Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists
Abstract
1. Introduction
2. Diagnosis, Staging, and Risk Assessment
2.1. Functional Assessment
2.2. Functional Tools
2.2.1. Somatostatin Receptor Imaging (SRI)
2.2.2. Positron Emission Tomography (PET)
2.3. Morphologic Imaging and Tools
2.3.1. Computed Tomography
2.3.2. Magnetic Resonance Imaging
2.3.3. Ultrasonography and Contrast-Enhanced Ultrasound (CEUS)
3. Clinical Setting
3.1. Pancreatic NENs
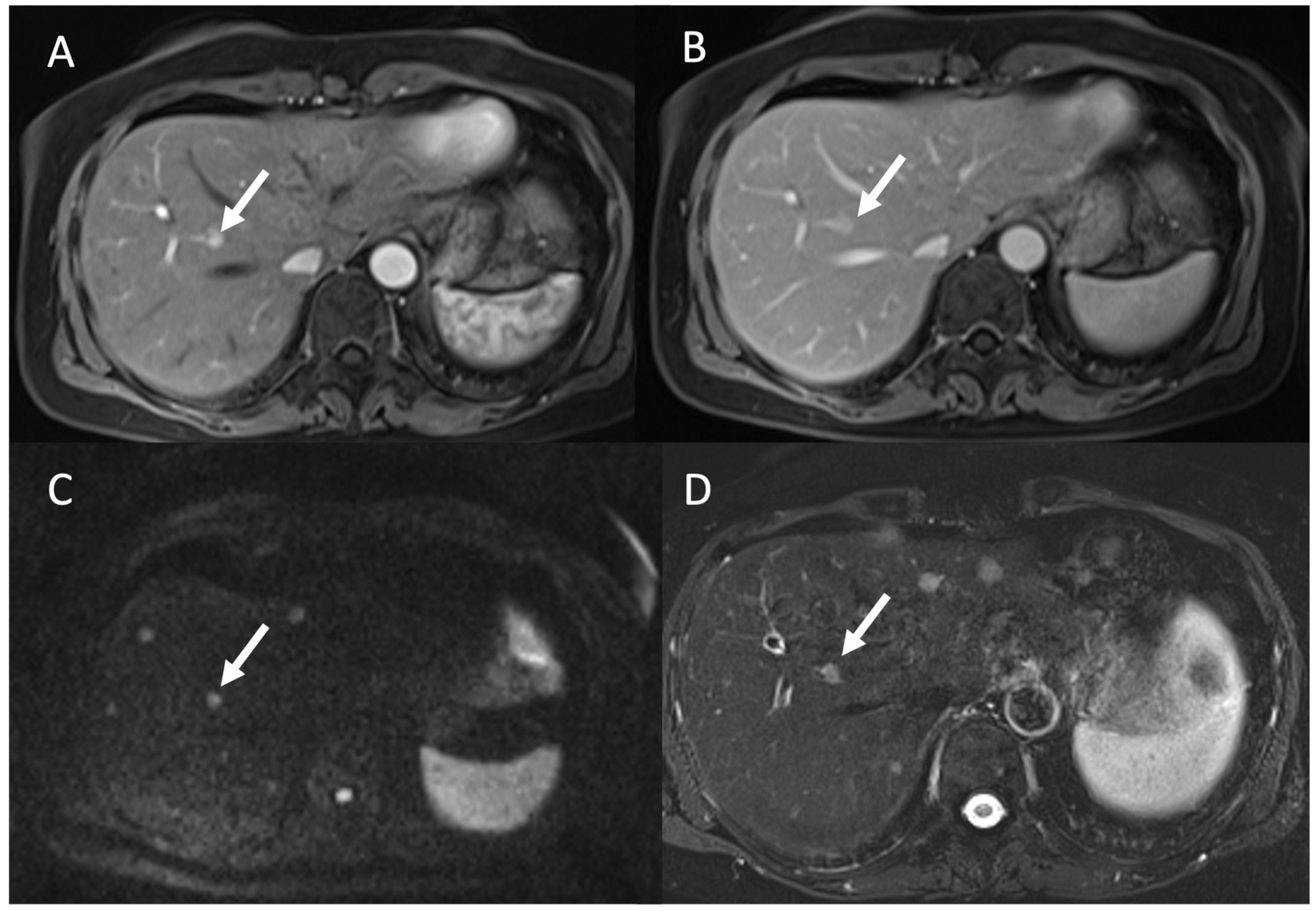
3.2. Liver Metastases
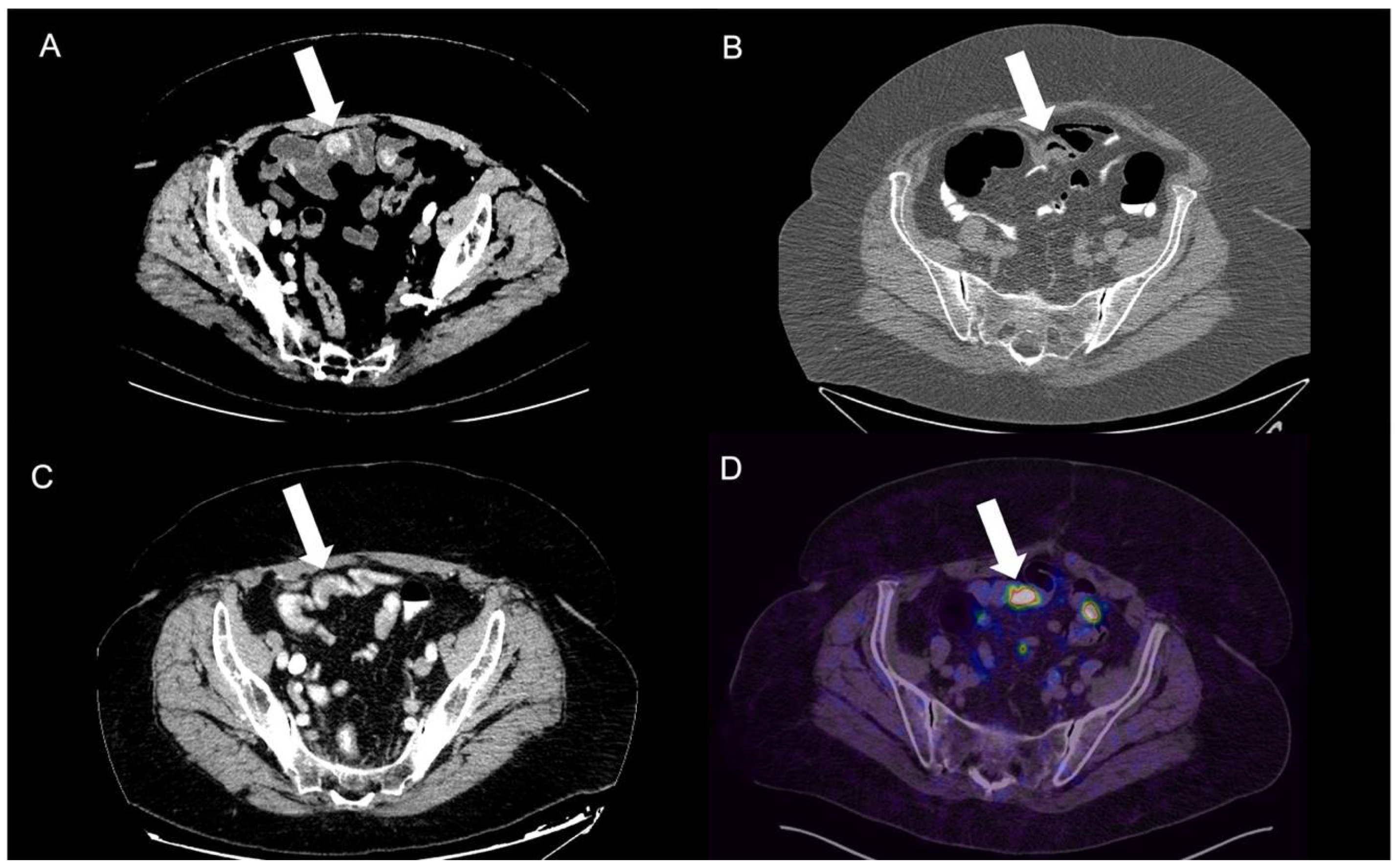
3.3. Gastrointestinal Tract NEN
4. Follow-Up and Treatment Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.; Abdalla, E.; Fleming, J.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Eh, T.; Ch, T. Imaging of gastroenteropancreatic neuroendocrine tumors. World J. Clin. Oncol. 2011, 2, 28. [Google Scholar] [CrossRef]
- Pantelis, A.; Panagopoulou, P.; Lapatsanis, D. Artificial Intelligence and Machine Learning in the Diagnosis and Management of Gastroenteropancreatic Neuroendocrine Neoplasms—A Scoping Review. Diagnostics 2022, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 1234. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Sahani, D.; Bonaffini, P.; Castillo, C.F.-D.; Blake, M. Gastroenteropancreatic Neuroendocrine Tumors: Role of Imaging in Diagnosis and Management. Radiology 2013, 11, 38–61. [Google Scholar] [CrossRef]
- Ganeshan, D.; Bhosale, P.; Yang, T.; Kundras, V. Imaging Features of Carcinoid Tumors of the Gastrointestinal Tract. 2013, 201, 773–786. AJR Am. J. Roentgenol. 2013, 201, 773–786. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; de Lutio di Castelguidone, E.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- De Felice, F.; Boldrini, L.; Greco, C.; Nardone, V.; Salvestrini, V.; Desideri, I. ESTRO vision 2030: The young Italian Association of Radiotherapy and Clinical Oncology (yAIRO) commitment statement. Radiol. Med. 2021, 126, 1374–1376. [Google Scholar] [CrossRef]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zheng-Pywell, R.; Chen, H.; Bibb, J.; Chen, H.; Rose, J. Management of Gastrointestinal Neuroendocrine Tumors. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419884058. [Google Scholar] [CrossRef]
- Liang, W.; Yang, P.; Huang, R.; Xu, L.; Wang, J.; Liu, W.; Zhang, L.; Wan, D.; Huang, Q.; Lu, Y.; et al. A Combined Nomogram Model to Preoperatively Predict Histologic Grade in Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2019, 25, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Bhosale, P.; Yano, M.; Itani, M.; Elsayes, A.; Halperin, D.; Bergsland, E.; Morani, A. New frontiers in imaging including radiomics updates for pancreatic neuroendocrine neoplasms. Abdom. Radiol. 2020, 47, 3078–3100. [Google Scholar] [CrossRef]
- Faviana, P.; Boldrini, L.; Gentile, C.; Erba, P.; Sammarco, E.; Bartoli, F.; Esposito, E.; Galli, L.; Lippolis, P.; Bardi, M. Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs. Diagnostics 2022, 12, 1960. [Google Scholar] [CrossRef]
- Coriat, R.; Walter, T.; Terris, B.; Couvelard, A.; Ruszniewski, P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist 2016, 21, 1191. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.; Olsen, I.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Med. 2021, 126, 1388–1395. [Google Scholar] [CrossRef]
- Cozzi, D.; Bicci, E.; Cavigli, E.; Danti, G.; Bettarini, S.; Tortoli, P.; Lorenzo; Mazzoni, N.; Busoni, S.; Pradella, S.; et al. Chest Radiology Radiomics in pulmonary neuroendocrine tumours (NETs). Radiol. Med. 2022, 127, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Karlafti, E.; Charalampidou, M.; Fotiadou, G.; Deka, I.A.; Raptou, G.; Kyriakidis, F.; Panidis, S.; Ioannidis, A.; Protopapas, A.; Netta, S.; et al. Ampullary Large-Cell Neuroendocrine Carcinoma, a Diagnostic Challenge of a Rare Aggressive Neoplasm: A Case Report and Literature Review. Diagnostics 2022, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic-Jovanovic, M.; Grubor, N.; Milosevic, S.; Jankovic, A.; Stosic, K.; Ostojic, S.; Ninic, A.; Micev, M.; Kovac, J. Total Pancreatectomy for Multicentric Cystic Neuroendocrine Tumor of the Pancreas: A Case Report. Diagnostics 2022, 12, 1003. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, L.; Klimstra, D. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am. J. Surg. Pathol. 2011, 35, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.; Morgensztern, D.; Carter, C. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef]
- El Ghannudi, S.; Ouvrard, E.; Mikail, N.; Freschini, B.; Schindler, T.; Imperiale, A. Cutting-Edge Imaging of Cardiac Metastases from Neuroendocrine Tumors: Lesson from a Case Series. Diagnostics 2022, 12, 1182. [Google Scholar] [CrossRef]
- Zilli, A.; Arcidiacono, P.; Conte, D.; Massironi, S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: Lights and shadows. Dig. Liver Dis. 2018, 50, 6–14. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Rottenburger, C.; Nicolas, G.; Wild, D. Gastroenteropancreatic neuroendocrine tumours (GEP-NET)–Imaging and staging. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 45–57. [Google Scholar] [CrossRef]
- Ramage, J.; Davies, A.H.G.; Ardill, J.; Bax, N.; Caplin, M.; Grossman, A.; Hawkins, R.; McNicol, A.; Reed, N.; Sutton, R. Watkinson, Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005, 54, iv1–iv16. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Yao, S.; Yu, M.; Wu, W.; Xue, H.; Kiesewetter, D.; Zhu, Z.; Li, F.; Zhao, Y.; et al. Glucagon-Like Peptide-1 Receptor PET/CT with 68Ga-NOTA-Exendin-4 for Detecting Localized Insulinoma: A Prospective Cohort Study. J. Nucl. Med. 2016, 57, 715. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Sotiropoulos, G.; Radtke, A.; Malago, M.; Bockisch, A.; Kuehl, H.; Li, J.; Broelsc, C. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann. Surg. 2010, 252, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Langer, S.; Federspiel, B.; Oxbøl, J.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Knigge, U.; Kjaer, A. 68Ga-DOTATOC PET and gene expression profile in patients with neuroendocrine carcinomas: Strong correlation between PET tracer uptake and gene expression of somatostatin receptor subtype 2. Am. J. Nucl. Med. Mol. Imag. 2016, 6, 59. [Google Scholar]
- Binderup, T.; Knigge, U.; Loft, A.; Mortensen, J.; Pfeifer, A.; Federspiel, B.; Hansen, C.; Højgaard, L.; Kjaer, A. Functional imaging of neuroendocrine tumors: A head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J. Nucl. Med. 2010, 51, 704–712. [Google Scholar] [CrossRef]
- Simsek, D.; Kuyumcu, S.; Turkmen, C.; Sanli, Y.; Aykan, F.; Unal, S.; Adalet, I. Can Complementary 68Ga-DOTATATE and 18F-FDG PET/CT Establish the Missing Link Between Histopathology and Therapeutic Approach in Gastroenteropancreatic Neuroendocrine Tumors? J. Nucl. Med. 2014, 55, 1811–1817. [Google Scholar] [CrossRef]
- Maxwell, J.; Howe, J. Imaging in neuroendocrine tumors: An update for the clinician. Int. J. Endocr. Oncol. 2015, 2, 159–168. [Google Scholar] [CrossRef]
- Kaewput, C.; Vinjamuri, S. Role of Combined 68Ga DOTA-Peptides and 18F FDG PET/CT in the Evaluation of Gastroenteropancreatic Neuroendocrine Neoplasms. Diagnostics 2022, 12, 280. [Google Scholar] [CrossRef]
- Shetty, D.; Patel, D.; Le, K.; Bui, C.; Mansberg, R. Pitfalls in Gallium-68 PSMA PET/CT Interpretation—A Pictorial Review. Tomography 2018, 4, 182–193. [Google Scholar] [CrossRef]
- de Herder, W.; Kwekkeboom, D.; Feelders, R.; van Aken, M.; Lamberts, S.W.J.; van der Lely, A.; Krenning, E. Somatostatin receptor imaging for neuroendocrine tumors. Pituitary 2006, 9, 243–248. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Grassetto, G.; Rubello, D.; Fanti, S. PET/CT with 68Gallium-DOTA-peptides in NET: An overview. Eur. J. Radiol. 2011, 80, e116–e119. [Google Scholar] [CrossRef]
- Bauckneht, M.; Albano, D.; Annunziata, S.; Santo, G.; Guglielmo, P.; Frantellizzi, V.; Branca, A.; Ferrari, C.; Vento, A.; Mirabile, A.; et al. Somatostatin Receptor PET/CT Imaging for the Detection and Staging of Pancreatic NET: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 598. [Google Scholar] [CrossRef]
- Hussein, M.; Cafarelli, F.; Paparella, M.; Rennie, W.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Fanti, S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. 2012, 39, 52–60. [Google Scholar] [CrossRef]
- Poeppel, T.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, I.; Henze, M.; Engelbrecht, S.; Eisenhut, M.; Runz, A.; Schäfer, M.; Schilling, T.; Haufe, S.; Herrmann, T.; Haberkorn, U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. 2007, 34, 1617–1626. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Kayani, I.; Quigley, A.; Soh, J.; Caplin, M.; Bomanji, J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J. Nucl. Med. 2010, 51, 875–882. [Google Scholar] [CrossRef]
- Naswa, N.; Sharma, P.; Kumar, A.; Nazar, A.; Kumar, R.; Chumber, S.; Bal, C. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study, AJR. Am. J. Roentgenol. 2011, 197, 1221–1228. [Google Scholar] [CrossRef]
- Krausz, Y.; Freedman, N.; Rubinstein, R.; Lavie, E.; Orevi, M.; Tshori, S.; Salmon, A.; Glaser, B.; Chisin, R.; Mishani, E.; et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: Comparison with 111In-DTPA-octreotide (OctreoScan®). Mol. Imaging Biol. 2011, 13, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Tomassetti, P.; Castellucci, P.; Campana, D.; Montini, G.; Rubello, D.; Nanni, C.; Rizzello, A.; Franchi, R.; Fanti, S. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur. J. Nucl. Med. Mol. 2008, 35, 1431–1438. [Google Scholar] [CrossRef]
- Calabrò, D.; Argalia, G.; Ambrosini, V. Role of PET/CT and Therapy Management of Pancreatic Neuroendocrine Tumors. Diagnostics 2020, 10, 1059. [Google Scholar] [CrossRef]
- Sharma, P.; Arora, S.; Mukherjee, A.; Pal, S.; Sahni, P.; Garg, P.; Khadgawat, R.; Thulkar, S.; Bal, C.; Kumar, R. Predictive value of 68Ga-DOTANOC PET/CT in patients with suspicion of neuroendocrine tumors: Is its routine use justified? Clin. Nucl. Med. 2014, 39, 37–43. [Google Scholar] [CrossRef]
- Sharma, P.; Naswa, N.; KC, S.; Alvarado, L.; Dwivedi, A.; Yadav, Y.; Kumar, R.; Ammini, A.; Bal, C. Comparison of the prognostic values of 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. Eur. J. Nucl. Med. Mol. 2014, 41, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Arora, S.; Dhull, V.; Naswa, N.; Kumar, R.; Ammini, A.; Bal, C. Evaluation of (68)Ga-DOTANOC PET/CT imaging in a large exclusive population of pancreatic neuroendocrine tumors. Abdom Imag. 2015, 40, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rufini, V.; Baum, R.; Castaldi, P.; Treglia, G.; De Gaetano, A.; Carreras, C.; Kaemmerer, D.; Hommann, M.; Hörsch, D.; Bonomo, L.; et al. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom Imag. 2012, 37, 1004–1020. [Google Scholar] [CrossRef]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: A meta-analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Bomanji, J.; Benkert, P.; Maecke, H.; Ell, P.; Reubi, J.; Caplin, M. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT Within Patients with Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2013, 54, 364–372. [Google Scholar] [CrossRef]
- De Camargo Etchebehere, E.C.S.; De Oliveira Santos, A.; Gumz, B.; Vicente, A.; Hoff, P.; Corradi, G.; Ichiki, W.; De Almeida Filho, J.; Cantoni, S.; Camargo, E.; et al. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-Octreotide SPECT/CT, and Whole-Body MR Imaging in Detection of Neuroendocrine Tumors: A Prospective Trial. J. Nucl. Med. 2014, 55, 1598–1604. [Google Scholar] [CrossRef]
- Durgapal, P.; Sharma, R.; Kandasamy, D.; Bal, C. Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-Nal3-octreotide for localization of clinically and biochemically suspected insulinoma. Q. J. Nucl. Med. Mol. Imag. 2016, 60, 69–76. [Google Scholar]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; KlÖppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Bozkurt, M.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imag. 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Severi, S.; Nanni, O.; Bodei, L.; Sansovini, M.; Ianniello, A.; Nicoletti, S.; Scarpi, E.; Matteucci, F.; Gilardi, L.; Paganelli, G. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imag. 2013, 40, 881–888. [Google Scholar] [CrossRef]
- Delpassand, E.; Samarghandi, A.; Mourtada, J.; Zamanian, S.; Espenan, G.; Sharif, R.; MacKenzie, S.; Kosari, K.; Barakat, O.; Naqvi, S.; et al. Long-Term Survival, Toxicity Profile, and role of F-18 FDG PET/CT scan in Patients with Progressive Neuroendocrine Tumors Following Peptide Receptor Radionuclide Therapy with High Activity In-111 Pentetreotide. Theranostics 2012, 2, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Orlefors, H.; Sundin, A.; Garske, U.; Juhlin, C.; Oberg, K.; Skogseid, B.; Langstrom, B.; Bergstrom, M.; Eriksson, B. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and computed tomography. J. Clin. Endocrinol. Metab. 2005, 90, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Perrucci, L.; Pellegrino, F.; Baroni, L.; Merlo, A.; Tilli, M.; Rambaldi, I.; Maietti, E.; Carnevale, A.; Bartolomei, M.; et al. Diagnostic contribution of contrast-enhanced CT as compared to unenhanced low-dose CT in PET/CT staging and treatment response assessment of 18FDG-avid lymphomas: A prospective study. J. Nucl. Med. 2021, 62, 1372–1379. [Google Scholar] [CrossRef]
- Pellegrino, F.; Scabbia, F.; Merlo, A.; Perrucci, L.; Aliberti, L.; Urso, A.; Ambrosio, M.; Cuneo, A.; Galeotti, R.; Giganti, M. Spontaneously reversible adrenal nodules in primary diffuse large B-cell testicular lymphoma mimicking an extranodal involvement: A case report. Radiol. Case Rep. 2021, 16, 2168–2173. [Google Scholar] [CrossRef]
- Koopmans, K.; Neels, O.; Kema, I.; Elsinga, P.; Sluiter, W.; Vanghillewe, K.; Brouwers, A.; Jager, P.; De Vries, E. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J. Clin. Oncol. 2008, 26, 1489–1495. [Google Scholar] [CrossRef]
- Adams, S.; Baum, R.; Rink, T.; Schumm-Dräger, P.; Usadel, K.; Hör, G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur. J. Nucl. Med. 1998, 25, 79–83. [Google Scholar] [CrossRef]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Muffatti, F.; Lena, M.S.; Pasetto, S.; Bezzi, C.; Bettinardi, V.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET Improve Preoperative Evaluation of Aggressiveness in Resectable Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 192. [Google Scholar] [CrossRef]
- Garin, E.; Le Jeune, F.; Devillers, A.; Cuggia, M.; De Lajarte-Thirouard, A.; Bouriel, C.; Boucher, E.; Raoul, J. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J. Nucl. Med. 2009, 50, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef]
- Masui, T.; Doi, R.; Ito, T.; Kami, K.; Ogawa, K.; Harada, D.; Uemoto, S. Diagnostic value of 18F-fluorodeoxyglucose positron emission tomography for pancreatic neuroendocrine tumors with reference to the World Health Organization classification. Oncol. Lett. 2010, 1, 155. [Google Scholar] [CrossRef]
- Cloyd, J.; Poultsides, G. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J. Gastroenterol. 2015, 21, 9512. [Google Scholar] [CrossRef]
- Fusco, R.; Setola, S.; Raiano, N.; Granata, V.; Cerciello, V.; Pecori, B.; Petrillo, A. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: Dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol. Medica 2022, 127, 733–742. [Google Scholar] [CrossRef]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT radiomics in predicting the overall survival of patients with stage III clear cell renal carcinoma after radical nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef]
- Masci, G.; Ciccarelli, F.; Mattei, F.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef]
- Gregucci, F.; Fiorentino, A.; Mazzola, R.; Ricchetti, F.; Bonaparte, I.; Surgo, A.; Figlia, V.; Carbonara, R.; Caliandro, M.; Ciliberti, M.; et al. Radiomic analysis to predict local response in locally advanced pancreatic cancer treated with stereotactic body radiation therapy. Radiol. Med. 2022, 127, 100–107. [Google Scholar] [CrossRef]
- Ruf, J.; Schiefer, J.; Furth, C.; Kosiek, O.; Kropf, S.; Heuck, F.; Denecke, T.; Pavel, M.; Pascher, A.; Wiedenmann, B. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J. Nucl. Med. 2011, 52, 697–704. [Google Scholar] [CrossRef]
- Carnevale, M.A.; D’Amato, E.; Pellegrino, F.; Toma, I.; Perrucci, L.; Di Ciesco, C.A.; Labaj, O. Pneumatosis cystoides intestinalis during the treatment with paclitaxel for metastatic ovarian cancertle. EuroMediter. Biomed. J. 2020, 15, 130–133. [Google Scholar] [CrossRef]
- Pinto, A.; Scaglione, M.; Romano, L. Perforation of a gastrojejunal anastomosis due to acute pancreatitis revealed by helical computed tomography. Acta Radiol. 2003, 44, 572–573. [Google Scholar] [CrossRef]
- Scaglione, M.; Grassi, R.; Pinto, A.; Giovine, S.; Gagliardi, N.; Stavolo, C.; Romano, L. Positive predictive value and negative predictive value of spiral CT in the diagnosis of closed loop obstruction complicated by intestinal ischemia. Radiol. Med. 2004, 107, 69–77. [Google Scholar]
- Scaglione, M.; Romano, S.; Pinto, F.; Flagiello, F.; Farina, R.; Acampora, C.; Romano, L. Helical CT diagnosis of small bowel obstruction in the acute clinical setting. Eur. J. Radiol. 2004, 50, 15–22. [Google Scholar] [CrossRef]
- Lassandro, F.; Scaglione, M.; Rossi, G.; Grassi, R.; Romano, L. Portomesenteric vein gas: Diagnostic and prognostic value. Emerg. Radiol. 2002, 9, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, M.; Romano, L.; Bocchini, G.; Sica, G.; Guida, F.; Pinto, A.; Grassi, R. Multidetector computed tomography of pancreatic, small bowel, and mesenteric traumas. Semin. Roentgenol. 2012, 47, 362–370. [Google Scholar] [CrossRef]
- Patlas, M.; Alabousi, A.; Scaglione, M.; Romano, L.; Soto, J. Cross-sectional imaging of nontraumatic peritoneal and mesenteric emergencies. Can. Assoc. Radiol. J. 2013, 64, 148–153. [Google Scholar] [CrossRef]
- FIacobellis; Narese, D.; Berritto, D.; Brillantino, A.; Di Serafino, M.; Guerrini, S.; Grassi, R.; Scaglione, M.; Mazzei, M.; Romano, L. Large Bowel Ischemia/Infarction: How to Recognize It and Make Differential Diagnosis? A Review. Diagnostics 2021, 11, 998. [Google Scholar] [CrossRef]
- Scaglione, M.; Galluzzo, M.; Santucci, D.; Trinci, M.; Messina, L.; Laccetti, E.; Faiella, E.; Zobel, B.B. Small bowel obstruction and intestinal ischemia: Emphasizing the role of MDCT in the management decision process. Abdom. Radiol. 2022, 47, 1541–1555. [Google Scholar] [CrossRef]
- Scaglione, M.; Pinto, A.; Romano, S.; Scialpi, M.; Volterrani, L.; Rotondo, A.; Romano, L. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: The problems and the possibilities. J. Pancreas 2005, 6, 278. [Google Scholar]
- Tamm, E.; Bhosale, P.; Lee, J.; Rohren, E. State-of-the-art Imaging of Pancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2016, 25, 375–400. [Google Scholar] [CrossRef]
- Scialpi, M.; Reginelli, A.; D’Andrea, A.; Gravante, S.; Falcone, G.; Baccari, P.; Manganaro, L.; Palumbo, B.; Cappabianca, S. Pancreatic tumors imaging: An update. Int. J. Surg. 2016, 28, S142–S155. [Google Scholar] [CrossRef]
- Atwi, N.; Sabottke, C.; Pitre, D.; Smith, D.; Danrad, R.; Dharaiya, E.; Kambadakone, A.; Pandharipande, P.; Toshav, A. Follow-up Recommendation Rates Associated with Spectral Detector Dual-Energy CT of the Abdomen and Pelvis: A Retrospective Comparison to Single-Energy CT. J. Am. Coll. Radiol. 2020, 17, 940–950. [Google Scholar] [CrossRef]
- Johnson, T.; Krauß, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Assadsangabi, R.; Babaei, R.; Songco, C.; Ivanovic, V.; Bobinski, M.; Chen, Y.; Nabavizadeh, S. Multimodality oncologic evaluation of superficial neck and facial lymph nodes. Radiol. Med. 2021, 126, 1074–1084. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Di Matteo, A.; Ceccarelli, L.; Farah, S.; Villota-Eraso, C.; Di Carlo, M.; Giovagnoni, A. Ultrasound and magnetic resonance imaging as diagnostic tools for sarcopenia in immune-mediated rheumatic diseases (IMRDs). Radiol. Med. 2022, 127, 1277–1291. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Z.; Tao, R.; Guo, Y.; Li, J.; Zhang, J.; Chen, K. Dual energy spectral CT imaging of insulinoma-Value in preoperative diagnosis compared with conventional multi-detector CT. Eur. J. Radiol. 2012, 81, 2487–2494. [Google Scholar] [CrossRef]
- Kamaoui, I.; De-Luca, V.; Ficarelli, S.; Mennesson, N.; Lombard-Bohas, C.; Pilleul, F. Value of CT enteroclysis in suspected small-bowel carcinoid tumors. Am. J. Roentgenol. 2010, 194, 629–633. [Google Scholar] [CrossRef]
- Morse, B.; Al-Toubah, T.; Montilla-Soler, J. Anatomic and Functional Imaging of Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2020, 21, 1–16. [Google Scholar] [CrossRef]
- Niederle, B.; Pape, U.; Costa, F.; Gross, D.; Kelestimur, F.; Knigge, U.; Öberg, K.; Pavel, M.; Perren, A.; Toumpanakis, C.; et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology 2016, 103, 125–138. [Google Scholar] [CrossRef]
- Soyer, P.; Aout, M.; Hoeffel, C.; Vicaut, E.; Placé, V.; Boudiaf, M. Helical CT-enteroclysis in the detection of small-bowel tumours: A meta-analysis. Eur. Radiol. 2013, 23, 388–399. [Google Scholar] [CrossRef]
- Hakim, F.; Alexander, J.; Huprich, J.; Grover, M.; Enders, F. CT-enterography may identify small bowel tumors not detected by capsule endoscopy: Eight years experience at Mayo Clinic Rochester. Dig. Dis. Sci. 2011, 56, 2914–2919. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Simonetti, I.; Fusco, R.; Sergio; Setola, V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef]
- Scola, E.; Desideri, I.; Bianchi, A.; Gadda, D.; Busto, G.; Fiorenza, A.; Amadori, T.; Mancini, S.; Miele, V.; Fainardi, E. Assessment of brain tumors by magnetic resonance dynamic susceptibility contrast perfusion-weighted imaging and computed tomography perfusion: A comparison study. Radiol. Med. 2022, 127, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Guo, W.; Zeng, P.; Liu, Y.; Lang, N.; Yuan, H. A preliminary study using spinal MRI-based radiomics to predict high-risk cytogenetic abnormalities in multiple myeloma. Radiol. Med. 2021, 126, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.; Jaschke, W.; Virgolini, I. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.; Morelli, J.; Neumann, R.; Haug, A.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.; Rist, C. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur. J. Nucl. Med. Mol. Imag. 2013, 40, 897–907. [Google Scholar] [CrossRef]
- Brenner, R.; Metens, T.; Bali, M.; Demetter, P.; Matos, C. Pancreatic neuroendocrine tumor: Added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur. J. Radiol. 2012, 81, e746–e749. [Google Scholar] [CrossRef] [PubMed]
- G. d’Assignies; Fina, P.; Bruno, O.; Vullierme, M.; Tubach, F.; Paradis, V.; Sauvanet, A.; Ruszniewski, P.; Vilgrain, V. High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: Comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology 2013, 268, 390–399. [Google Scholar] [CrossRef]
- Ronot, M.; Clift, A.; Baum, R.; Singh, A.; Kulkarni, H.; Frilling, A.; Vilgrain, V. Morphological and Functional Imaging for Detecting and Assessing the Resectability of Neuroendocrine Liver Metastases. Neuroendocrinology 2018, 106, 74–88. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T.; Lumbroso, J.; Caillet, H.; Laplanche, A.; Boige, V.; Ducreux, M.; Duvillard, P.; Elias, D.; Schlumberger, M.; et al. Detection of liver metastases from endocrine tumors: A prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cwikła, J.; Buscombe, J.; Caplin, M.; Watkinson, A.; Walecki, J.; Gorczyca-Wiśniewska, E.; Hilson, A. Diagnostic imaging of carcinoid metastases to the abdomen and pelvis. Med. Sci. Monit. 2004, 10, 9–16. [Google Scholar] [PubMed]
- Chambers, A.; Pasieka, J.; Dixon, E.; Rorstad, O. Role of imaging in the preoperative staging of small bowel neuroendocrine tumors. J. Am. Coll. Surg. 2010, 211, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Sundin, Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 803–818. [CrossRef]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 127, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.; Aversana, F.D.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Maio, F.; Avallone, A.; Nasti, G.; Palaia, R.; Albino, V.; Grassi, R.; Izzo, F.; Petrillo, A. Qualitative assessment of EOB-GD-DTPA and Gd-BT-DO3A MR contrast studies in HCC patients and colorectal liver metastases. Infect. Agent. Cancer 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.; Dell’aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; de Lutio di Castelguidone, E.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid–enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Med. 2022, 127, 1098–1105. [Google Scholar] [CrossRef]
- Pecoraro, M.; Cipollari, S.; Marchitelli, L.; Messina, E.; Del Monte, M.; Galea, N.; Ciardi, M.; Francone, M.; Catalano, C.; Panebianco, V. Cross-sectional analysis of follow-up chest MRI and chest CT scans in patients previously affected by COVID-19. Radiol. Med. 2021, 126, 1273. [Google Scholar] [CrossRef]
- Cappabianca; Di Grezia, G.; Grassi, V.; Rotondo, A. The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: Our experience and literature review. Radiol Med. 2011, 116, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Smits, M.; Boxerman, J.; Huang, R.; Barboriak, D.; Weller, M.; Chung, C.; Tsien, C.; Brown, P.; Shankar, L.; et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro. Oncol. 2020, 22, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Werther, G. Best Practice & Research Clinical Endocrinology and Metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 591–601. [Google Scholar] [CrossRef]
- Orditura, M.; Petrillo, A.; Ventriglia, J.; Diana, A.; Laterza, M.; Fabozzi, A.; Savastano, B.; Franzese, E.; Conzo, G.; Santini, L. Pancreatic neuroendocrine tumors: Nosography, management and treatment. Int. J. Surg. 2016, 1, S156–S162. [Google Scholar] [CrossRef]
- Halefoglu, A.; Ozagari, A. Tumor grade estımatıon of clear cell and papıllary renal cell carcınomas usıng contrast-enhanced MDCT and FSE T2 weıghted MR ımagıng: Radıology-pathology correlation. Radiol. Med. 2021, 126, 1139–1148. [Google Scholar] [CrossRef]
- Attili, F.; Capurso, G.; Vanella, G.; Fuccio, L.; Fave, G.; Costamagna, G.; Larghi, A. Diagnostic and therapeutic role of endoscopy in gastroenteropancreatic neuroendocrine neoplasms. Dig. Liver Dis. 2014, 46, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Amato, D.; Albino, V.; Patrone, R.; Izzo, F.; Petrillo, A. Beyond the vascular profile: Conventional DWI, IVIM and kurtosis in the assessment of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7284–7293. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Belli, A.; Danti, G.; Bicci, E.; Cutolo, C.; Petrillo, A.; Izzo, F. Diffusion weighted imaging and diffusion kurtosis imaging in abdominal oncological setting: Why and when. Infect. Agent. Cancer 2022, 17, 25. [Google Scholar] [CrossRef]
- Bruno, F.; Granata, V.; Bellisari, F.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Satake, H.; Ishigaki, S.; Ito, R.; Naganawa, S. Breast Radiology Radiomics in breast MRI: Current progress toward clinical application in the era of artificial intelligence. Radiol. Med. 2022, 127, 39–56. [Google Scholar] [CrossRef]
- Jensen, J.; Helpern, J. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Filippo; Grande, D.; Luca; Sconfienza, M. An update in musculoskeletal tumors: From quantitative imaging to radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Zhang, X.; Zhu, H.; Liu, Y.; Wei, Y.; Sun, Y. Non-gaussian models of 3-Tesla diffusion-weighted MRI for the differentiation of pancreatic ductal adenocarcinomas from neuroendocrine tumors and solid pseudopapillary neoplasms. Magn. Reson. Imag. 2021, 83, 68–76. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Zeng, P.; Ma, L.; Liu, J.; Song, Z.; Liu, J.; Yuan, H. The diagnostic value of intravoxel incoherent motion diffusion-weighted imaging for distinguishing nonhypervascular pancreatic neuroendocrine tumors from pancreatic ductal adenocarcinomas. Eur. J. Radiol. 2022, 150. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Paul; Summers, E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Anwar; et al. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use on behalf of the Italian Working Group on Magnetic Resonance. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef]
- Schraml, C.; Schwenzer, N.; Sperling, O.; Aschoff, P.; Lichy, M.; Uller, M.M.; Brendle, C.; Werner, M.; Claussen, C.; Pfannenberg, C. Staging of neuroendocrine tumours: Comparison of [68Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imag. 2013, 13, 63–72. [Google Scholar] [CrossRef]
- Hope, T.; Pampaloni, M.; Nakakura, E.; VanBrocklin, H.; Slater, J.; Jivan, S.; Aparici, C.; Yee, J.; Bergsland, E. Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom. Imag. 2015, 40, 1432–1440. [Google Scholar] [CrossRef]
- Beiderwellen, K.; Poeppel, T.; Hartung-Knemeyer, V.; Buchbender, C.; Kuehl, H.; Bockisch, A.; Lauenstein, T. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: Initial results. Investig. Radiol. 2013, 48, 273–279. [Google Scholar] [CrossRef]
- Ierardi, A.; Stellato, E.; Pellegrino, G.; Bonelli, C.; Cellina, M.; Renzulli, M.; Biondetti, P.; Carrafiello, G. Fluid-dynamic control microcatheter used with glue: Preliminary experience on its feasibility and safety. Radiol. Med. 2022, 127, 272–276. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.; Bozkurt, M.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Ergün, E.L.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Ierardi, A.; Carnevale, A.; Angileri, S.; Pellegrino, F.; Renzulli, M.; Golfieri, R.; Zhang, D.; Sun, H.; Giganti, M.; Dionigi, G.; et al. Outcomes following minimally invasive imagine-guided percutaneous ablation of adrenal glands. Gland Surg. 2020, 9, 859–866. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Pellegrino, F.; Di Stefano, G.; Bonelli, C.; Renzulli, M.; Giganti, M.; Carrafiello, G. Uterine Myomas: Extravascular Treatment. Semin. Ultrasound CT. MR. 2021, 42, 56–74. [Google Scholar] [CrossRef]
- Francica, G.; Meloni, M.; De Sio, I.; Smolock, A.; Brace, C.; Iadevaia, M.; Santambrogio, R.; Sironi, S.; Scaglione, M.; Lee, F. Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: Safety and efficacy. Eur. J. Radiol. 2016, 85, 739–743. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.; Hunt, S.; Park, B. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef]
- Spinelli, M.; Balbaa, M.F.; Mauro; Gallazzi, B.; Mohamed; Eid, E.-E.; Hesham; Kotb, T.; Shafei, E.; Anna; et al. Role of percutaneous CT-guided radiofrequency ablation in treatment of intra-articular, in close contact with cartilage and extra-articular osteoid osteomas: Comparative analysis and new classification system. Radiol. Med. 2022, 127, 1142–1150. [Google Scholar] [CrossRef]
- Gurgitano, M.; Salvatore; Angileri, A.; Giovanni; Rodà, M.; Liguori, A.; Pandolfi, M.; Ierardi, A.; Bradford; Wood, J.; et al. Vascular and Interventional Radiology Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Anna; Scrofani, R.; Marra, P.; Coniglio, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Paolo; Rio, D.; Ziglioli, F.; et al. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Agazzi, G.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D.; et al. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.-R.; He, Y.; Chen, J.-Q.; Yang, H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): A comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar] [CrossRef]
- Carnevale, A.; Pellegrino, F.; Cossu, A.; Ierardi, A.; Parenti, G.; Carrafiello, G.; Giganti, M. Current concepts in ablative procedures for primary benign liver lesions: A step forward to minimize the invasiveness of treatment when deemed necessary. Med. Oncol. 2020, 37, 31. [Google Scholar] [CrossRef]
- Stefanini, M.; Simonetti, G. Interventional Magnetic Resonance Imaging Suite (IMRIS): How to build and how to use. Radiol. Med. 2022, 127, 1063–1067. [Google Scholar] [CrossRef]
- Mansur, A.; Garg, T.; Shrigiriwar, A.; Etezadi, V.; Georgiades, C.; Habibollahi, P.; Huber, T.; Camacho, J.; Nour, S.; Sag, A.; et al. Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors. Diagnostics 2022, 12, 1300. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Kos-Kudla, B.; Blicharz-Dorniak, J.; Strzelczyk, J.; Baldys-Waligórska, A.; Bednarczuk, T.; Bolanowski, M.; Boratyn-Nowicka, A.; Borowska, M.; Cichocki, A.; Cwikla, J.; et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2017, 68, 79–110. [Google Scholar] [CrossRef]
- Anderson, M.; Carpenter, S.; Thompson, N.; Nostrant, T.; Elta, G.; Scheiman, J. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am. J. Gastroenterol. 2000, 95, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsusaki, S. The Utility of Endoscopic-Ultrasonography-Guided Tissue Acquisition for Solid Pancreatic Lesions. Diagnostics 2022, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yasuda, I.; Hanaoka, T.; Hayashi, Y.; Araki, Y.; Motoo, I.; Kajiura, S.; Ando, T.; Fujinami, H.; Tajiri, K.; et al. Diagnostic Fine-Needle Biopsy of Small Solid Pancreatic Lesions Using a Franseen Needle during Endoscopic Ultrasound Examination. Diagnostics 2020, 11, 27. [Google Scholar] [CrossRef]
- Ishii, T.; Katanuma, A.; Toyonaga, H.; Chikugo, K.; Nasuno, H.; Kin, T.; Hayashi, T.; Takahashi, K. Role of Endoscopic Ultrasound in the Diagnosis of Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Sugihara, Y.; Yamazaki, H.; Koutani, H.; Tamura, T.; Tsuda, I.; Emori, T.; Kawaji, Y.; Hatamaru, K.; Yamashita, Y.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasound for Diagnosis of the Aggressiveness of Pancreatic Neuroendocrine Neoplasm. Diagnostics 2022, 12, 2988. [Google Scholar] [CrossRef]
- Manta, R.; Nardi, E.; Pagano, N.; Ricci, C.; Sica, M.; Castellani, D.; Bertani, H.; Piccoli, M.; Mullineris, B.; Tringali, A.; et al. Pre-operative Diagnosis of Pancreatic Neuroendocrine Tumors with Endoscopic Ultrasonography and Computed Tomography in a Large Series. J. Gastrointestin. Liver Dis. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- James, P.; Tsolakis, A.; Zhang, M.; Belletrutti, P.; Mohamed, R.; Roberts, D.; Heitman, S. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: A meta-analysis. Gastrointest. Endosc. 2015, 81, 848–856.e1. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Ciaravino, V.; De Robertis, R.; Martini, P.T.; Cardobi, N.; Cingarlini, S.; Amodio, A.; Landoni, L.; Capelli, P.; D’Onofrio, M. Imaging presentation of pancreatic neuroendocrine neoplasms. Insights Imag. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, M.; Kim, H. Diagnosis of Pancreatic Neuroendocrine Tumors. Clin. Endosc. 2017, 50, 537. [Google Scholar] [CrossRef]
- Partelli, S.; Cirocchi, R.; Crippa, S.; Cardinali, L.; Fendrich, V.; Bartsch, D.; Falconi, M. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br. J. Surg. 2017, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Peterson, M.; Federle, M.; Baron, R.; Haradome, H.; Kawamori, Y.; Nawano, S.; Araki, T. Islet cell tumor of the pancreas: Biphasic CT versus MR imaging in tumor detection. Radiology 2000, 216, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sundin, A.; Vullierme, M.; Kaltsas, G.; Plöckinger, U.; Åkerström, G.; Annibale, B.; Arnold, R.; Bajetta, E.; Barkmanova, J.; Chen, Y.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological examinations. Neuroendocrinology 2009, 90, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Shah, A.; Wei, W.; Varadhachary, G.; Johnson, V.; Shah, V.; Kundra, V. Carcinoid tumours: Predicting the location of the primary neoplasm based on the sites of metastases. Eur. Radiol. 2012, 23, 400–407. [Google Scholar] [CrossRef]
- Kang, Y.; Cho, J.-H.; Se; Hwang, H. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: A systematic review and meta-analysis. Radiol. Med. 2022, 127, 1124–1133. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 127, 1079–1084. [Google Scholar] [CrossRef]
- Chan, M.; Ma, K.A. Chan, Surgical management of neuroendocrine tumor-associated liver metastases: A review. Gland Surg. 2018, 7, 28–35. [Google Scholar] [CrossRef]
- Alagusundaramoorthy, S.; Gedaly, R. Role of surgery and transplantation in the treatment of hepatic metastases from neuroendocrine tumor. World J. Gastroenterol. 2014, 20, 14348. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Coppa, J.; Miceli, R.; Bhoori, S.; Bongini, M.; Camerini, T.; Milione, M.; Regalia, E.; Spreafico, C.; et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases from Neuroendocrine Tumors. Am. J. Transpl. 2016, 16, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Ercolani, G.; Neri, F.; Cescon, M.; Stacchini, G.; Del Gaudio, M.; Cucchetti, A.; Pinna, A. Liver transplantation for hepatic tumors: A systematic review. World J. Gastroenterol. 2014, 20, 5345–5352. [Google Scholar] [CrossRef]
- Veenendaal, L.; Rinkes, I.B.; Lips, C.; van Hillegersberg, R. Liver metastases of neuroendocrine tumours; early reduction of tumour load to improve life expectancy. World J. Surg. Oncol. 2006, 4, 35. [Google Scholar] [CrossRef]
- Barat, M.; Cottereau, A.; Kedra, A.; Dermine, S.; Palmieri, L.; Coriat, R.; Dautry, R.; Tselikas, L.; Soyer, P.; Dohan, A. The Role of Interventional Radiology for the Treatment of Hepatic Metastases from Neuroendocrine Tumor: An Updated Review. J. Clin. Med. 2020, 9, 2302. [Google Scholar] [CrossRef] [PubMed]
- Maire, F.; Lombard-Bohas, C.; O’Toole, D.; Vullierme, M.; Rebours, V.; Couvelard, A.; Pelletier, A.; Zappa, M.; Pilleul, F.; Hentic, O.; et al. Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: A prospective randomized study. Neuroendocrinology 2012, 96, 294–300. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Nani, R.; Aliberti, C.; Fiorentini, C.; Guadagni, S. Updates of colorectal cancer liver metastases therapy: Review on DEBIRI. Hepatic Oncol. 2020, 7, HEP16. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.; Knuth, J.; Keily, J.; McDermott, J.; Weber, S.; Chen, H.; Rilling, W.; Quebbeman, E.; Agarwal, D.; Pitt, H. Hepatic neuroendocrine metastases: Chemo- or bland embolization? J. Gastrointest. Surg. 2008, 12, 1951–1960. [Google Scholar] [CrossRef]
- Bloomston, M.; Al-Saif, O.; Klemanski, D.; Pinzone, J.; Martin, E.; Palmer, B.; Guy, G.; Khabiri, H.; Ellison, E.; Shah, M. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: Lessons learned. J. Gastrointest. Surg. 2007, 11, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Choi, J.; Cantor, A.; Kvols, L. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control. 2006, 13, 72–78. [Google Scholar] [CrossRef]
- Osborne, D.; Zervos, E.; Strosberg, J.; Boe, B.; Malafa, M.; Rosemurgy, A.; Yeatman, T.; Carey, L.; Duhaine, L.; Kvols, L. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann. Surg. Oncol. 2006, 13, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yao, J.; Ahrar, K.; Wallace, M.; Morello, F.; Madoff, D.; Murthy, R.; Hicks, M.; Ajani, J. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: The M.D. Anderson experience. Cancer J. 2003, 9, 261–267. [Google Scholar] [CrossRef]
- Brown, K.; Koh, B.; Brody, L.; Getrajdman, G.; Susman, J.; Fong, Y.; Blumgart, L. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J. Vasc. Interv. Radiol. 1999, 10, 397–403. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Cao, C.D.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef]
- Clouse, M.; Perry, L.; Stuart, K.; Stokes, K. Hepatic Arterial Chemoembolization for Metastatic Neuroendocrine Tumors. Digestion 1994, 55, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Clift, A.; Braat, A.; Alsafi, A.; Wasan, H.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef]
- Braat, A.; Kappadath, S.; Ahmadzadehfar, H.; Stothers, C.; Frilling, A.; Deroose, C.; Flamen, P.; Brown, D.; Sze, D.; Mahvash, A.; et al. Radioembolization with 90Y Resin Microspheres of Neuroendocrine Liver Metastases: International Multicenter Study on Efficacy and Toxicity, Cardiovasc. Intervent. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wild, A.; Halappa, V.; Kumar, R.; Ellsworth, S.; Ziegler, M.; Garg, T.; Rosati, L.; Su, Z.; Hacker-Prietz, A.; et al. Neuroendocrine tumor liver metastases treated with yttrium-90 radioembolization. Contemp. Clin. Trials. 2016, 50, 143–149. [Google Scholar] [CrossRef]
- Peker, A.; Çiçek, O.; Soydal, Ç.; Küçük, N.; Bilgiç, S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn Interv Radiol. 2015, 21, 54–59. [Google Scholar] [CrossRef]
- Memon, K.; Lewandowski, R.; Mulcahy, M.; Riaz, A.; Ryu, R.; Sato, K.; Gupta, R.; Nikolaidis, P.; Miller, F.; Yaghmai, V.; et al. Radioembolization for neuroendocrine liver metastases: Safety, imaging, and long-term outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 887–894. [Google Scholar] [CrossRef]
- Barbier, C.; Garske-Román, U.; Sandström, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. Mol. Imag. 2016, 43, 1425–1431. [Google Scholar] [CrossRef]
- Paprottka, P.; Hoffmann, R.; Haug, A.; Sommer, W.; Raebler, F.; Trumm, C.; Schmidt, G.; Ashoori, N.; Reiser, M.; Jakobs, T. Radioembolization of symptomatic, unresectable neuroendocrine hepatic metastases using yttrium-90 microspheres. Cardiovasc. Intervent. Radiol. 2012, 35, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Devcic, Z.; Rosenberg, J.; Braat, A.; Techasith, T.; Banerjee, A.; Sze, D.; Lam, M. The Efficacy of Hepatic 90Y Resin Radioembolization for Metastatic Neuroendocrine Tumors: A Meta-Analysis. J. Nucl. Med. 2014, 55, 1404–1410. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur. J. Radiol. 2018, 100, 23–29. [Google Scholar] [CrossRef]
- Ceelen, F.; Theisen, D.; De Albéniz, X.; Auernhammer, C.; Haug, A.; D’Anastasi, M.; Paprottka, P.; Rist, C.; Reiser, M.; Sommer, W. Towards new response criteria in neuroendocrine tumors: Which changes in MRI parameters are associated with longer progression-free survival after radioembolization of liver metastases? J. Magn. Reson. Imag. 2015, 41, 361–368. [Google Scholar] [CrossRef]
- Sommer, W.; Ceelen, F.; García-Albéniz, X.; Paprottka, P.; Auernhammer, C.; Armbruster, M.; Nikolaou, K.; Haug, A.; Reiser, M.; Theisen, D. Defining predictors for long progression-free survival after radioembolisation of hepatic metastases of neuroendocrine origin. Eur. Radiol. 2013, 23, 3094–3103. [Google Scholar] [CrossRef]
- Filippi, L.; Scopinaro, F.; Pelle, G.; Cianni, R.; Salvatori, R.; Schillaci, O.; Bagni, O. Molecular response assessed by 68Ga-DOTANOC and survival after 90Y microsphere therapy in patients with liver metastases from neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imag. 2016, 43, 432–440. [Google Scholar] [CrossRef]
- Fendrich, V.; Langer, P.; Celik, I.; Bartsch, D.; Zielke, A.; Ramaswamy, A.; Rothmund, M. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann. Surg. 2006, 244, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Modlin, I.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet. Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Bocchini, M.; Nicolini, F.; Severi, S.; Bongiovanni, A.; Ibrahim, T.; Simonetti, G.; Grassi, I.; Mazza, M. Biomarkers for Pancreatic Neuroendocrine Neoplasms (PanNENs) Management—An Updated Review. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, U.; Xin, S.; Hartenstein, A.; Auer, T.; Dräger, F.; Froböse, K.; Jann, H.; Mogl, M.; Amthauer, H.; Geisel, D.; et al. Automatized Hepatic Tumor Volume Analysis of Neuroendocrine Liver Metastases by Gd-EOB MRI-A Deep-Learning Model to Support Multidisciplinary Cancer Conference Decision-Making. Cancers 2021, 13, 2726. [Google Scholar] [CrossRef]
- Haider, M.; Jiang, B.; Parker, J.; Bullock, A.; Goehler, A.; Tsai, L. Use of MRI and Ga-68 DOTATATE for the detection of neuroendocrine liver metastases. Abdom. Radiol. 2022, 47, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Drolet, S.; Daigle, C.; Deshaies, I.; Ouellet, J.; Ball, C.; Dixon, E.; Marceau, J.; Ouellet, J. Additional value of gadoxetic acid-enhanced MRI to conventional extracellular gadolinium-enhanced MRI for the surgical management of colorectal and neuroendocrine liver metastases. HPB 2020, 22, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Raso, M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Gao, J.; Li, J.; Li, M.; Zhou, Z.; Peng, Y. Performance evaluation of a deep learning image reconstruction (DLIR) algorithm in “double low” chest CTA in children: A feasibility study. Radiol. Med. 2021, 126, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Sandor, A.; Modlin, I. A retrospective analysis of 1570 appendiceal carcinoids. Am. J. Gastroenterol. 1998, 93, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.; Kidd, M.; Latich, I.; Zikusoka, M.; Shapiro, M. Current status of gastrointestinal carcinoids. Gastroenterology 2005, 128, 1717–1751. [Google Scholar] [CrossRef]
- Turaga, K.; Kvols, L. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. Cancer J. Clin. 2011, 61, 113–132. [Google Scholar] [CrossRef]
- Chang, S.; Choi, D.; Soon, J.; Won, J.; Park, M.; Sang, W.; Da, K.; Jang, K. Neuroendocrine neoplasms of the gastrointestinal tract: Classification, pathologic basis, and imaging features. Radiographics 2007, 27, 1667–1679. [Google Scholar] [CrossRef]
- Heller, M.; Shah, A. Imaging of neuroendocrine tumors. Radiol. Clin. North Am. 2011, 49, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Paski, S.; Semrad, C. Small bowel tumors, Gastrointest. Endosc. Clin. N. Am. 2009, 19, 461–479. [Google Scholar] [CrossRef]
- Paulsen, S.; Huprich, J.; Fletcher, J.; Booya, F.; Young, B.; Fidler, J.; Johnson, C.; Barlow, J. CT enterography as a diagnostic tool in evaluating small bowel disorders: Review of clinical experience with over 700 cases. Radiographics 2006, 26, 641–657. [Google Scholar] [CrossRef]
- Pickhardt, P. Differential Diagnosis of Polypoid Lesions Seen at CT Colonography (Virtual Colonoscopy)1. Radiographics 2004, 24, 1535–1556. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Meyerhardt, J.; Mangu, P.; Flynn, P.; Korde, L.; Loprinzi, C.; Minsky, B.; Petrelli, N.; Ryan, K.; Schrag, D.; Wong, S.; et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 2013, 31, 4465–4470. [Google Scholar] [CrossRef]
- Pellegrino, F.; Carnevale, A.; Bisi, R.; Cavedagna, D.; Reverberi, R.; Uccelli, L.; Leprotti, S.; Giganti, M. Best Practices on Radiology Department Workflow: Tips from the Impact of the COVID-19 Lockdown on an Italian University Hospital. Healthcare 2022, 10, 1771. [Google Scholar] [CrossRef]
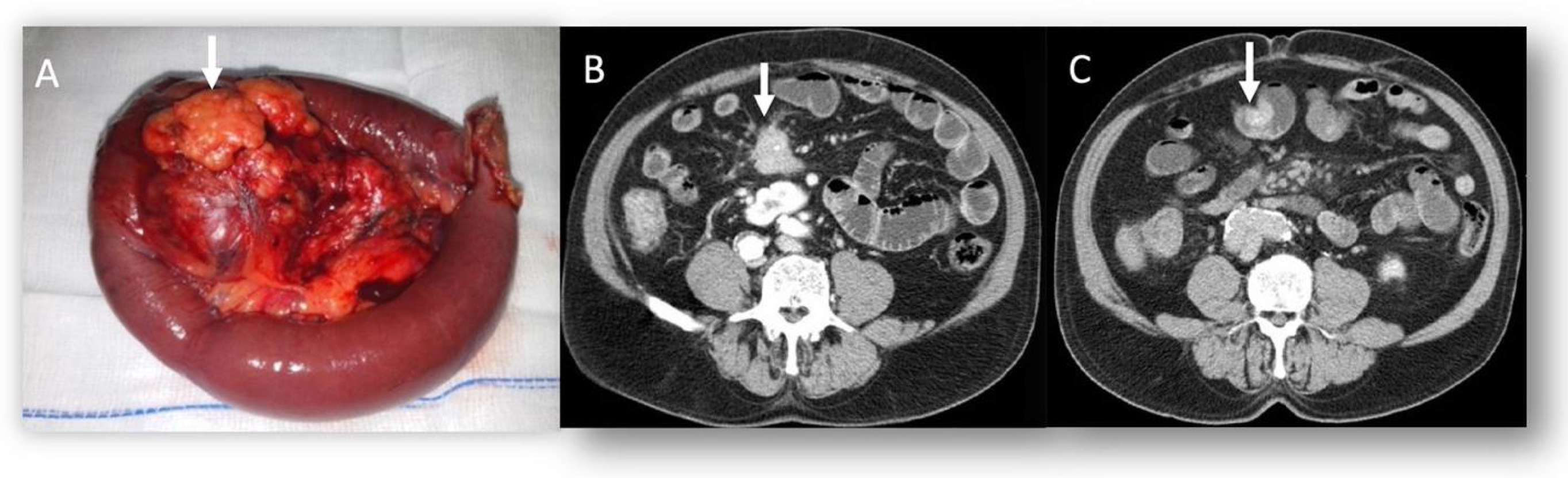
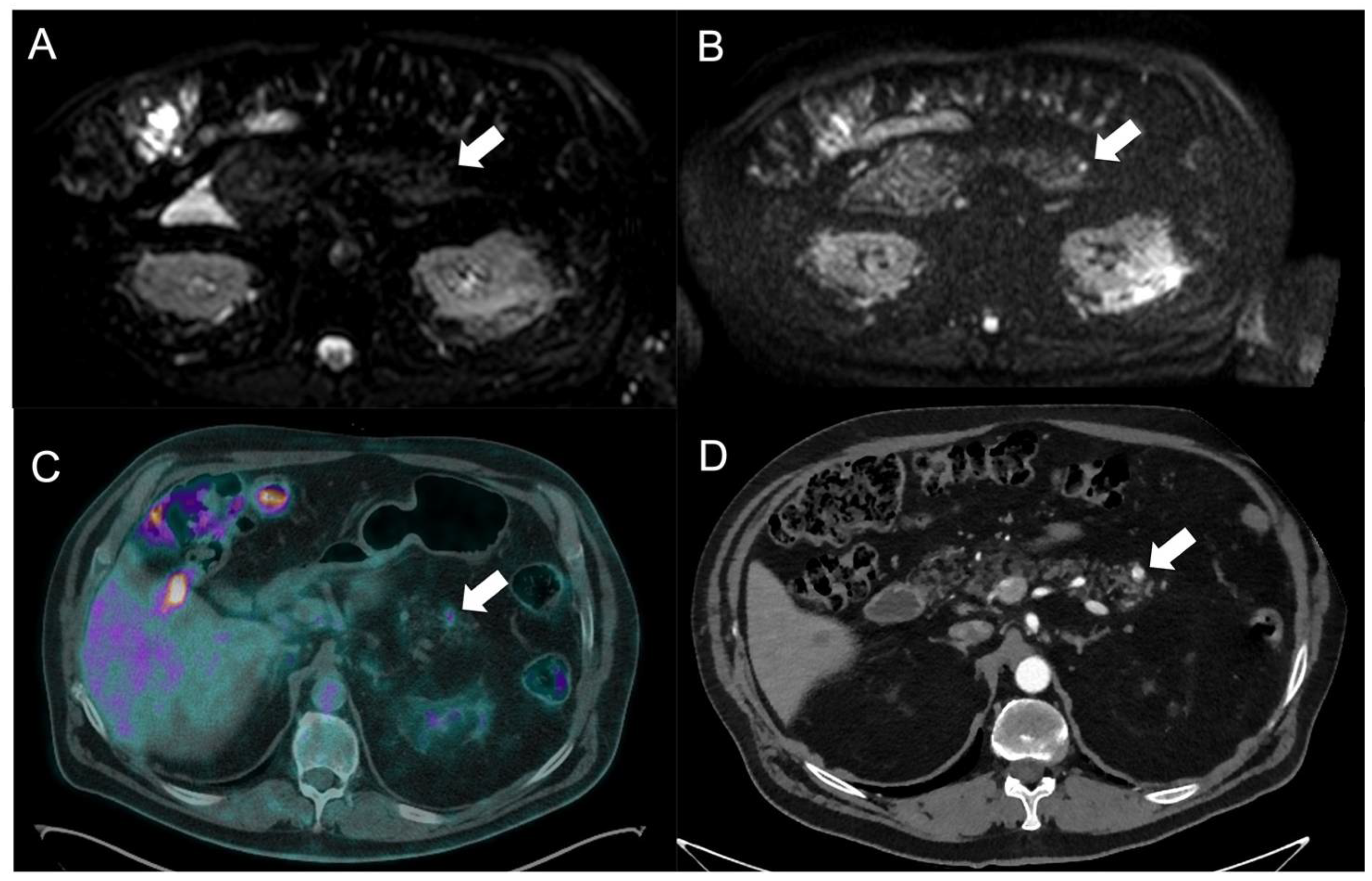
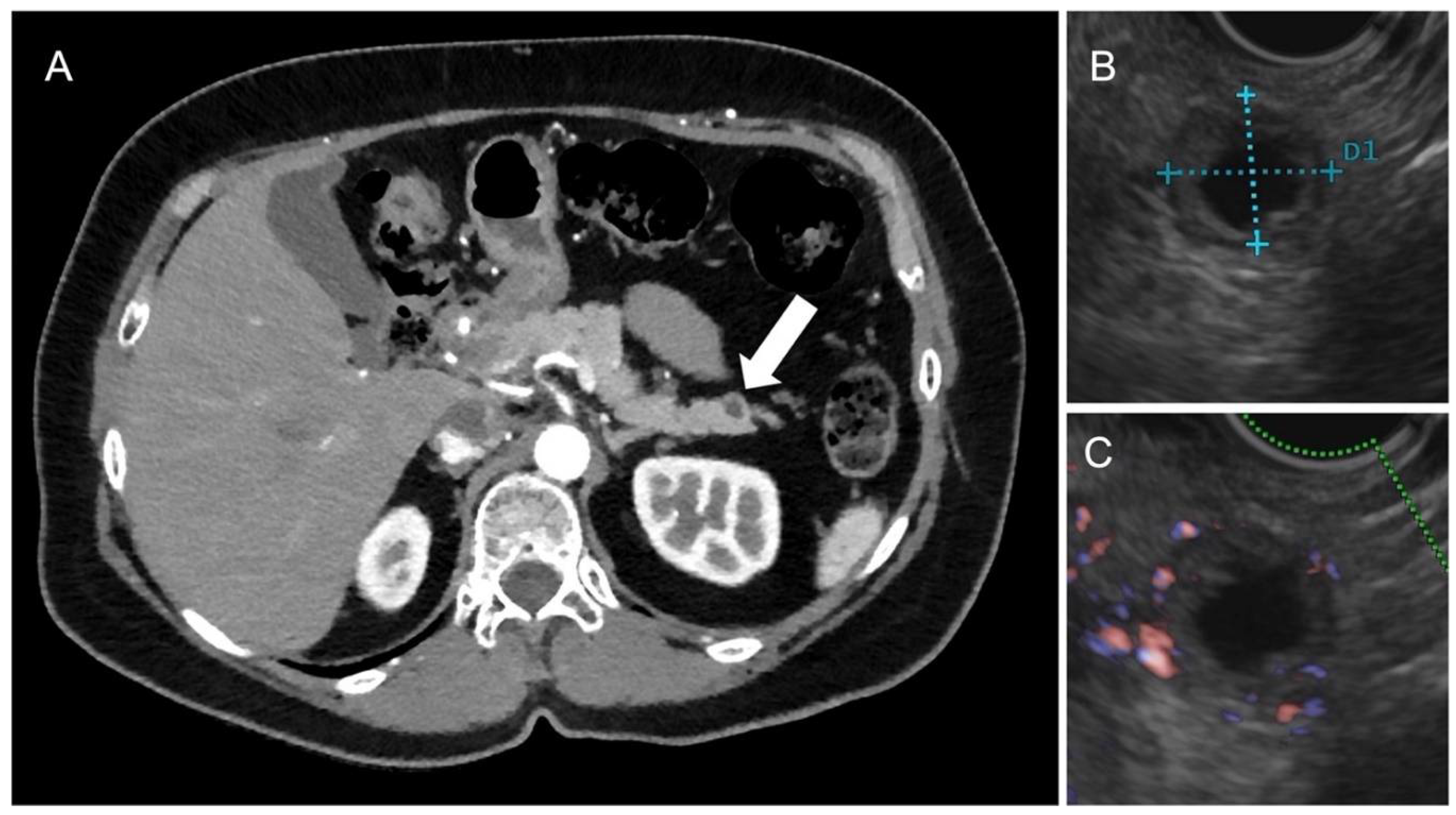
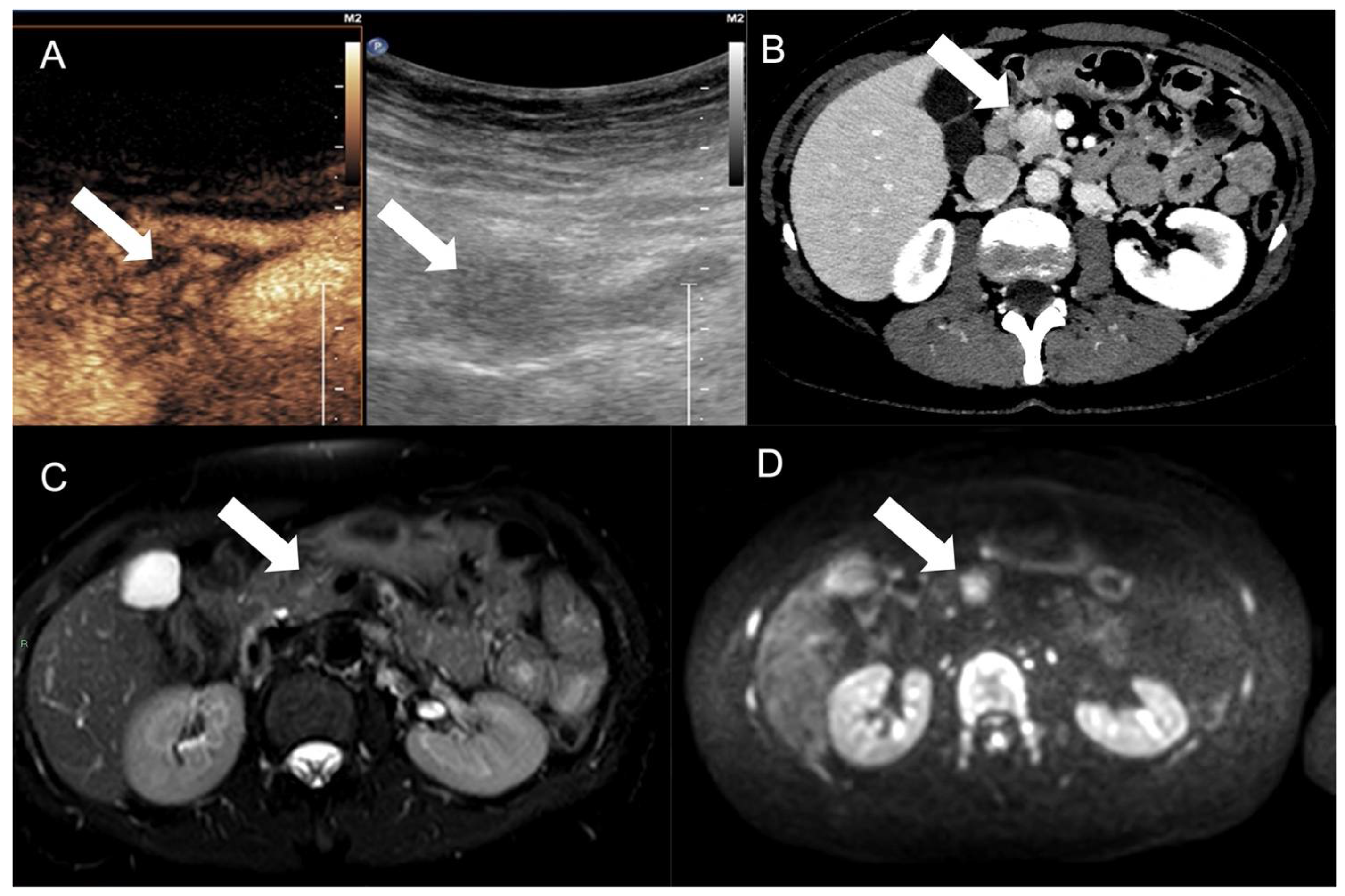
| Hypoglycemia and hypokalemia | Insulinomas |
| Weight loss, abdominal pain, and jaundice | Ppomas |
| Watery diarrhea, achlorhydria, and hypokalemia | Vipomas |
| Diabetes, dermatitis (necrolytic migratory erythema), deep vein thrombosis, and depression | Glucagonomas |
| Cholelithiasis, hyperglycemia, and steatorrhea | Somatostatinomas |
| Carcinoid syndrome with watery diarrhea, hypotension, bronchospasm, flushing, and right-sided heart disease | Liver metastasis from small bowel lesions |
| Terminology | Differentiation | Grade | Mitotic Rate * | Ki-67 Index |
|---|---|---|---|---|
| NET, G1 | Well differentiated | Low | <2 | <3% |
| NET, G2 | Well differentiated | Intermediate | 2–20 | 3–20% |
| NET, G3 | Well differentiated | High | >20 | >20% |
| NEC, small-cell type | Poorly differentiated | High | >20 | >20% |
| NEC, large-cell type | >20 | >20% | ||
| MiNEN | Well or poorly differentiated | Variable | Variable | Variable |
| Advantages | Disadvantages | Use | |
|---|---|---|---|
| US | -no ionizing radiations -widely available -inexpensive | -interoperator variability -not recommended for other sites in the gastrointestinal tract -low sensitivity in pancreatic lesions | -initial diagnosis of liver metastases -surveillance in some patients with liver metastases -liver lesion characterization with contrast-enhanced ultrasound (CEUS) -tool to guide liver lesion biopsies -intraoperative use for lesions detection |
| EUS | -possibility of fine-needle biopsy of the lesion with Ki-67 evaluation -increases the overall PNEN detection rate after a CT scan | -inadequacy of sampling due to site (pancreatic body or tail), small size, rich stromal fibrosis, or cystic and necrotic components -inexperience of endosonologists and cytopathologists | -detection of small pancreatic NENs -histological diagnosis -intratumoral vascularity with contrast-enhanced endoscopic ultrasound |
| CT contrast enhanced | -good sensitivity and specificity -widely available | -ionizing radiation | -diagnosis, staging, treatment response assessment, and surveillance of primary neuroendocrine tumors -lung lesions assessment |
| MRI contrast enhanced | -no ionizing radiation -ability to characterize lesions using pre and postcontrast sequences and DWI -cholangiopancreatography sequences to assess the relationship between the lesion and the pancreatic duct | -low sensitivity for detecting small lesions in the duodenum, stomach, and small intestine | -liver, pancreas, brain, and bone evaluation |
| Fluorodeoxyglucose PET | -complementary information distinguishing between slowly proliferating and aggressive tumors (higher glucose metabolism in G3 and high G2 NENs) | -ionizing radiation -low sensitivity for neuroendocrine tumors | -optional in NEN evaluation -prognostication (worse prognosis if FDG uptake) and post-therapy assessment |
| 68Ga-DOTA-SSA PET/CT | -good sensitivity and specificity | -low sensitivity for insulinomas | -staging with primary tumor location and metastatic detection -restaging with the assessment of residual, recurrent, or progressive disease -patient selection for peptide receptor radionuclide therapy |
| Abdomen Multidetector CT Protocol | |
| Voltage | 120 kVp |
| Effective amperage | 200 mAs |
| Rotation time | 0.5 s |
| Detector collimation | 1.5 mm |
| Section thickness | 3.0 mm |
| Pitch | 0.75 |
| Increment | 1.5 mm |
| Coverage | Image from the 11th vertebral body through the iliac crest |
| Oral contrast material | Negative oral contrast material (500 mL of water 30 min before examination and 250 mL of water immediately before examination) |
| Nonenhanced CT | |
| Contrast-enhanced CT | 100–125 mL isomolar or osmolar iodinated contrast material (370 mg/mL) at 4–5 mL/s |
| Image Acquisition Phase | |
| Arterial phase | 15–25 s (CT angiography in preoperative setting) 25–30 s |
| Pancreatic parenchymal phase | 40–45 s |
| Portal venous phase | 60–70 s |
| Multiplanar reformation | Axial, sagittal, and coronal planes; section thickness, 3 mm |
| Thorax/Neck Multidetector CT Protocol | |
| Thorax–Neck–Abdomen | Amount of contrast media and injection rates adjusted to what is required to perform a proper CT of the abdomen |
| Neck | 1.5–2 mL/kg body weight of contrast media 300–350 mg/mL injected at 2.5 mL/s using a 40 s scanning delay |
| Thorax | 1–1.5 mL/kg body weight of contrast media 300–350 mg/mL injected at 1.5 mL/s using a 60 s scanning delay |
| CT Enteroclysis | |
| Patient preparation | 2000 mL of hyperosmolar fluid, such as mannitol, with a 50% concentration, or, alternatively, warm tap water administered through a nasogastrojejunal tube by using a dedicated (150–200 mL/min rate) power injector Intravenous glucagon or anticholinergic drug (recommended) |
| Contrast-enhanced CT | 120–150 mL isomolar or osmolar iodinated contrast media at 3 mL/s Arterial phase using a 25 s scanning delay Venous phase using a 60 s scanning delay |
| ESMO | NCCN | ENETS | |
|---|---|---|---|
| Small-bowel NEN | Grades 1–2: Laboratory tests and CT or MRI every 3–6 months. Grade 3: Every 2–3 months. Octreoscan after 18–24 months if SRS positive. | Clinical review at 3–12 months with biomarkers and CT or MRI as clinically indicated; then, review every 6–12 months for maximum of 10 years. | Grade 1: US, CT, or MRI at 6 and 12 months, then yearly or longer; octreoscan (or gallium-68-based PET) at baseline and every 2 years. Grade 2–3: US, CT, or MRI every 3 months indefinitely; octreoscan (or gallium-68-based PET) at 3 months and yearly. |
| Pancreatic NEN | Grades 1–2: Laboratory tests and CT or MRI every 3–6 months. Grade 3: Every 2–3 months. Octreoscan after 18–24 months if SSTR positive. | Clinical review at 3–12 months with biomarkers and CT or MRI as clinically indicated; then, review every 6–12 months for maximum of 10 years. | Grade 1: US, CT, or MRI at 6 and 12 months, then yearly or longer; octreoscan (or gallium-68–based PET) at baseline and every 2 years. Grades 2–3: US, CT, or MRI every 3 months indefinitely; octreoscan (or gallium-68-based PET) at 3 months and yearly. |
| Appendiceal NEN | Not reported | Not follow-up: <2 cm completely resected by appendicectomy “as clinically indicated”. | No follow-up: <1 cm completely resected with appendicectomy; appendiceal NET >1 cm completely resected with right hemicolectomy without lymph node involvement. |
| Rectal NEN | Not reported | Not follow-up: <1 cm with negative margins. | No follow-up: completely resected rectal NETs <1 cm. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, F.; Granata, V.; Fusco, R.; Grassi, F.; Tafuto, S.; Perrucci, L.; Tralli, G.; Scaglione, M. Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography 2023, 9, 217-246. https://doi.org/10.3390/tomography9010018
Pellegrino F, Granata V, Fusco R, Grassi F, Tafuto S, Perrucci L, Tralli G, Scaglione M. Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography. 2023; 9(1):217-246. https://doi.org/10.3390/tomography9010018
Chicago/Turabian StylePellegrino, Fabio, Vincenza Granata, Roberta Fusco, Francesca Grassi, Salvatore Tafuto, Luca Perrucci, Giulia Tralli, and Mariano Scaglione. 2023. "Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists" Tomography 9, no. 1: 217-246. https://doi.org/10.3390/tomography9010018
APA StylePellegrino, F., Granata, V., Fusco, R., Grassi, F., Tafuto, S., Perrucci, L., Tralli, G., & Scaglione, M. (2023). Diagnostic Management of Gastroenteropancreatic Neuroendocrine Neoplasms: Technique Optimization and Tips and Tricks for Radiologists. Tomography, 9(1), 217-246. https://doi.org/10.3390/tomography9010018








