Reporting Diagnostic Reference Levels for Paediatric Patients Undergoing Brain Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
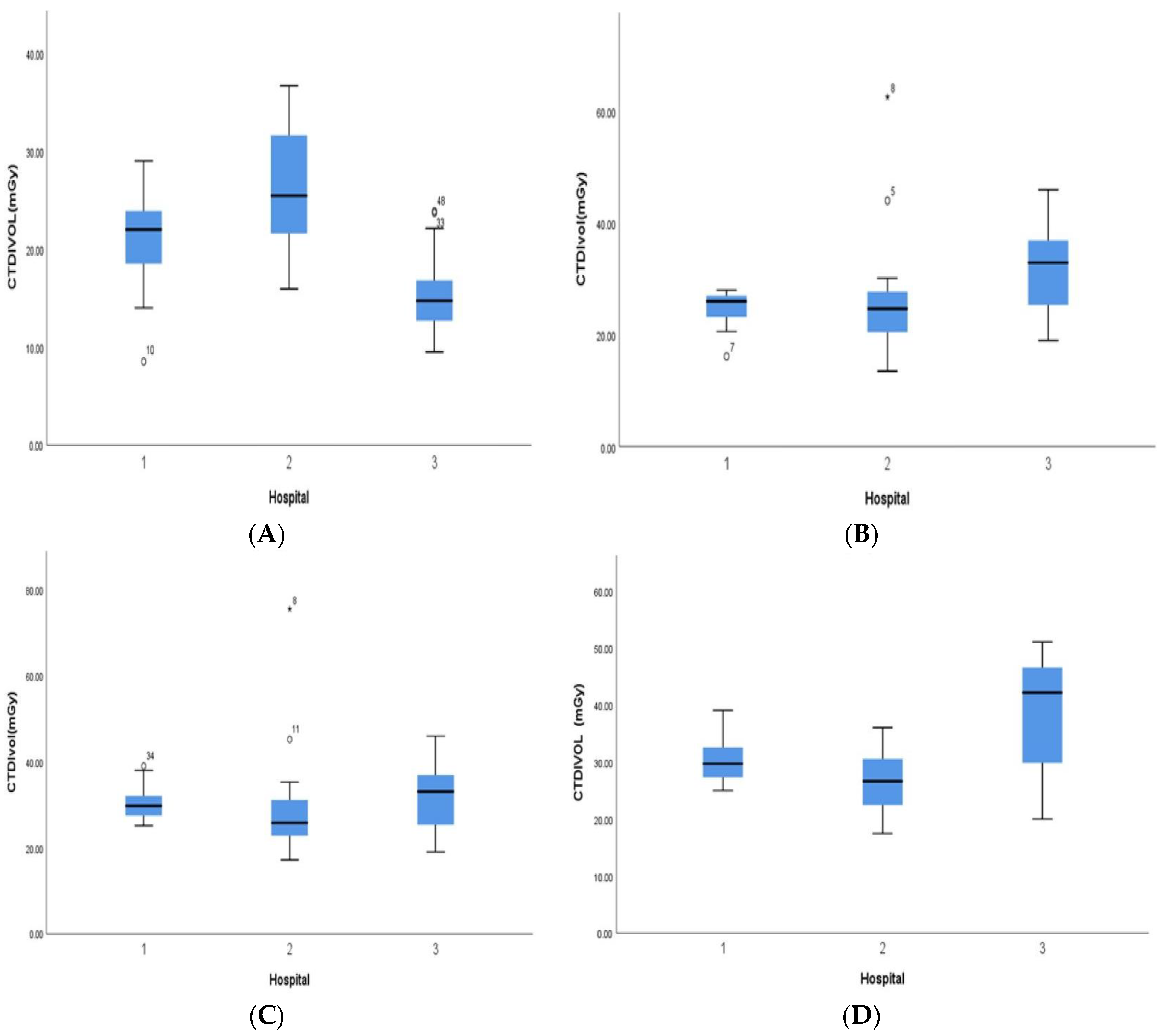
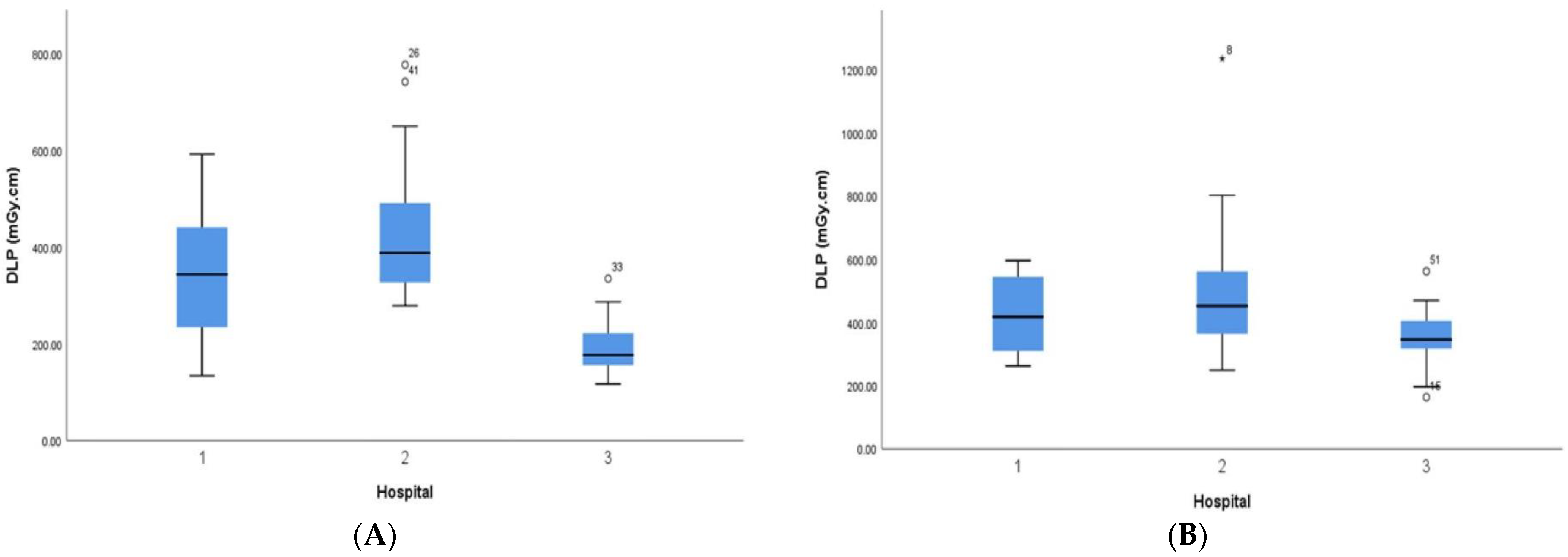
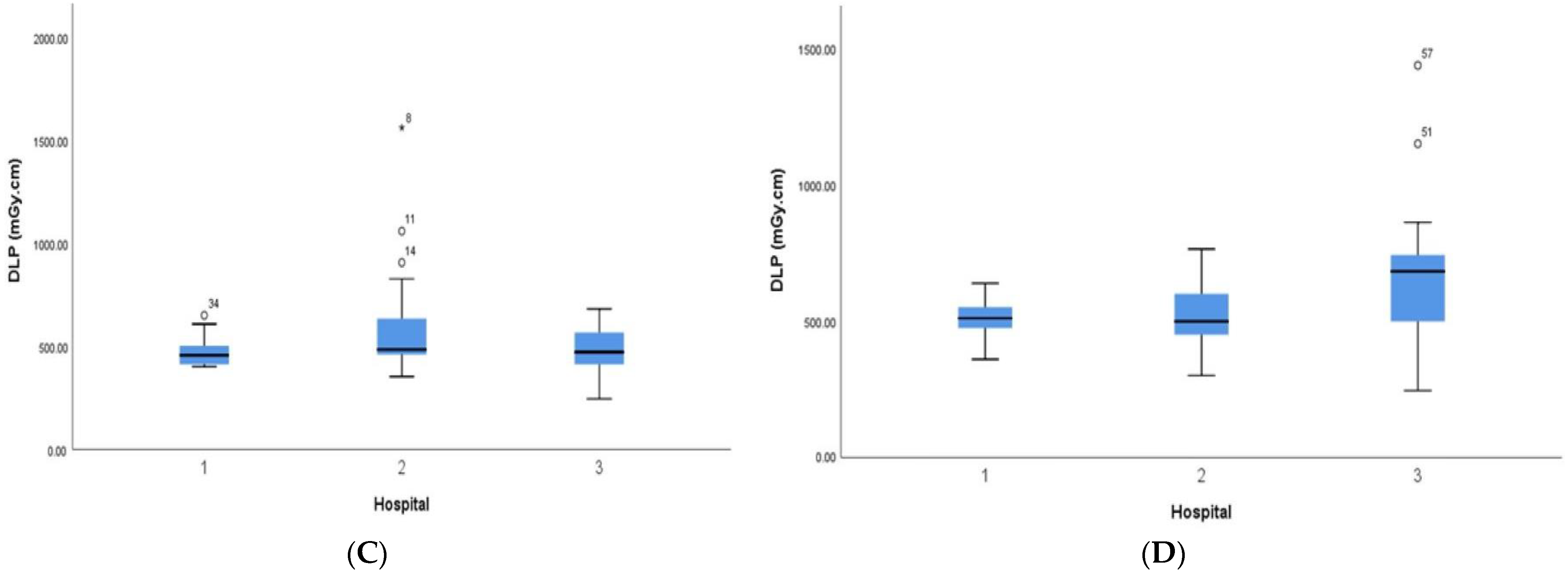
3. Results
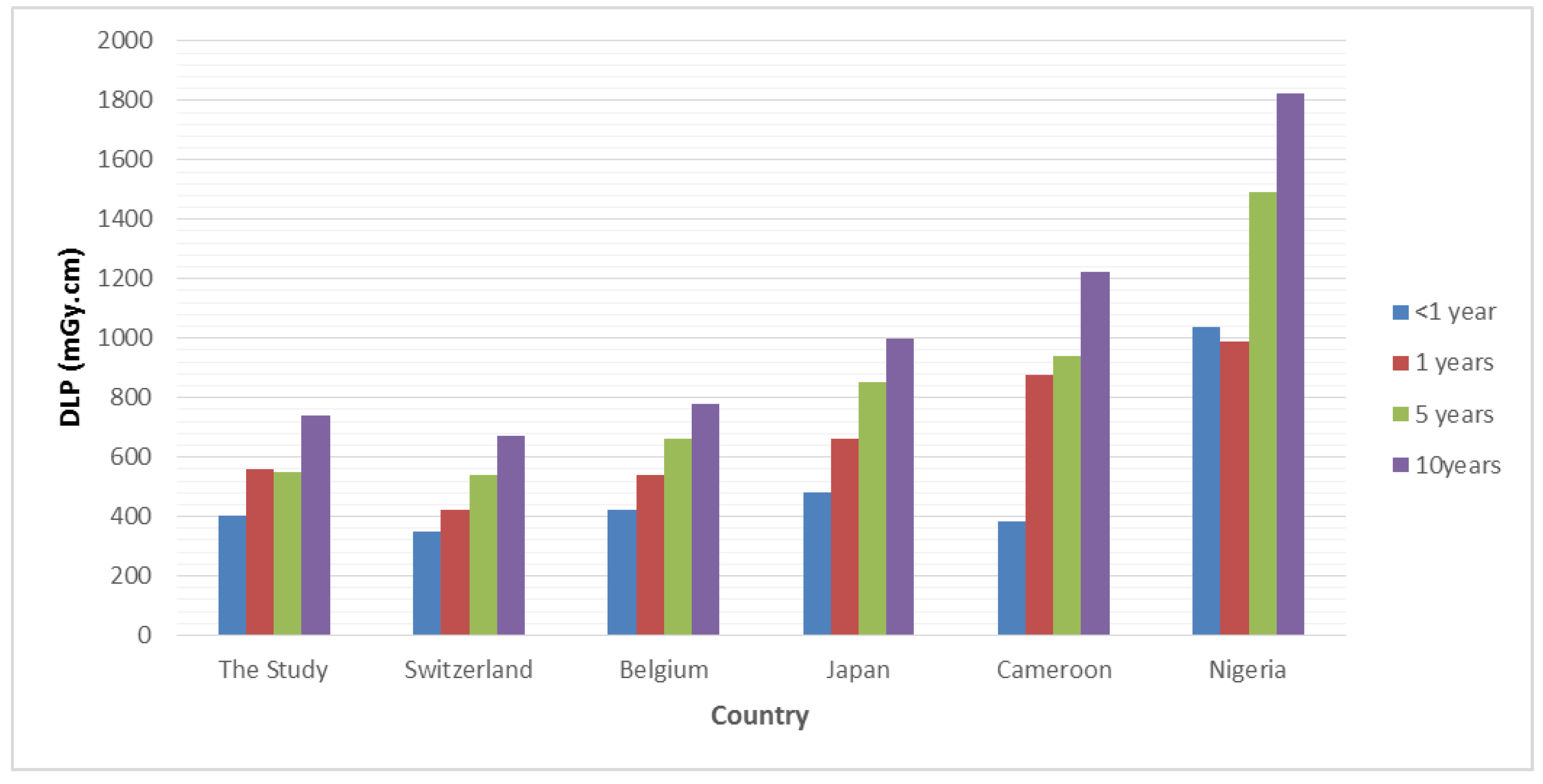
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyanka; Kadavigere, R.; Sukumar, S.; Pendem, S. Diagnostic reference levels for computed tomography examinations in pediatric population—A systematic review. J. Cancer Res. Ther. 2021, 17, 845–852. [Google Scholar] [PubMed]
- Bibbo, G.; Brown, S.; Linke, R. Diagnostic reference levels of paediatric computed tomography examinations performed at a dedicated Australian paediatric hospital. J. Med. Imaging Radiat. Oncol. 2016, 60, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed]
- Vawda, Z.; Pitcher, R.; Akudugu, J.; Groenewald, W. Diagnostic reference levels for paediatric computed tomography. S. Afr. J. Radiol. 2015, 19, a846. [Google Scholar] [CrossRef]
- Vañó, E.; Miller, D.; Martin, C.; Rehani, M.; Kang, K.; Rosenstein, M.; Ortiz-López, P.; Mattsson, S.; Padovani, R.; Rogers, A. Diagnostic reference levels in medical imaging: ICRP Publication 135. Ann. ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef]
- Bosmans, H.; Damilakis, J.; Ducou le Pointe, H.; Foley, S.J. Radiation Protection No. 185 European Guidelines on Diagnostic Reference Levels for Paediatric Imaging; European Commission: Brussels, Belgium, 2018.
- International Atomic Energy Agency. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; IAEA Safety Standards Series No. GSR Part 3; IAEA: Vienna, Austria, 2014. [Google Scholar]
- Almén, A.; Guðjónsdóttir, J.; Heimland, N.; Højgaard, B.; Waltenburg, H.; Widmark, A. Paediatric diagnostic reference levels for common radiological examinations using the European guidelines. Br. J. Radiol. 2022, 95, 20210700. [Google Scholar] [CrossRef]
- The Saudi Food and Drug Authority (SFDA). National Diagnostic Reference Levels (NDRL) Computed Tomography (CT)—Adult. 2022. Available online: https://www.sfda.gov.sa/sites/default/files/2023-02/NDRL-En.pdf (accessed on 15 May 2023).
- Bawazeer, O.; Saleem, R.; Alhazmi, M.; Asiri, N.; Mohammed, T.; Alsaab, A.; Algethami, M.; Sedayo, A.; Ajlouni, A. Assessment of pediatric radiation doses in brain CT procedures. Radioprotection 2022, 57, 305–310. [Google Scholar] [CrossRef]
- Alashban, Y.; Shubayr, N. Establishing diagnostic reference levels for CT examinations in the south region of Saudi Arabia. Radiat. Phys. Chem. 2022, 201, 110–407. [Google Scholar] [CrossRef]
- Alhailiy, A.; Brennan, P.; McEntee, M.; Kench, P.; Ryan, E. Diagnostic reference levels in cardiac computed tomography angiography: A systematic review. Radiat. Prot. Dosim. 2017, 178, 63–72. [Google Scholar] [CrossRef]
- Wagner, F.; Bize, J.; Racine, D.; Le Coultre, R.; Verdun, F.; Trueb, P.R.; Treier, R. Derivation of new diagnostic reference levels for neuro-paediatric computed tomography examinations in Switzerland. J. Radiol. Prot. 2018, 38, 1013. [Google Scholar] [CrossRef]
- The Federal Agency for Nuclear Control (FANC). CT Scanners. 2020. Available online: https://afcn.fgov.be/fr/professionnels/professions-medicales/applications-radiologiques/nrd/CTscanners (accessed on 20 May 2023).
- Japan Network for Research and Information on Medical Exposure. National Diagnostic Reference Levels in Japan. 2020. Available online: http://www.radher.jp/J-RIME/report/DRL2020_Engver.pdf (accessed on 15 May 2023).
- Kamdem, E.F.; Samba, O.N.; Manemo, C.T.; Kouam, B.B.F.; Abogo, S.; Tambe, J.; Amougou, J.C.M.; Guegang, E.; Zeh, O.F.; Moifo, B. Establishment of Local Diagnostic Reference Level for Routine Paediatric Computed Tomography Examinations in Bafoussam West Cameroon. Radiat. Prot. Dosim. 2022, 198, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, E.U.; Adejoh, T.; Erim, A.E. Dose benchmarks for paediatric head computed tomography examination in Nigeria. Radiat. Prot. Dosim. 2019, 185, 464–471. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Malchair, F.; Maccia, C. Practical advices for optimal CT scanner dose in children. Radioprotection 2020, 55, 117–122. [Google Scholar] [CrossRef]
- Zarb, F.; Rainford, L.; McEntee, M.F. AP diameter shows the strongest correlation with CTDI and DLP in abdominal and chest CT. Radiat. Prot. Dosim. 2010, 140, 266–273. [Google Scholar] [CrossRef]
- Kotre, C.; Reay, J.; Chapple, C. The influence of patient size on patient doses in cardiology. Radiat. Prot. Dosim. 2005, 117, 222–224. [Google Scholar] [CrossRef]
- Alhailiy, A.B.; Ekpo, E.U.; Kench, P.L.; Ryan, E.A.; Brennan, P.C.; McEntee, M. The associated factors for radiation dose variation in cardiac CT angiography. Br. J. Radiol. 2019, 92, 20180793. [Google Scholar] [CrossRef]
- Meeson, S.; Alvey, C.; Golding, S. The in vivo relationship between cross-sectional area and CT dose index in abdominal multidetector CT with automatic exposure control. J. Radiol. Prot. 2010, 30, 139. [Google Scholar] [CrossRef]
- Hayton, A.; Wallace, A.; Marks, P.; Edmonds, K.; Tingey, D.; Johnston, P. Australian diagnostic reference levels for multi detector computed tomography. Australas. Phys. Eng. Sci. Med. 2013, 36, 19–26. [Google Scholar] [CrossRef]
- Natale, V.; Rajagopalan, A. Worldwide variation in human growth and the World Health Organization growth standards: A systematic review. BMJ Open 2014, 4, e003735. [Google Scholar] [CrossRef]
- Hahn, F.J.; Chu, W.-K.; Cheung, J.Y. CT measurements of cranial growth: Normal subjects. Am. J. Neuroradiol. 1984, 5, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Alrehily, F. Diagnostic reference levels of radiographic and CT examinations in Saudi Arabia: A systematic review. Radiat. Prot. Dosim. 2022, 198, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | ICRP Age (Years) Mean (% or Interquartile Range) | ||||
|---|---|---|---|---|---|
| <1 | 1 | 5 | 10 | ||
| No. of patients | 60 | 59 | 60 | 47 | |
| Patient age, years | 6 M (IQR 2.9) | 3 Y (2.4) | 7 Y (6.9) | 13 Y (11.15) | |
| Gender | Boy | 33 (55%) | 31 (52.5%) | 44 (73%) | 30 (64%) |
| Girl | 27 (45%) | 28 (47.5%) | 16 (27%) | 17 (36%) | |
| Patient height, cm | 61.9 (IQR 53.5–69.3) | 95.5 (IQR 85.5–108) | 123.7 (IQR 115–135) | 135.1 (IQR 134.3–146.8) | |
| Patient weight, kg | 6.4 (IQR 3.5–8.1) | 14 (IQR 11–16.6) | 25.2 (IQR 19.8–27.2) | 44.7 (IQR 36.5–55) | |
| Body mass index, kg/m2 | 15.7 (IQR 13–17.4) | 15.2 (IQR 13.9–16.5) | 17.6 (IQR 14.4–19.4) | 20.5 (IQR 16.3–21.6) | |
| Transverse width, mm | 111.3 (IQR 92.4–124.5) | 132.3 (IQR 126–137.7) | 139.2 (IQR 132.1–144.7) | 141.6 (IQR 134.7–148.3) | |
| AP width, mm | 130.1 (IQR 114–140.6) | 156 (IQR 148–167.6) | 168.4 (IQR 160.9–177.1) | 170.7 (IQR 165.4–176.5) | |
| Cross-Sectional Area, cm2 | 115.5 (IQR 83.5–142.1) | 163.4 (IQR 146.4–183.1) | 183.8 (IQR 174.4–191.9) | 190 (IQR 180–200.6) | |
| scan length, mm | 154.7 (IQR 121.3–190) | 177.5 (IQR 142.6–210) | 179.4 (IQR 143.2–210) | 191.8 (IQR 162.7–220) | |
| Scanning Characteristics | ICRP Age (Years) Mean (% or Interquartile Range) | |||
|---|---|---|---|---|
| <1 | 1 | 5 | 10 | |
| Tube voltage, kV | 100 Median (IQR 100–120) | 120 Median (IQR 100–120) | 120 Median (IQR 100–120) | 120 Median (IQR 120–120) |
| Tube current, mA | 176.6 (IQR 125–243.5) | 183.5 (IQR 125–247) | 182.6 (IQR 145–249) | 173.8 (IQR 140–210) |
| slice thickness | 2.5 Median (IQR 2.5 –5) | 2.5 Median (IQR 2.5 –5) | 2.5 Median (IQR 2.5–5) | 2.5 Median (IQR 2.5–5) |
| Pitch | 0.53 Median (IQR 0.5–1) | 0.65 Median (IQR 0.5–1) | 0.65 Median (IQR 0.5–1) | 0.65 Median (IQR 0.5–1) |
| IR | 60 (100) | 59 (100) | 60 (100) | 47 (100) |
| CTDIvol | 20.9 (IQR 15.1–24.5) | 27.6 (IQR 22.7–30.7) | 30.5 (IQR 25.2–34.5) | 32.3(IQR 27–38.1) |
| Total DLP, mGy cm | 312.6 (IQR 189.3–403.9) | 470.3 (IQR 365–561.4) | 519.5 (IQR 418.8–554.9) | 574 (IQR 454–681) |
| Age Group | CTDIvol (mGy) | DLP (mGy cm) | ||||
|---|---|---|---|---|---|---|
| 75th | Median | 25th | 75th | Median | 25th | |
| <1 year | 25 | 22 | 15 | 404 | 281 | 191 |
| 1 years | 30 | 26 | 23 | 560 | 452 | 367 |
| 5 years | 34 | 29 | 25 | 548 | 474 | 426 |
| 10 years | 45 | 30 | 20 | 742 | 551 | 439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhailiy, A.; Alkhybari, E.; Alghamdi, S.; Fisal, N.; Aldosari, S.; Albeshan, S. Reporting Diagnostic Reference Levels for Paediatric Patients Undergoing Brain Computed Tomography. Tomography 2023, 9, 2029-2038. https://doi.org/10.3390/tomography9060159
Alhailiy A, Alkhybari E, Alghamdi S, Fisal N, Aldosari S, Albeshan S. Reporting Diagnostic Reference Levels for Paediatric Patients Undergoing Brain Computed Tomography. Tomography. 2023; 9(6):2029-2038. https://doi.org/10.3390/tomography9060159
Chicago/Turabian StyleAlhailiy, Ali, Essam Alkhybari, Sultan Alghamdi, Nada Fisal, Sultan Aldosari, and Salman Albeshan. 2023. "Reporting Diagnostic Reference Levels for Paediatric Patients Undergoing Brain Computed Tomography" Tomography 9, no. 6: 2029-2038. https://doi.org/10.3390/tomography9060159
APA StyleAlhailiy, A., Alkhybari, E., Alghamdi, S., Fisal, N., Aldosari, S., & Albeshan, S. (2023). Reporting Diagnostic Reference Levels for Paediatric Patients Undergoing Brain Computed Tomography. Tomography, 9(6), 2029-2038. https://doi.org/10.3390/tomography9060159





