Initial CT Imaging Predicts Mortality in Severe Traumatic Brain Injuries in Pediatric Population—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
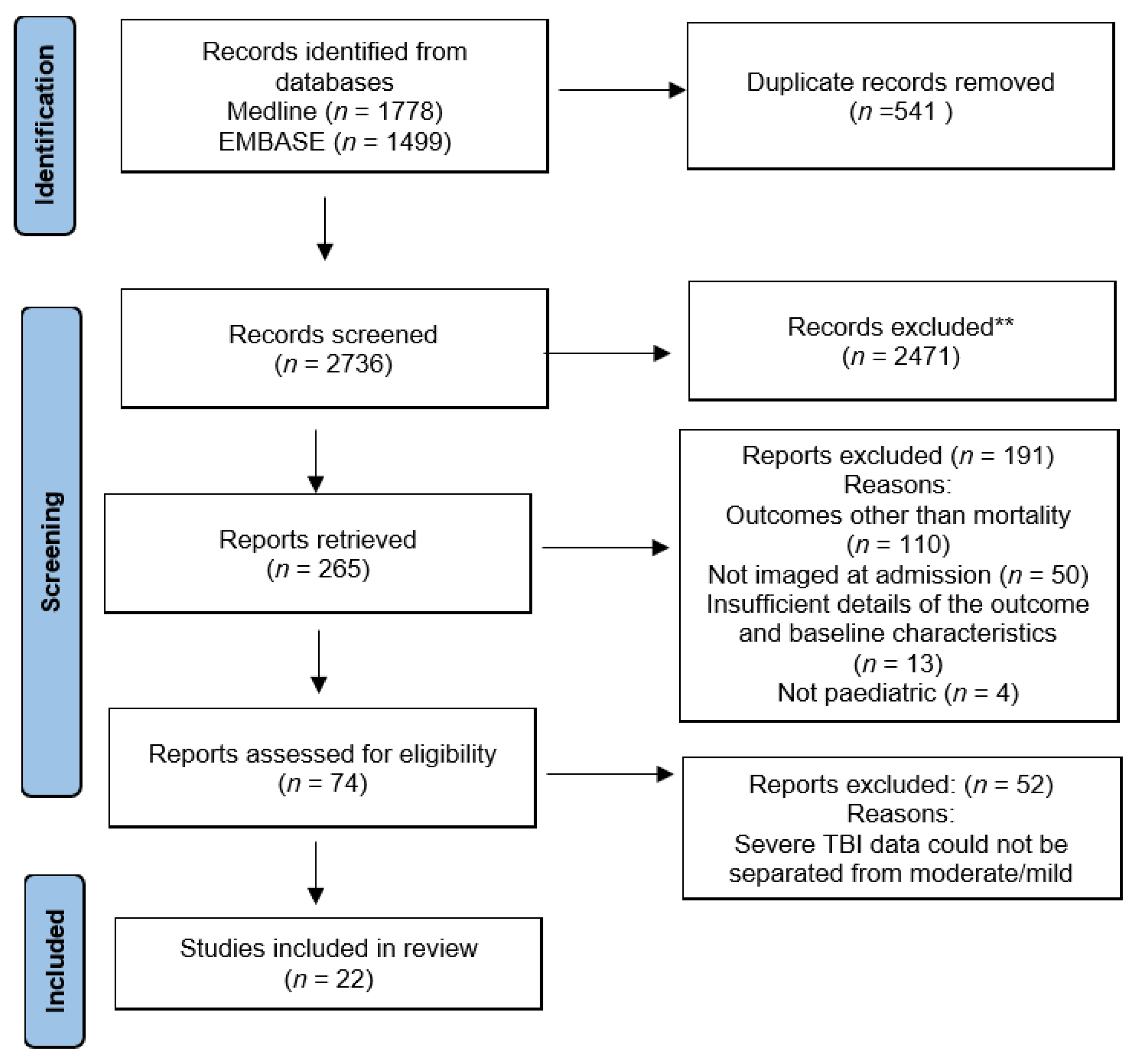
3.1. Literature Search and Article Selection
3.2. Summary of Demographics and Outcome
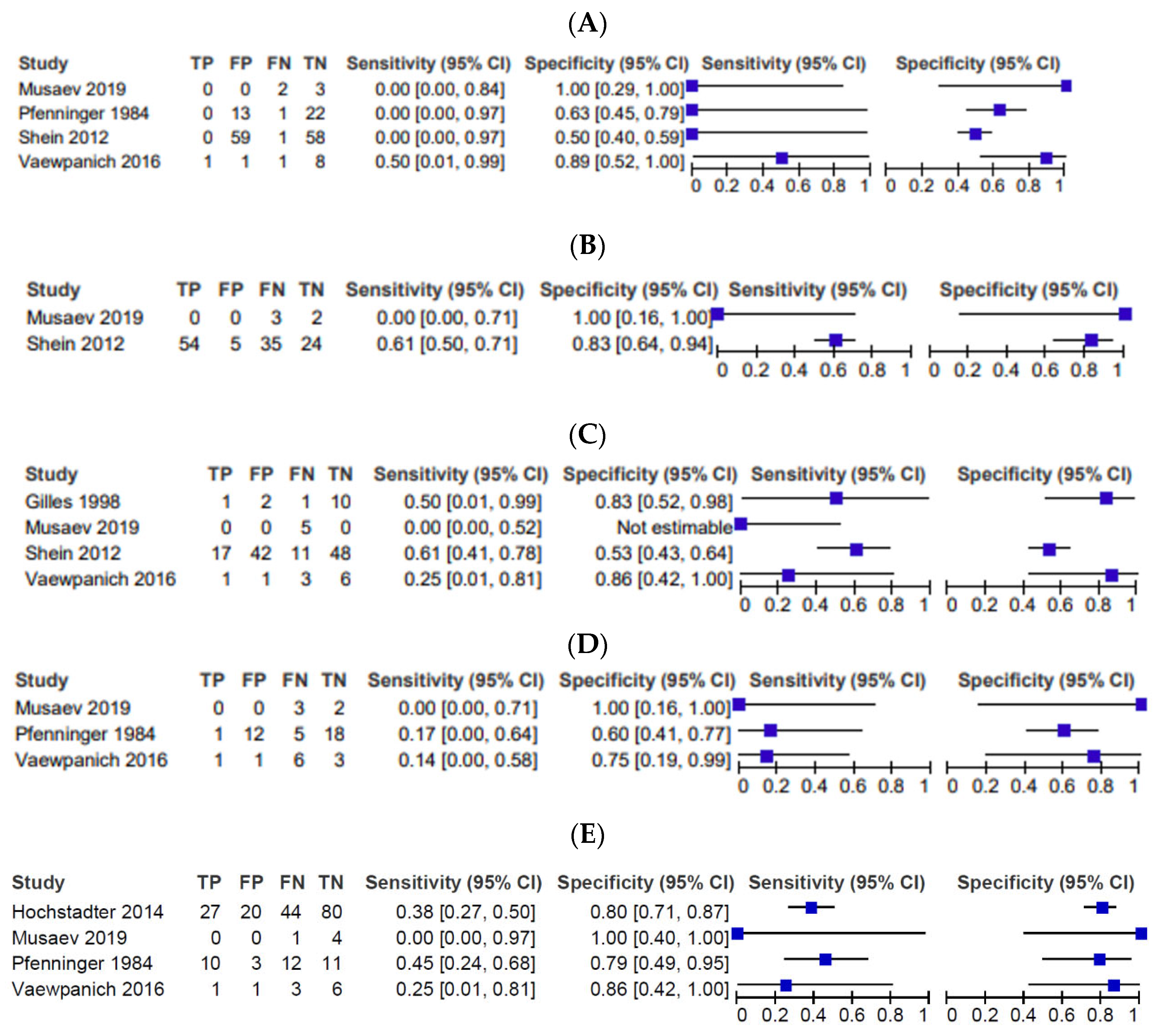
3.3. Imaging Findings
3.4. Risk of Bias
4. Discussion
4.1. Cerebral Edema
4.2. SDH
4.3. EDH
4.4. tSAH
4.5. Skull Fractures
4.6. Sex
4.7. Additional Findings
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| TBIs | traumatic brain injuries |
| GCS | Glasgow Coma Scale |
| GOS | Glasgow Outcome Scale |
| SN | sensitivity |
| SP | specificity |
| PPV | positive predictive value |
| NPV | negative predictive value |
| AUC | area under curve |
| ROC | receiver operating characteristic |
| EDH | extradural hematoma |
| SDH | subdural hematoma |
| tSAH | traumatic subarachnoid hemorrhage |
References
- Wilson, J.T.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Feickert, H.J.; Drommer, S.; Heyer, R. Severe head injury in children: Impact of risk factors on outcome. J. Trauma 1999, 47, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hochstadter, E.; Stewart, T.C.; Alharfi, I.M.; Ranger, A.; Fraser, D.D. Subarachnoid hemorrhage prevalence and its association with short-term outcome in pediatric severe traumatic brain injury. Neurocrit. Care 2014, 21, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Josan, V.A.; Sgouros, S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2006, 22, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakov, S.V.; Semenova, Z.B.; Lukianov, V.I.; Sorokina, E.G.; Karaseva, O.V. Prognosis of Severe Traumatic Brain Injury Outcomes in Children. Acta Neurochir. Suppl. 2018, 126, 11–16. [Google Scholar]
- Vaewpanich, J.; Reuter-Rice, K. Continuous electroencephalography in pediatric traumatic brain injury: Seizure characteristics and outcomes. Epilepsy Behav. EB 2016, 62, 225–230. [Google Scholar] [CrossRef]
- Vavilala, M.S.; Lujan, S.B.; Qiu, Q.; Bell, M.J.; Ballarini, N.M.; Guadagnoli, N.; Depetris, M.A.; Faguaga, G.A.; Baggio, G.M.; Busso, L.O.; et al. Intensive care treatments associated with favorable discharge outcomes in Argentine children with severe traumatic brain injury: For the South American Guideline Adherence Group. PloS ONE 2017, 12, e0189296. [Google Scholar] [CrossRef]
- Rosario, B.L.; Horvat, C.M.; Wisniewski, S.R.; Bell, M.J.; Panigrahy, A.; Zuccoli, G.; Narayanan, S.; Balasubramani, G.K.; Beers, S.R.; Adelson, P.D. Presenting Characteristics Associated With Outcome in Children With Severe Traumatic Brain Injury: A Secondary Analysis From a Randomized, Controlled Trial of Therapeutic Hypothermia. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2018, 19, 957–964. [Google Scholar] [CrossRef]
- Pfenninger, J. Early prediction of outcome after severe head injury in children. Z. Kinderchir. Organ Dtsch. Schweiz. Osterreichischen Ges. Kinderchir. Sur.g Infancy Child 1984, 39, 223–228. [Google Scholar] [CrossRef]
- Shein, S.L.; Bell, M.J.; Kochanek, P.M.; Tyler-Kabara, E.C.; Wisniewski, S.R.; Feldman, K.; Makoroff, K.; Scribano, P.V.; Berger, R.P. Risk factors for mortality in children with abusive head trauma. J. Pediatr. 2012, 161, 716–722.e1. [Google Scholar] [CrossRef]
- Musaev, S.; Musaeva, Y. The clinical characteristics of posttraumatic epilepsy following moderate-to-severe traumatic brain injury in children. Neurol. Sci. 2019, 405, 87. [Google Scholar] [CrossRef]
- Gilles, E.E.; Nelson, M.D. Cerebral complications of nonaccidental head injury in childhood. Pediatr. Neurol. 1998, 19, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Praharaj, S.S.; Mohanty, A.; Kolluri, V.S. Prognostic factors in children with severe diffuse brain injuries: A study of 74 patients. Pediatr. Neurosurg. 2001, 34, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Rath, G.P.; Lamsal, R.; Sinha, S.; Bithal, P.K. Intracranial Pressure Monitoring in Children with Severe Traumatic Brain Injury: A Retrospective Study. J. Pediatr. Neurosci. 2019, 14, 7–15. [Google Scholar]
- Semple, P.L.; Bass, D.H.; Peter, J.C. Severe head injury in children--a preventable but forgotten epidemic. S. Afr. Med. J. Suid-Afr. Tydskr. Vir. Geneeskd. 1998, 88, 440–444. [Google Scholar]
- Kan, C.H.; Saffari, M.; Khoo, T.H. Prognostic factors of severe traumatic brain injury outcome in children aged 2-16 years at a major neurosurgical referral centre. Malays J. Med. Sci. MJMS 2009, 16, 25–33. [Google Scholar] [PubMed]
- Chiaretti, A.; Piastra, M.; Pulitanò, S.; Pietrini, D.; De Rosa, G.; Barbaro, R.; Di Rocco, C. Prognostic factors and outcome of children with severe head injury: An 8-year experience. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2002, 18, 129–136. [Google Scholar] [CrossRef]
- Davidson, A. Social Determinants of Health: A Comparative Approach, 2nd, ed.; Don Mills Ont, Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Ducrocq, S.C.; Meyer, P.G.; Orliaguet, G.A.; Blanot, S.; Laurent-Vannier, A.; Renier, D.; Carli, P.A. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: Experience of a French pediatric trauma center. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2006, 7, 461–467. [Google Scholar] [CrossRef]
- Levin, H.S.; Aldrich, E.F.; Saydjari, C.; Eisenberg, H.M.; Foulkes, M.A.; Bellefleur, M.; Luerssen, T.G.; Jane, J.A.; Marmarou, A.; Marshall, L.F. Severe head injury in children: Experience of the Traumatic Coma Data Bank. Neurosurgery 1992, 31, 435–443; Discussion 443–444. [Google Scholar] [CrossRef]
- Trabold, F.; Meyer, P.G.; Blanot, S.; Carli, P.A.; Orliaguet, G.A. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004, 30, 108–112. [Google Scholar] [CrossRef]
- Poomthavorn, P.; Maixner, W.; Zacharin, M. Pituitary function in paediatric survivors of severe traumatic brain injury. Arch. Dis. Child 2008, 93, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Chaiwat, O.; Sharma, D.; Udomphorn, Y.; Armstead, W.M.; Vavilala, M.S. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma 2009, 26, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.S.; Green, R.; Virani, K.; Wong, H.; Eddy, K.; Vandorpe, R. Admission Perfusion CT for Classifying Early In-Hospital Mortality of Patients With Severe Traumatic Brain Injury: A Pilot Study. AJR Am. J. Roentgenol. 2020, 214, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.; Langlands, G.; Doucette, S.; Phillips, S. CT Perfusion in Acute Stroke Predicts Final Infarct Volume- Inter-observer Study. Can. J. Neurol. Sci. 2016, 43, 93–97. [Google Scholar] [CrossRef]
- Wannamaker, R.; Guinand, T.; Menon, B.K.; Demchuk, A.; Goyal, M.; Frei, D.; Bharatha, A.; Jovin, T.G.; Shankar, J.; Krings, T.; et al. Computed Tomographic Perfusion Predicts Poor Outcomes in a Randomized Trial of Endovascular Therapy. Stroke 2018, 49, 1426–1433. [Google Scholar] [CrossRef]
- Shankar, J.J.; Vandorpe, R. CT perfusion for confirmation of brain death. AJNR Am. J. Neuroradiol. 2013, 34, 1175–1179. [Google Scholar] [CrossRef]
- MacDonald, D.; Stewart-Perrin, B.; Shankar, J.J.S. The Role of Neuroimaging in the Determination of Brain Death. J. Neuroimaging 2018, 28, 374–379. [Google Scholar] [CrossRef]
- Shankar, J.J.S.; Stewart-Perrin, B.; Quraishi, A.U.; Bata, I.; Vandorpe, R. Computed Tomography Perfusion Aids in the Prognostication of Comatose Postcardiac Arrest Patients. Am. J. Cardiol. 2018, 121, 874–878. [Google Scholar] [CrossRef]
- Shankar, J.J.S. Variation in CT perfusion protocol has implications on defining irreversibly damaged ischemic brain parenchyma. Eur. Radiol. 2021, 31, 8315–8316. [Google Scholar] [CrossRef]
- Alcock, S.; Batoo, D.; Ande, S.R.; Grierson, R.; Essig, M.; Martin, D.; Trivedi, A.; Sinha, N.; Leeies, M.; Zeiler, F.A.; et al. Early diagnosis of mortality using admission CT perfusion in severe traumatic brain injury patients (ACT-TBI), Protocol for a prospective cohort study. BMJ Open 2021, 11, e047305. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| patients should be imaged with CT scan within 24 h of admission the study must talk about prognostication outcomes the study contains clinical picture including Glasgow Comma Scale (GCS) the injury must be a severe TBI (GCS ≤ 8) the study must include discharge outcomes with Glasgow Outcome Scale (GOS) the study must contain patient demographics (age, sex) the study must contain at least 5 patients both single- and multi-center are acceptable | any studies with non-acute traumatic events any non-brain or non-calvarium injuries adult studies (over 18 years) any non-traumatic brain injury mild and moderate traumatic brain injuries all systematic reviews, meta-analyses, reviews, and book chapters |
| Total Patients | 2219 | Number of Studies That Reported Finding |
|---|---|---|
| Male | 1461 | 22 |
| Female | 747 | 22 |
| Subdural hematoma | 293/797 = 36.7% | 11 |
| Epidural hematoma | 77/879 = 8.8% | 11 |
| Traumatic Subarachnoid hemorrhage | 347/1154 = 30.0% | 14 |
| Skull fractures | 244/771 = 31.6% | 10 |
| Brain edema | 416/924 = 45.0% | 10 |
| Dead | 564/2219 = 25.4% | 22 |
| Survived | 1651/2219 = 74.4% | 22 |
| SN | SP | PPV | NPV | AUC | |
|---|---|---|---|---|---|
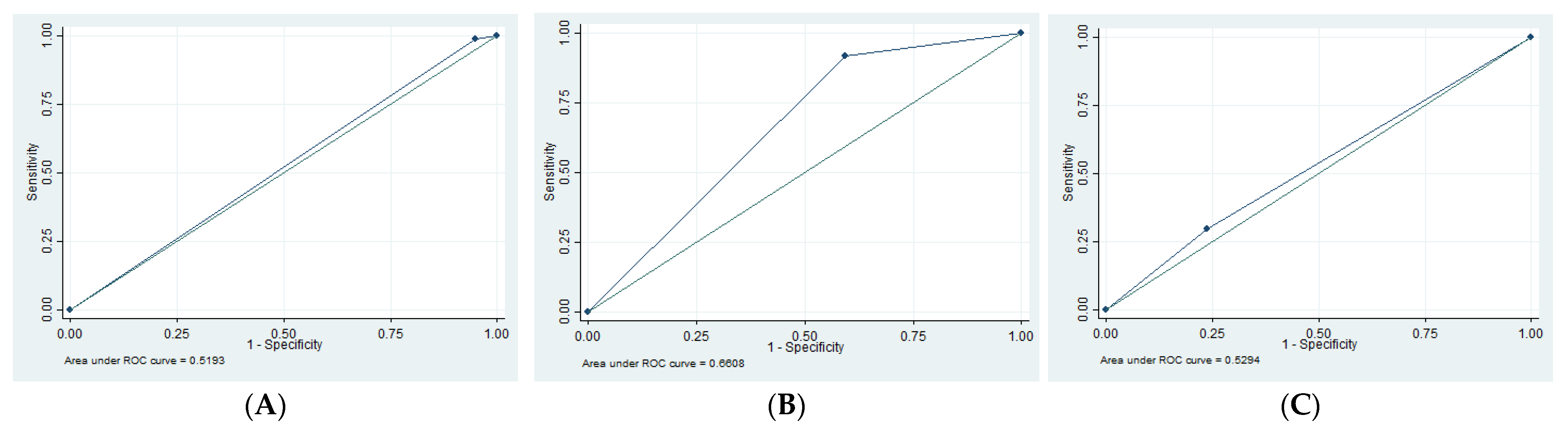
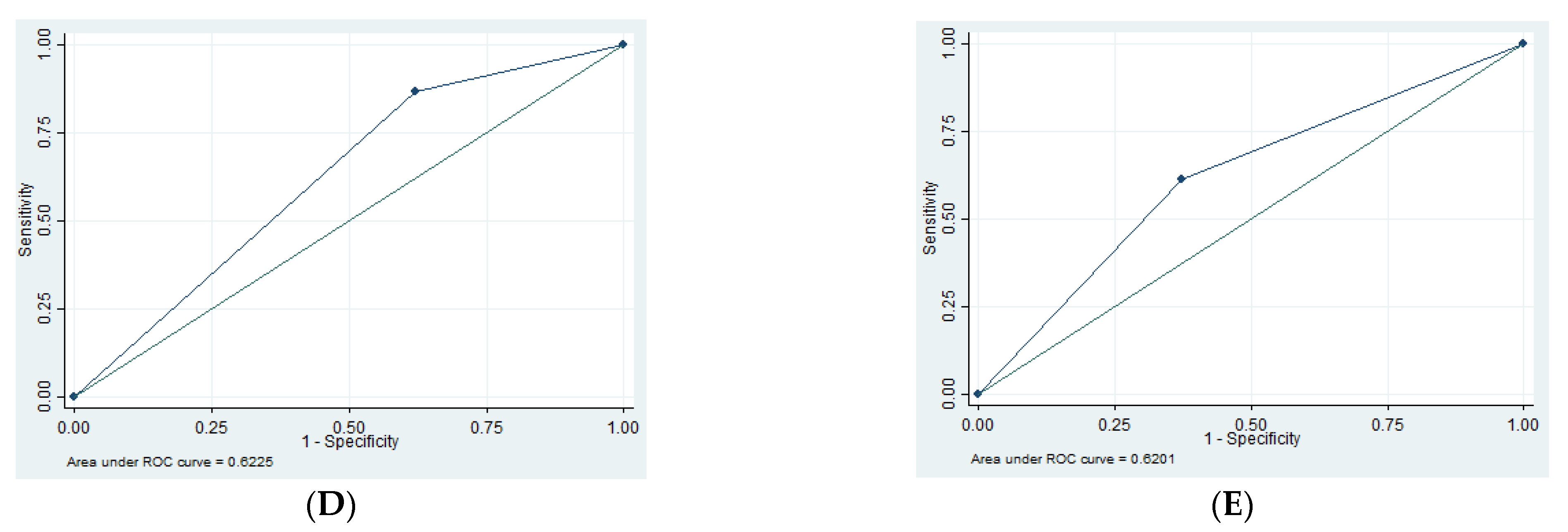
| EDH | 1.35 | 94.79 | 16.67 | 55.49 | 0.52 |
| SDH | 13.3 | 62.16 | 12.50 | 63.89 | 0.62 |
| tSAH | 61.29 | 62.73 | 38.78 | 80.80 | 0.62 |
| SF | 29.69 | 76.19 | 48.72 | 58.72 | 0.53 |
| Edema | 91.53 | 40.63 | 58.70 | 83.87 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goubran, D.; Batoo, D.; Linton, J.; Shankar, J. Initial CT Imaging Predicts Mortality in Severe Traumatic Brain Injuries in Pediatric Population—A Systematic Review and Meta-Analysis. Tomography 2023, 9, 541-551. https://doi.org/10.3390/tomography9020044
Goubran D, Batoo D, Linton J, Shankar J. Initial CT Imaging Predicts Mortality in Severe Traumatic Brain Injuries in Pediatric Population—A Systematic Review and Meta-Analysis. Tomography. 2023; 9(2):541-551. https://doi.org/10.3390/tomography9020044
Chicago/Turabian StyleGoubran, Doris, Divjeet Batoo, Janice Linton, and Jai Shankar. 2023. "Initial CT Imaging Predicts Mortality in Severe Traumatic Brain Injuries in Pediatric Population—A Systematic Review and Meta-Analysis" Tomography 9, no. 2: 541-551. https://doi.org/10.3390/tomography9020044
APA StyleGoubran, D., Batoo, D., Linton, J., & Shankar, J. (2023). Initial CT Imaging Predicts Mortality in Severe Traumatic Brain Injuries in Pediatric Population—A Systematic Review and Meta-Analysis. Tomography, 9(2), 541-551. https://doi.org/10.3390/tomography9020044






