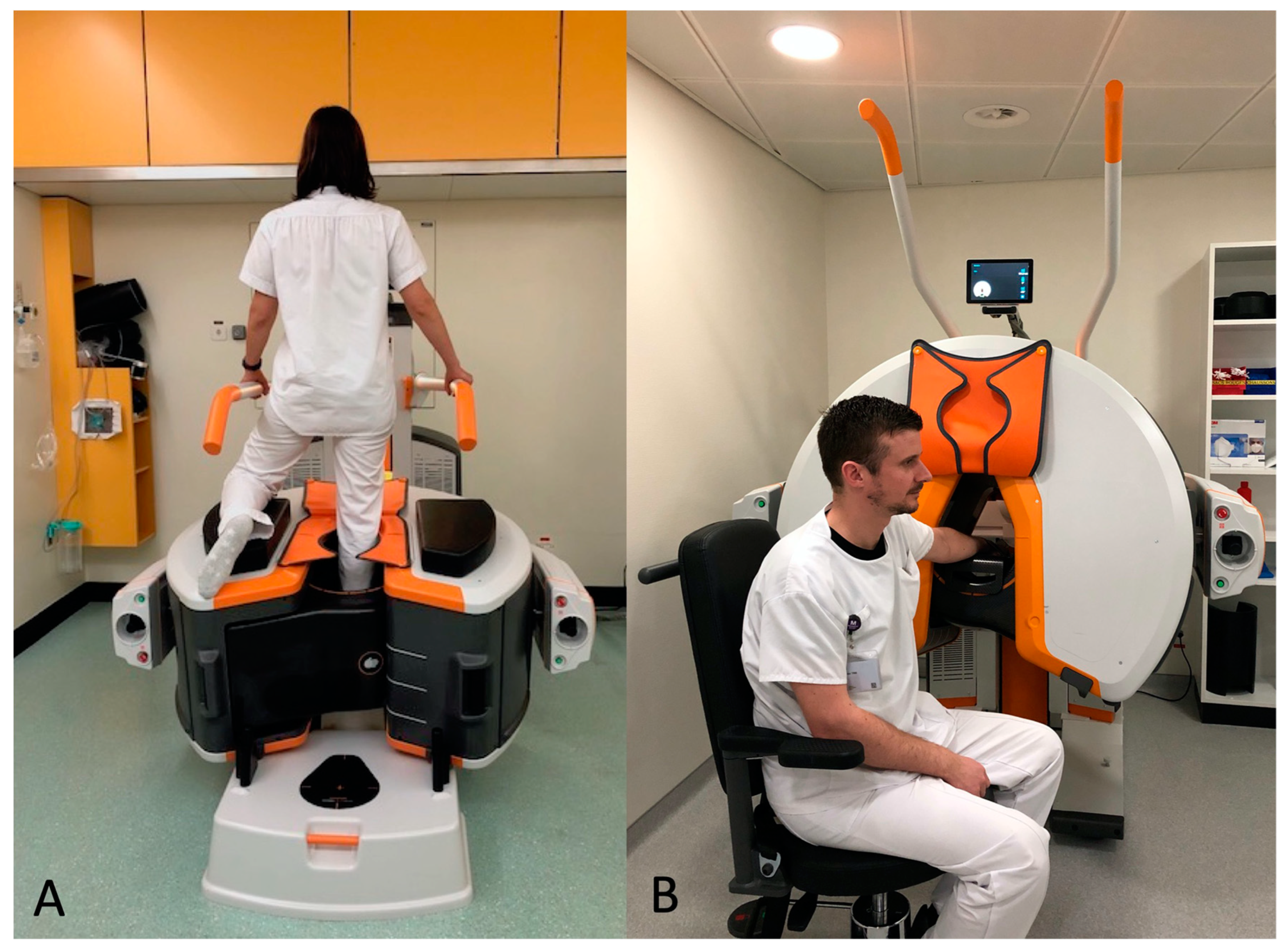
2.2. DR and CBCT-A Acquisition Protocol and Image Reconstruction
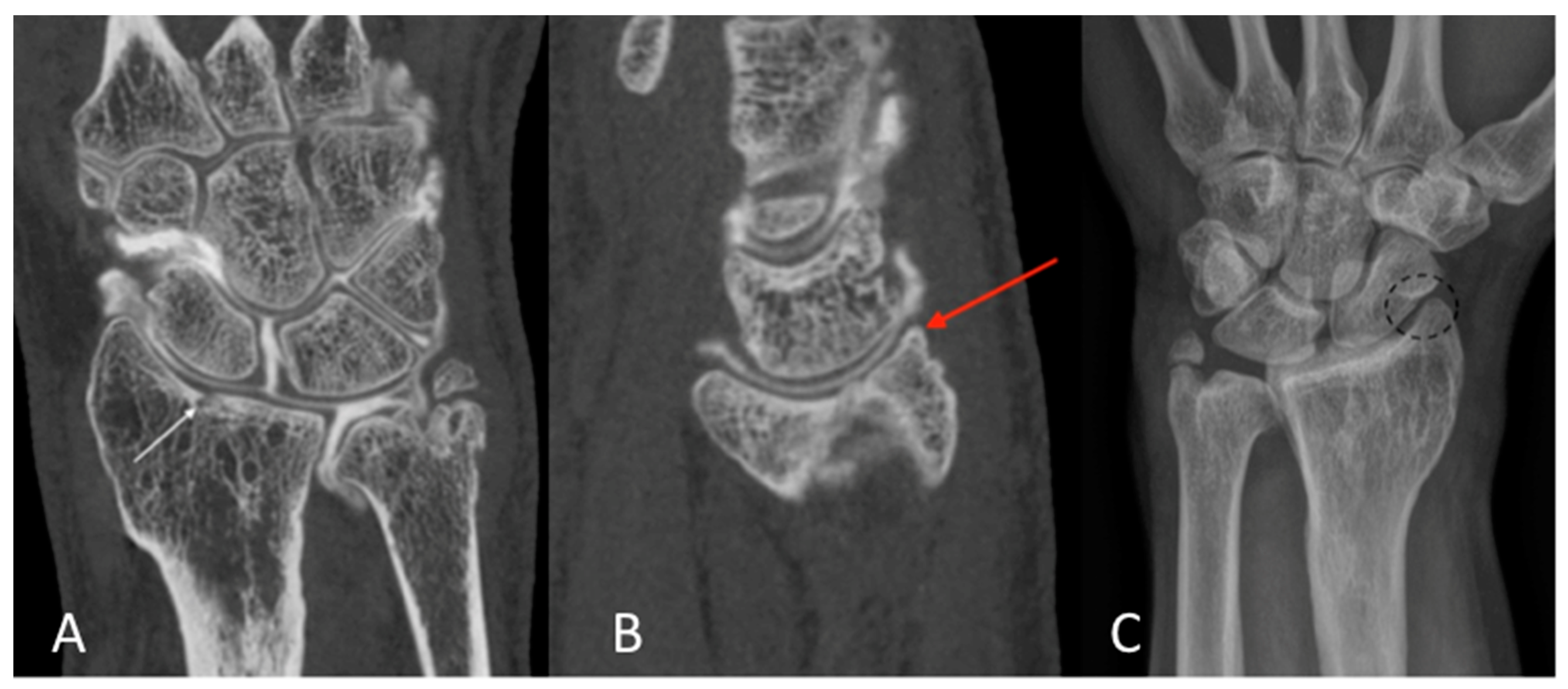
2.3. Qualitative Image Analysis for CBCT-A
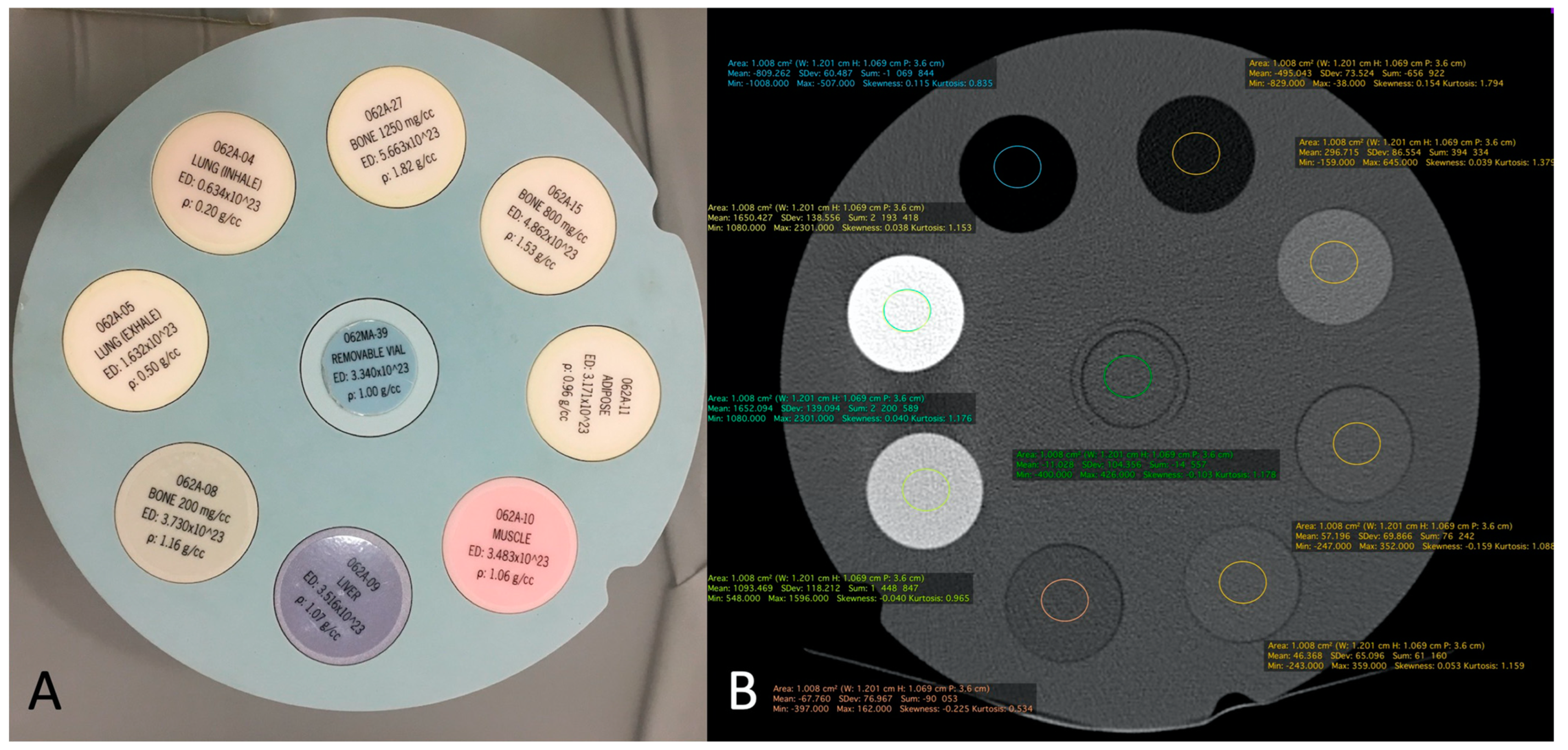
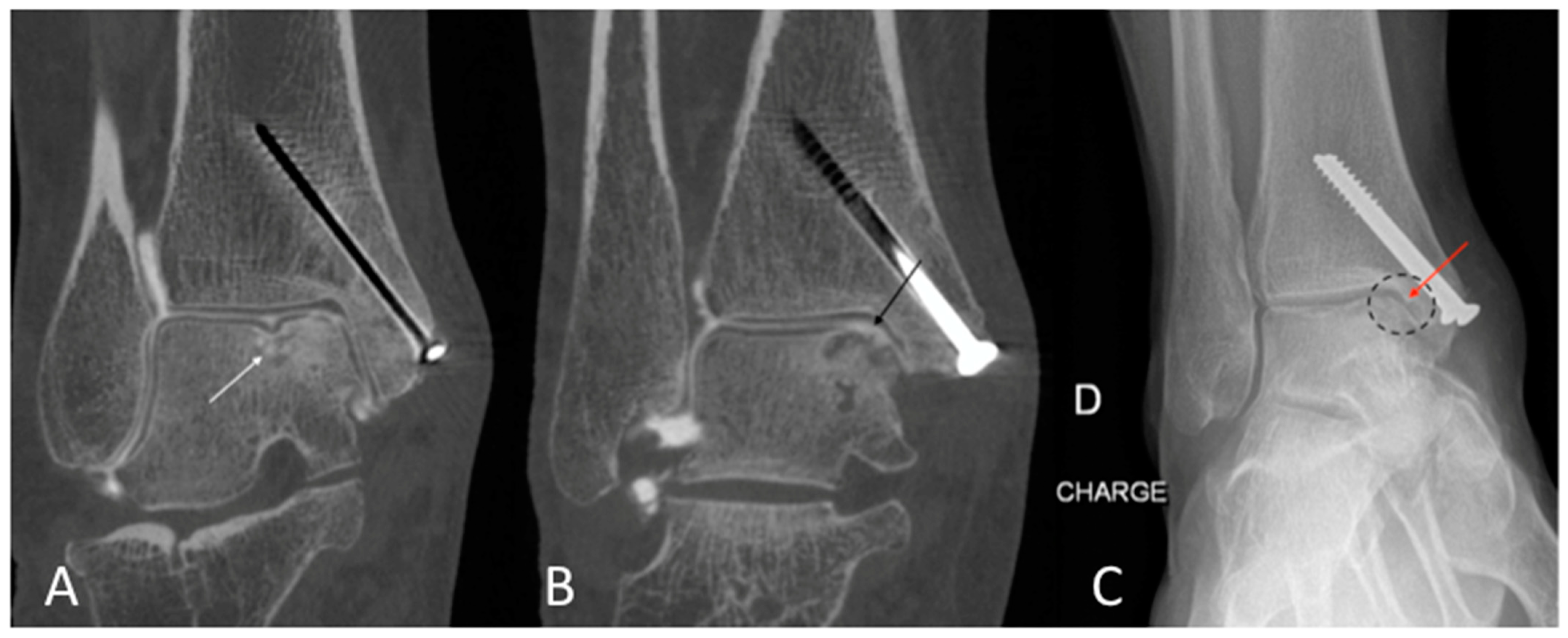
2.4. Quantitative Image Analysis of CBCT-A
2.5. Radiation Dose Measurement of DR and CBCT-A
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacobson, J.A.; Girish, G.; Jiang, Y.; Sabb, B.J. Radiographic evaluation of arthritis: Degenerative joint disease and variations. Radiology 2008, 248, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Guermazi, A.; Kwoh, C.K. Diagnosis and Longitudinal Assessment of Osteoarthritis: Review of Available Imaging Techniques. Rheum. Dis. Clin. N. Am. 2016, 42, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Hafezi-Nejad, N.; Carrino, J.A. Conventional and novel imaging modalities in osteoarthritis: Current state of the evidence. Curr. Opin. Rheumatol. 2015, 27, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, D.; Guermazi, A.; Crema, M.D.; Roemer, F.W. Imaging in osteoarthritis: What have we learned and where are we going? Minerva Med. 2011, 102, 15–32. [Google Scholar] [PubMed]
- Hayashi, D.; Roemer, F.W.; Guermazi, A. Imaging for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, S.; Qu, X.; Wang, Z.; Zheng, J.; Xie, X.; Ma, G.; Zhang, Z.; Ma, X. Evaluation of trabecular structure changes in osteoarthritis of the temporomandibular joint with cone beam computed tomography imaging. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 124, 315–322. [Google Scholar] [CrossRef]
- Roemer, F.W.; Eckstein, F.; Hayashi, D.; Guermazi, A. The role of imaging in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 31–60. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Seki, K.; Yoshino, T.; Ogisawa, S.; Arai, Y.; Tonogi, M.; Iinuma, T. Diagnostic Study of Mandibular Cortical Index Classification Using Dental Cone-Beam Computed Tomography Findings: A Preliminary Cross-Sectional Study. Reports 2023, 6, 48. [Google Scholar] [CrossRef]
- Namatevs, I.; Nikulins, A.; Edelmers, E.; Neimane, L.; Slaidina, A.; Radzins, O.; Sudars, K. Modular Neural Networks for Osteoporosis Detection in Mandibular Cone-Beam Computed Tomography Scans. Tomography 2023, 9, 1772–1786. [Google Scholar] [CrossRef]
- Abuzaid, M.M.; Elshami, W.; Jayachandran, D.; Korappil, N.; Tekin, H.O. Establishment of Diagnostic Reference Levels in Cone Beam Computed Tomography Scans in the United Arab Emirates. Tomography 2022, 8, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Larheim, T.A.; Abrahamsson, A.-K.; Kristensen, M.; Arvidsson, L.Z. Temporomandibular joint diagnostics using CBCT. Dentomaxillofacial Radiol. 2015, 44, 20140235. [Google Scholar] [CrossRef] [PubMed]
- Carrino, J.A.; Al Muhit, A.; Zbijewski, W.; Thawait, G.K.; Stayman, J.W.; Packard, N.; Senn, R.; Yang, D.; Foos, D.H.; Yorkston, J.; et al. Dedicated cone-beam CT system for extremity imaging. Radiology 2014, 270, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Thawait, G.; Gang, G.J.; Zbijewski, W.; Reigel, T.; Brown, T.; Corner, B.; Demehri, S.; Siewerdsen, J.H. Characterization of 3D joint space morphology using an electrostatic model (with application to osteoarthritis). Phys. Med. Biol. 2015, 60, 947–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prakash, P.; Zbijewski, W.; Gang, G.J.; Ding, Y.; Stayman, J.W.; Yorkston, J.; Carrino, J.A.; Siewerdsen, J.H. Task-based modeling and optimization of a cone-beam CT scanner for musculoskeletal imaging. Med. Phys. 2011, 38, 5612–5629. [Google Scholar] [CrossRef] [PubMed]
- Zbijewski, W.; De Jean, P.; Prakash, P.; Ding, Y.; Stayman, J.W.; Packard, N.; Senn, R.; Yang, D.; Yorkston, J.; Machado, A.; et al. A dedicated cone-beam CT system for musculoskeletal extremities imaging: Design, optimization, and initial performance characterization. Med. Phys. 2011, 38, 4700–4713. [Google Scholar] [CrossRef]
- Sharma, L.; Eckstein, F.; Song, J.; Guermazi, A.; Prasad, P.; Kapoor, D.; Cahue, S.; Marshall, M.; Hudelmaier, M.; Dunlop, D. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008, 58, 1716–1726. [Google Scholar] [CrossRef]
- De Vos, W.; Casselman, J.; Swennen, G.R. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2009, 38, 609–625. [Google Scholar] [CrossRef]
- Boudabbous, S.; Arditi, D.; Paulin, E.; Syrogiannopoulou, A.; Becker, C.; Montet, X. Model-Based Iterative Reconstruction (MBIR) for the Reduction of Metal Artifacts on CT. AJR Am. J. Roentgenol. 2015, 205, 380–385. [Google Scholar] [CrossRef]
- Altman, R.D.; Gold, G.E. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr. Cartil. 2007, 15 (Suppl. A), A1–A56. [Google Scholar] [CrossRef]
- Koivisto, J.; van Eijnatten, M.; Kiljunen, T.; Shi, X.Q.; Wolff, J. Effective Radiation Dose in the Wrist Resulting from a Radiographic Device, Two CBCT Devices and One MSCT Device: A Comparative Study. Radiat. Prot. Dosim. 2018, 179, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.G.; Landis, J.R.; Freeman, J.L.; Freeman, D.H.; Lehnen, R.G. General Methodology for Analysis of Experiments with Repeated Measurement of Categorical Data. Biometrics 1977, 33, 133–158. [Google Scholar] [CrossRef]
- Posadzy, M.; Desimpel, J.; Vanhoenacker, F. Cone beam CT of the musculoskeletal system: Clinical applications. Insights Imaging 2018, 9, 35–45. [Google Scholar] [CrossRef]
- Koskinen, S.K.; Haapamäki, V.V.; Salo, J.; Lindfors, N.C.; Kortesniemi, M.; Seppälä, L.; Mattila, K.T. CT arthrography of the wrist using a novel, mobile, dedicated extremity cone-beam CT (CBCT). Skelet. Radiol. 2013, 42, 649–657. [Google Scholar] [CrossRef]
- Huang, A.J.; Chang, C.Y.; Thomas, B.J.; MacMahon, P.J.; Palmer, W.E. Using cone-beam CT as a low-dose 3D imaging technique for the extremities: Initial experience in 50 subjects. Skelet. Radiol. 2015, 44, 797–809. [Google Scholar] [CrossRef]
- Kokkonen, H.T.; Suomalainen, J.S.; Joukainen, A.; Kröger, H.; Sirola, J.; Jurvelin, J.S.; Salo, J.; Töyräs, J. In vivo diagnostics of human knee cartilage lesions using delayed CBCT arthrography. J. Orthop. Res. 2014, 32, 403–412. [Google Scholar] [CrossRef]
- Newman, S.; Ahmed, H.; Rehmatullah, N. Radiographic vs. MRI vs. arthroscopic assessment and grading of knee osteoarthritis—are we using appropriate imaging? J. Exp. Orthop. 2022, 9, 2. [Google Scholar] [CrossRef]
- Brett, A.; Bowes, M.A.; Conaghan, P.G. Comparison of 3D quantitative osteoarthritis imaging biomarkers from paired CT and MR images: Data from the IMI-APPROACH study. BMC Musculoskelet. Disord. 2023, 24, 76. [Google Scholar] [CrossRef]
- Arbabi, S.; Foppen, W.; Gielis, W.P.; van Stralen, M.; Jansen, M.; Arbabi, V.; de Jong, P.A.; Weinans, H.; Seevinck, P. MRI-based synthetic CT in the detection of knee osteoarthritis: Comparison with CT. J. Orthop. Res. 2023, 41, 2530–2539. [Google Scholar] [CrossRef]
- Mys, K.; Stockmans, F.; Vereecke, E.; van Lenthe, G.H. Quantification of bone microstructure in the wrist using cone-beam computed tomography. Bone 2018, 114, 206–214. [Google Scholar] [CrossRef]
- Swennen, G.R.; Schutyser, F. Three-dimensional cephalometry: Spiral multi-slice vs cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 410–416. [Google Scholar] [CrossRef]
- Koivisto, J.; Kiljunen, T.; Kadesjo, N.; Shi, X.Q.; Wolff, J. Effective radiation dose of a MSCT, two CBCT and one conventional radiography device in the ankle region. J. Foot Ankle Res. 2015, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, J.; Kiljunen, T.; Wolff, J.; Kortesniemi, M. Assessment of effective radiation dose of an extremity CBCT, MSCT and conventional X ray for knee area using MOSFET dosemeters. Radiat. Prot. Dosim. 2013, 157, 515–524. [Google Scholar] [CrossRef]


| Grades | Description |
|---|---|
| 0 | Normal, with no radiographic findings of OA |
| 1 | Doubtful joint space narrowing Doubtful osteophytes |
| 2 | Possible joint space narrowing Definite osteophytes |
| 3 | Multiple and moderately sized osteophytes Definite joint space narrowing Small pseudocystic with sclerotic walls Possible deformity of the bone contour |
| 4 | Multiple and large osteophytes Severe joint space narrowing Marked sclerosis Definite bone deformity |
| Parameters | CBCT | DR |
|---|---|---|
| Energy | 80 kVp | 50 kVp |
| Current | 5 mA | 3.2 mAs |
| FOV | 216 × 216 mm | |
| Matrix | 884 × 884 | |
| Isotropic voxel size | 0.26 mm | |
| Rotation time | 25.18 s | |
| Exposure time | 21 s approximately | |
| Scan rotation angle | 216.5° | |
| CTDI (indicated) | 3.14 mGy (16 cm phantom) | |
| Focus-detector distance | 120 cm |
| Median | Rs (Spearman) | p Values | |
|---|---|---|---|
| Mean joint width (MJW) | 0.09876 | 0.9405 | 0.0213 |
| Kellegren and Lawrence | −1.000 | 0.6350 | <0.0001 |
| Osteophytes | 0.0 | 0.4848 | <0.0001 |
| Subchondral sclerosis | 0.0 | 0.3551 | 0.2972 |
| Erosions | 0.0 | 0.3393 | 0.1849 |
| GE CT Densities | Siemens MDCT Densities | CBCT with MBIR 1 Densities | CBCT with MBIR2 Densities | |
|---|---|---|---|---|
| Bone of 1250 mg/cc (ρ: 1.82 g/cc) | 1651.115 (SD: 149.550) | 1652.094 (SD: 139.094) | 1895.378 (SD: 142.514) | 1061 (SD: 97.320) |
| Bone of 800 mg/cc (ρ: 1.53 g/cc) | 1091.529 (SD: 93.369) | 1093.469 (SD: 118.212) | 1282.889 (SD: 118.033) | 858.058 (SD: 85.509) |
| Bone of 200 mg/cc (ρ: 1.16 g/cc) | 298.070 (SD: 78.129) | 296.715 (SD: 73.) | 298.396 (SD: 78.129) | 236.401 (SD: 59.354) |
| Adipose tissue (ρ: 0.96 g/cc) | −68.434 (SD: 77.762) | −67.760 (SD: 76.967) | −73.072 (SD: 71.203) | −70.934 (SD: 62.959) |
| Muscle (ρ: 1.06 g/cc) | 43.208 (SD: 68.922) | 46.368 (SD: 65.096) | 33.949 (SD: 74.198) | 15.139 (SD: 57.353) |
| Liver (ρ: 1.07 g/cc) | 56.396 (SD: 76.148) | 57.196 (SD: 69.866) | 42.652 (SD: 75.826) | 26.372 (SD: 55.516) |
| Lung (inhale) (ρ: 0.20 g/cc) | −810.597 (SD: 67.894) | −809.262 (SD: 60.487) | −862.192 (SD: 52.165) | −760.330 (SD: 59.773) |
| Lung (exhale) (ρ: 0.50 g/cc) | −493.688 (SD: 79.298) | −495.043 (SD: 73.524) | −560.997 (SD: 57.972) | −494–122 (SD: 56.538) |
| CBCT-A | DR | |
|---|---|---|
| Dose radiation for PA projection mGy | 0.037 mGy | 0.029 mGy |
| Dose radiation for oblique/lateral projection | 0.013 mGy | 0.033 mGy |
| 1 3D acquisition | 4.902 mGy | -- |
| Total dose radiation | 4.952 mGy | 0.062 mGy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamard, M.; Sans Merce, M.; Gorican, K.; Poletti, P.-A.; Neroladaki, A.; Boudabbous, S. The Role of Cone-Beam Computed Tomography CT Extremity Arthrography in the Preoperative Assessment of Osteoarthritis. Tomography 2023, 9, 2134-2147. https://doi.org/10.3390/tomography9060167
Hamard M, Sans Merce M, Gorican K, Poletti P-A, Neroladaki A, Boudabbous S. The Role of Cone-Beam Computed Tomography CT Extremity Arthrography in the Preoperative Assessment of Osteoarthritis. Tomography. 2023; 9(6):2134-2147. https://doi.org/10.3390/tomography9060167
Chicago/Turabian StyleHamard, Marion, Marta Sans Merce, Karel Gorican, Pierre-Alexandre Poletti, Angeliki Neroladaki, and Sana Boudabbous. 2023. "The Role of Cone-Beam Computed Tomography CT Extremity Arthrography in the Preoperative Assessment of Osteoarthritis" Tomography 9, no. 6: 2134-2147. https://doi.org/10.3390/tomography9060167
APA StyleHamard, M., Sans Merce, M., Gorican, K., Poletti, P.-A., Neroladaki, A., & Boudabbous, S. (2023). The Role of Cone-Beam Computed Tomography CT Extremity Arthrography in the Preoperative Assessment of Osteoarthritis. Tomography, 9(6), 2134-2147. https://doi.org/10.3390/tomography9060167






