Radiation Dose Reduction Opportunities in Vascular Imaging
Abstract
1. Introduction
2. Methods
3. Results
3.1. Computed Tomography Radiation Reduction Methodologies
3.1.1. Image Acquisition Techniques
3.1.2. Image Reconstruction Techniques
3.1.3. Artificial Intelligence
3.1.4. Dual-Source CT
3.1.5. Dual-Energy CT
3.2. Non-Ionizing Radiation Imaging Modalities
3.2.1. Magnetic Resonance Angiography
3.2.2. Open and Larger Bore MRI
3.2.3. Contrast-Enhanced Ultrasound
3.3. Other Methods of Radiation Dose Reduction
3.3.1. Alternating Imaging Modalities
3.3.2. Protocol Driven Radiation Dose Reduction
3.4. High-Risk Populations
3.4.1. Children
3.4.2. Marfan Syndrome
3.4.3. Ehlers-Danlos Syndrome
3.4.4. Loeys-Deitz Syndrome
3.4.5. Turner Syndrome
3.4.6. Bicuspid Aortic Valve
3.4.7. Other High-Risk Populations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| AAPM | American Association of Physicists in Medicine |
| ACHD | adult congenital heart disease |
| ACR | American College of Radiology |
| ACTM | automatic tube current modulation |
| AI | artificial intelligence |
| AIS | acute ischemic stroke |
| AiCE | Advanced intelligent Clear-IQ Engine |
| ALARA | as low as reasonably achievable |
| ASIR | adaptive statistical iterative reconstruction |
| ASL | arterial spin labeling |
| BEIR | Biologic Effects of Ionizing Radiation |
| CAD | coronary artery disease |
| CaSc | calcium score |
| CCTA | coronary computed tomography angiography |
| CEUS | contrast-enhanced ultrasound |
| CE-MRA | contrast-enhanced magnetic resonance angiography |
| cm | centimeter |
| CMR | cardiac magnetic resonance |
| CMRA | cardiac magnetic resonance angiography |
| CNN | convolutional neural networks |
| CS TOF-MRA | compressed sensing time of flight magnetic resonance angiography |
| CT | computed tomography |
| CTA | computed tomography angiography |
| CTDIvol | Computed Tomography Dose Index Volume |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| DECT | dual-energy computed tomography |
| DL | deep learning |
| DLIR | deep learning image reconstruction |
| DLP | dose length product |
| DSCT | dual-source computed tomography |
| DWI | diffusion weighted imaging |
| DUS | duplex ultrasound |
| ECG | electrocardiogram |
| ECTCM | electrocardiogram-controlled tube current modulation |
| ED | emergency department |
| EMT | endovascular mechanical thrombectomy |
| FBP | filtered back projection |
| FPS | frames per second |
| FSD | flow-sensitive dephasing |
| ICH | intracranial hemorrhage |
| ICRP | International Commission on Radiological Protection |
| ICU | intensive care unit |
| IFDIR-MRA | inflow-dependent inversion recovery magnetic resonance angiography |
| IR | iterative reconstruction |
| keV | kiloelectronvolt |
| LCS | lung cancer screening |
| LDCT | low dose computed tomography |
| mGy | milligray |
| mL | milliliter |
| MRI | magnetic resonance imaging |
| MRA | magnetic resonance angiography |
| mSv | millisievert |
| NC-MRA | non-contrast magnetic resonance angiography |
| NCRP | National Council on Radiation Protection and Measurements |
| NDCT | normal dose computed tomography |
| PCI | percutaneous coronary intervention |
| PE | pulmonary embolus |
| PETRA-MRA | pointwise encoding time reduction magnetic resonance angiography |
| QISS | quiescent-interval slice-selective |
| SNR | signal to noise ratio |
| T | Tesla |
| TOF | time of flight |
| TOF-MRA | time of flight magnetic resonance angiography |
| TTE | transthoracic echocardiography |
| V/Q | ventilation/profusion |
| VLFF | very low frame rate fluoroscopy |
| VMI | virtual monoenergetic images |
| WT | tissue weighting factor |
| zTE-MRA | zero echo time magnetic resonance angiography |
References
- Costello, J.E.; Cecava, N.D.; Tucker, J.E.; Bau, J.L. CT Radiation Dose: Current Controversies and Dose Reduction Strategies. AJR Am. J. Roentgenol. 2013, 201, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Bickford, M.W.; Nance, J.W.; Zhang, L.; De Cecco, C.N.; Wichmann, J.L.; Vogl, T.J.; Schoepf, U.J. State-of-the-Art Pulmonary CT Angiography for Acute Pulmonary Embolism. AJR Am. J. Roentgenol. 2017, 208, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Baralo, B.; Samson, P.; Hoenig, D.; Smith, A. Percutaneous kidney stone surgery and radiation exposure: A review. Asian J. Urol. 2019, 7, 10–17. [Google Scholar] [CrossRef]
- National Research Council, Division on Earth and Life Studies, Board on Radiation Effects Research, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- AAPM Position Statements, Policies and Procedures—Details 2018. Available online: https://www.aapm.org/org/policies/details.asp?type=PP&id=2548 (accessed on 6 August 2022).
- Jordan, D.W.; Becker, M.; Brady, S.; Feng, J.C.; Jafari, M.E.; Johnson, L.M.; Keenan, M.A.; Kitchin, D.R.; Lee, R.K.; Sensakovic, W.; et al. ACR Appropriateness Criteria® Radiation Dose Assessment Introduction. ACR Appropr. Criteria® 2020, 4, 1–4. [Google Scholar]
- Ribeiro, A.; Husson, O.; Drey, N.; Murray, I.; May, K.; Thurston, J.; Oyen, W. Ionising radiation exposure from medical imaging—A review of Patient’s (un) awareness. Radiography 2020, 26, e25–e30. [Google Scholar] [CrossRef] [PubMed]
- Applegate, K.E. Protection of patients in diagnostic and interventional medical imaging: Collaboration is the key. Health Phys. 2015, 108, 221–223. [Google Scholar] [CrossRef]
- Radiation Dosimetry: CT. Accreditation Support 2020. Available online: https://accreditationsupport.acr.org/support/solutions/articles/11000056198-radiation-dosimetry-ct-revised-12-8-2020- (accessed on 7 August 2022).
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 9–34. [Google Scholar] [CrossRef]
- Hajhosseiny, R.; Rashid, I.; Bustin, A.; Munoz, C.; Cruz, G.; Nazir, M.S.; Grigoryan, K.; Ismail, T.F.; Preston, R.; Neji, R.; et al. Clinical comparison of sub-mm high-resolution non-contrast coronary CMR angiography against coronary CT angiography in patients with low-intermediate risk of coronary artery disease: A single center trial. J. Cardiovasc. Magn. Reson. 2021, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.E.; Obaid, D.R. Low-Dose Radiation Advances in Coronary Computed Tomography Angiography in the Diagnosis of Coronary Artery Disease. Curr. Cardiol. Rev. 2019, 15, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Bradley, J.A.; Ng, A.K.; Samson, P.; Robinson, C.; Lopez-Mattei, J.; Mitchell, J.D. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncology 2021, 3, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.B.; Mirkin, J.; Oliver, T.K.; Azzoli, C.G.; Berry, D.A.; Brawley, O.W.; Byers, T.; Colditz, G.; Gould, M.K.; Jett, J.R.; et al. Benefits and Harms of CT Screening for Lung Cancer: A systematic review. JAMA 2012, 307, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Demb, J.; Chu, P.; Yu, S.; Whitebird, R.; Solberg, L.; Miglioretti, D.L.; Smith-Bindman, R. Analysis of Computed Tomography Radiation Doses Used for Lung Cancer Screening Scans. JAMA Intern. Med. 2019, 179, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Koyama, H.; Seki, S.; Kishida, Y.; Yoshikawa, T. Radiation dose reduction techniques for chest CT: Principles and clinical results. Eur. J. Radiol. 2018, 111, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A.; Mahesh, M.; Bhargavan-Chatfield, M.; Chambers, C.E.; Elee, J.G.; Frush, D.P.; Miller, D.L.; Royal, H.D.; Milano, M.T.; Spelic, D.C.; et al. Patient Exposure from Radiologic and Nuclear Medicine Procedures in the United States: Procedure Volume and Effective Dose for the Period 2006–2016. Radiology 2020, 295, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, B.; Hein, F.; Meyer, T.; Hadamitzky, M.; Martinoff, S.; Schömig, A.; Hausleiter, J. Trends in radiation protection in CT: Present and future status. J. Cardiovasc. Comput. Tomogr. 2009, 3, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Klink, T.; Obmann, V.; Heverhagen, J.; Stork, A.; Adam, G.; Begemann, P. Reducing CT radiation dose with iterative reconstruction algorithms: The influence of scan and reconstruction parameters on image quality and CTDIvol. Eur. J. Radiol. 2014, 83, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Du, X.; Guo, D.; Cao, L.; Gao, Y.; Bai, M.; Li, P.; Liu, J.; Li, K. Noise-based tube current reduction method with iterative reconstruction for reduction of radiation exposure in coronary CT angiography. Eur. J. Radiol. 2012, 82, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.A.; McEntee, M.F.; Reed, W.; Kench, P.L. Radiation dose and diagnostic image quality associated with iterative reconstruction in coronary CT angiography: A systematic review. J. Med. Imaging Radiat. Oncol. 2016, 60, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Corbett, T. Radiation Dose Reduction Strategies in Coronary CTA. Radiol. Technol. 2020, 91, 404–406. [Google Scholar] [PubMed]
- Harder, A.M.D.; Willemink, M.J.; De Ruiter, Q.M.; De Jong, P.A.; Schilham, A.M.; Krestin, G.P.; Leiner, T.; Budde, R.P. Dose reduction with iterative reconstruction for coronary CT angiography: A systematic review and meta-analysis. Br. J. Radiol. 2016, 89, 20150068. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Schoepf, U.J.; Silverman, J.R.; Krazinski, A.W.; Canstein, C.; Deak, Z.; Grimm, J.; Geyer, L.L. Iterative Image Reconstruction Techniques: Cardiothoracic computed tomography applications. J. Thorac. Imaging 2014, 29, 198–208. [Google Scholar] [CrossRef]
- Abou-Issa, A.H.; Elganayni, F.; Al-Azzazy, M.Z. Effect of low tube kV on radiation dose and image quality in retrospective ECG-gated coronary CT angiography. Egypt. J. Radiol. Nucl. Med. 2011, 42, 327–333. [Google Scholar] [CrossRef][Green Version]
- Kambadakone, A. Artificial Intelligence and CT Image Reconstruction: Potential of a New Era in Radiation Dose Reduction. J. Am. Coll. Radiol. 2020, 17, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Choi, S.-Y.; Hwang, J.A.; Lim, S.; Lee, M.H.; Yi, B.H.; Cha, J.G. The potential for reduced radiation dose from deep learning-based CT image reconstruction. Medicine 2021, 100, e25814. [Google Scholar] [CrossRef]
- Shan, H.; Padole, A.; Homayounieh, F.; Kruger, U.; Khera, R.D.; Nitiwarangkul, C.; Kalra, M.K.; Wang, G. Competitive performance of a modularized deep neural network compared to commercial algorithms for low-dose CT image reconstruction. Nat. Mach. Intell. 2019, 1, 269–276. [Google Scholar] [CrossRef]
- Gupta, R.V.; Kalra, M.K.; Ebrahimian, S.; Kaviani, P.; Primak, A.; Bizzo, B.; Dreyer, K.J. Complex Relationship Between Artificial Intelligence and CT Radiation Dose. Acad. Radiol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, I.; Jacobsen, M.C.; Godoy, M.C.; Stefanidis, K.; Layman, R.R. Dual-energy CT in pulmonary vascular disease. Br. J. Radiol. 2022, 95, 20210699. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Eid, M.; De Cecco, C.N.; Jacobs, B.E.; Albrecht, M.H.; Varga-Szemes, A.; Tesche, C.; Caruso, D.; Laghi, A.; Schoepf, U.J. Dual-Energy Computed Tomography in Cardiothoracic Vascular Imaging. Radiol. Clin. North Am. 2018, 56, 521–534. [Google Scholar] [CrossRef]
- Hamid, S.; Nasir, M.U.; So, A.; Andrews, G.; Nicolaou, S.; Qamar, S.R. Clinical Applications of Dual-Energy CT. Korean J. Radiol. 2021, 22, 970–982. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Tong, H.; Dong, Y.; Ma, D.; Xu, L.; Yang, C. Meta-analysis of computed tomography angiography versus magnetic resonance angiography for intracranial aneurysm. Medicine 2018, 97, e10771. [Google Scholar] [CrossRef]
- Edelman, R.R.; Koktzoglou, I. Noncontrast MR angiography: An update. J. Magn. Reson. Imaging 2018, 49, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wu, W.; Su, C.; Zhu, Q.; Schmidt, M.; Sun, Y.; Forman, C.; Speier, P.; Hong, X.; Lu, S. High-resolution compressed sensing time-of-flight MR angiography outperforms CT angiography for evaluating patients with Moyamoya disease after surgical revascularization. BMC Med. Imaging 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Ravesh, M.S.; Tesch, K.; Lebenatus, A.; Koktzoglou, I.; Edelman, R.R.; Eden, M.; Langguth, P.; Graessner, J.; Jansen, O.; Both, M. Clinical Value of Noncontrast-Enhanced Radial Quiescent-Interval Slice-Selective ( QISS ) Magnetic Resonance Angiography for the Diagnosis of Acute Pulmonary Embolism Compared to Contrast-Enhanced Computed Tomography and Cartesian Balanced Steady-State Free Precession. J. Magn. Reson. Imaging 2020, 52, 1510–1524. [Google Scholar] [CrossRef]
- Zhang, F.; Ran, Y.; Zhu, M.; Lei, X.; Niu, J.; Wang, X.; Zhang, Y.; Li, S.; Zhu, J.; Gao, X.; et al. The Use of Pointwise Encoding Time Reduction With Radial Acquisition MRA to Assess Middle Cerebral Artery Stenosis Pre- and Post-stent Angioplasty: Comparison With 3D Time-of-Flight MRA and DSA. Front. Cardiovasc. Med. 2021, 8, 739332. [Google Scholar] [CrossRef]
- Shang, S.; Ye, J.; Dou, W.; Luo, X.; Qu, J.; Zhu, Q.; Zhang, H.; Wu, J. Validation of Zero TE–MRA in the Characterization of Cerebrovascular Diseases: A Feasibility Study. Am. J. Neuroradiol. 2019, 40, 1484–1490. [Google Scholar] [CrossRef]
- Heit, J.J.; Zaharchuk, G.; Wintermark, M. Advanced Neuroimaging of Acute Ischemic Stroke: Penumbra and Collateral Assessment. Neuroimaging Clin. North Am. 2018, 28, 585–597. [Google Scholar] [CrossRef]
- Babu-Narayan, S.V.; Giannakoulas, G.; Valente, A.M.; Li, W.; Gatzoulis, M.A. Imaging of congenital heart disease in adults. Eur. Heart J. 2015, 37, 1182–1195. [Google Scholar] [CrossRef]
- Kato, Y.; Ambale-Venkatesh, B.; Kassai, Y.; Kasuboski, L.; Schuijf, J.; Kapoor, K.; Caruthers, S.; Lima, J.A.C. Non-contrast coronary magnetic resonance angiography: Current frontiers and future horizons. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 591–612. [Google Scholar] [CrossRef]
- Debrey, S.M.; Yu, H.; Lynch, J.K.; Lovblad, K.-O.; Wright, V.L.; Janket, S.-J.; Baird, A.E. Diagnostic Accuracy of Magnetic Resonance Angiography for Internal Carotid Artery Disease: A systematic review and meta-analysis. Stroke 2008, 39, 2237–2248. [Google Scholar] [CrossRef]
- Heiss, R.; Nagel, A.M.; Laun, F.B.; Uder, M.; Bickelhaupt, S. Low-Field Magnetic Resonance Imaging: A New Generation of Breakthrough Technology in Clinical Imaging. Investig. Radiol. 2021, 56, 726–733. [Google Scholar] [CrossRef]
- Fischer, A. Revisiting the Physics behind MRI and the Opportunities that Lower Field Strengths Offer. 2020. Available online: https://marketing.webassets.siemens-healthineers.com/90472072ae6f7648/3230ad37f3ce/MAGNETOM_Flash_Free-Max_Edition_RSNA_Edition.pdf (accessed on 18 September 2022).
- Bredahl, K.; Mestre, X.M.; Coll, R.V.; Ghulam, Q.M.; Sillesen, H.; Eiberg, J. Contrast-Enhanced Ultrasound in Vascular Surgery: Review and Update. Ann. Vasc. Surg. 2017, 45, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Yusuf, G.T.; Daneshi, M.; Ramnarine, R.; Deganello, A.; Sellars, M.E.; Sidhu, P.S. Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom. Radiol. 2018, 43, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Marschner, C.A.; Rübenthaler, J.; Froelich, M.F.; Schwarze, V.; Clevert, D.-A. Benefits of contrast-enhanced ultrasonography for interventional procedures. Ultrasonography 2021, 40, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Trinci, M.; Piccolo, C.L.; Ferrari, R.; Galluzzo, M.; Ianniello, S.; Miele, V. Contrast-enhanced ultrasound (CEUS) in pediatric blunt abdominal trauma. J. Ultrasound 2018, 22, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Mastracci, T.M.; Katsargyris, A.; Verhoeven, E.L. The role of mandatory lifelong annual surveillance after thoracic endovascular repair. J. Vasc. Surg. 2012, 56, 1786–1793. [Google Scholar] [CrossRef]
- Patel, V.K.; Fruauff, A.; Esses, D.; Lipsitz, E.C.; Levsky, J.M.; Haramati, L.B. Implementation of an aortic dissection CT protocol with clinical decision support aimed at decreasing radiation exposure by reducing routine abdominopelvic imaging. Clin. Imaging 2020, 67, 108–112. [Google Scholar] [CrossRef]
- Weinrich, J.M.; Lenz, A.; Girdauskas, E.; Adam, G.; von Kodolitsch, Y.; Bannas, P. Current and Emerging Imaging Techniques in Patients with Genetic Aortic Syndromes. Rofo 2019, 192, 50–58. [Google Scholar] [CrossRef]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef]
- Borger, M.A.; Fedak, P.W.; Stephens, E.H.; Gleason, T.G.; Girdauskas, E.; Ikonomidis, J.S.; Khoynezhad, A.; Siu, S.C.; Verma, S.; Hope, M.D.; et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: Full online-only version. J. Thorac. Cardiovasc. Surg. 2018, 156, e41–e74. [Google Scholar] [CrossRef]
- Kubo, T. Vendor free basics of radiation dose reduction techniques for CT. Eur. J. Radiol. 2019, 110, 14–21. [Google Scholar] [CrossRef]
- Demchuk, A.M.; Menon, B.K.; Goyal, M. Comparing Vessel Imaging: Noncontrast Computed Tomography/Computed Tomographic Angiography Should Be the New Minimum Standard in Acute Disabling Stroke. Stroke 2016, 47, 273–281. [Google Scholar] [CrossRef] [PubMed]
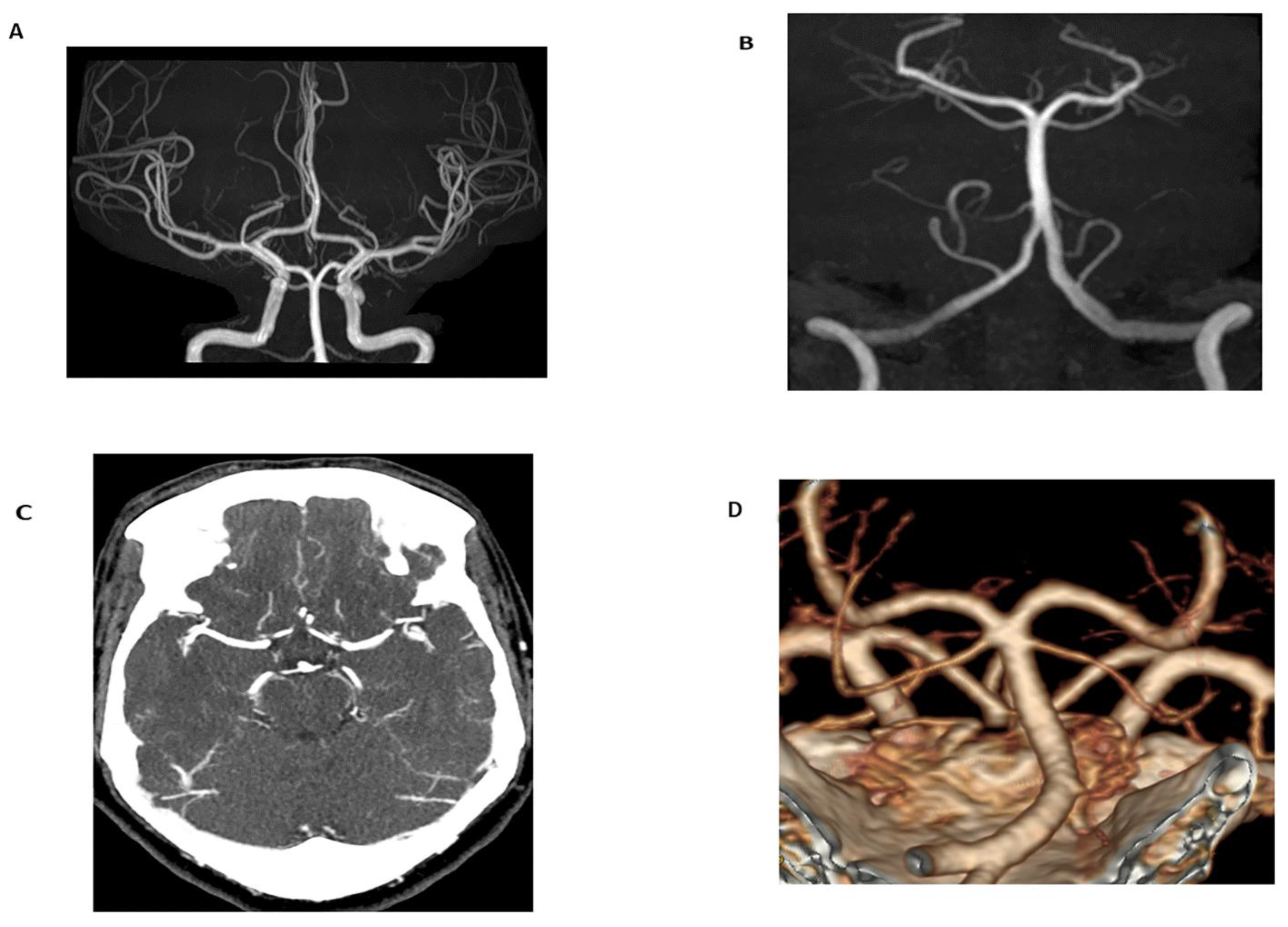
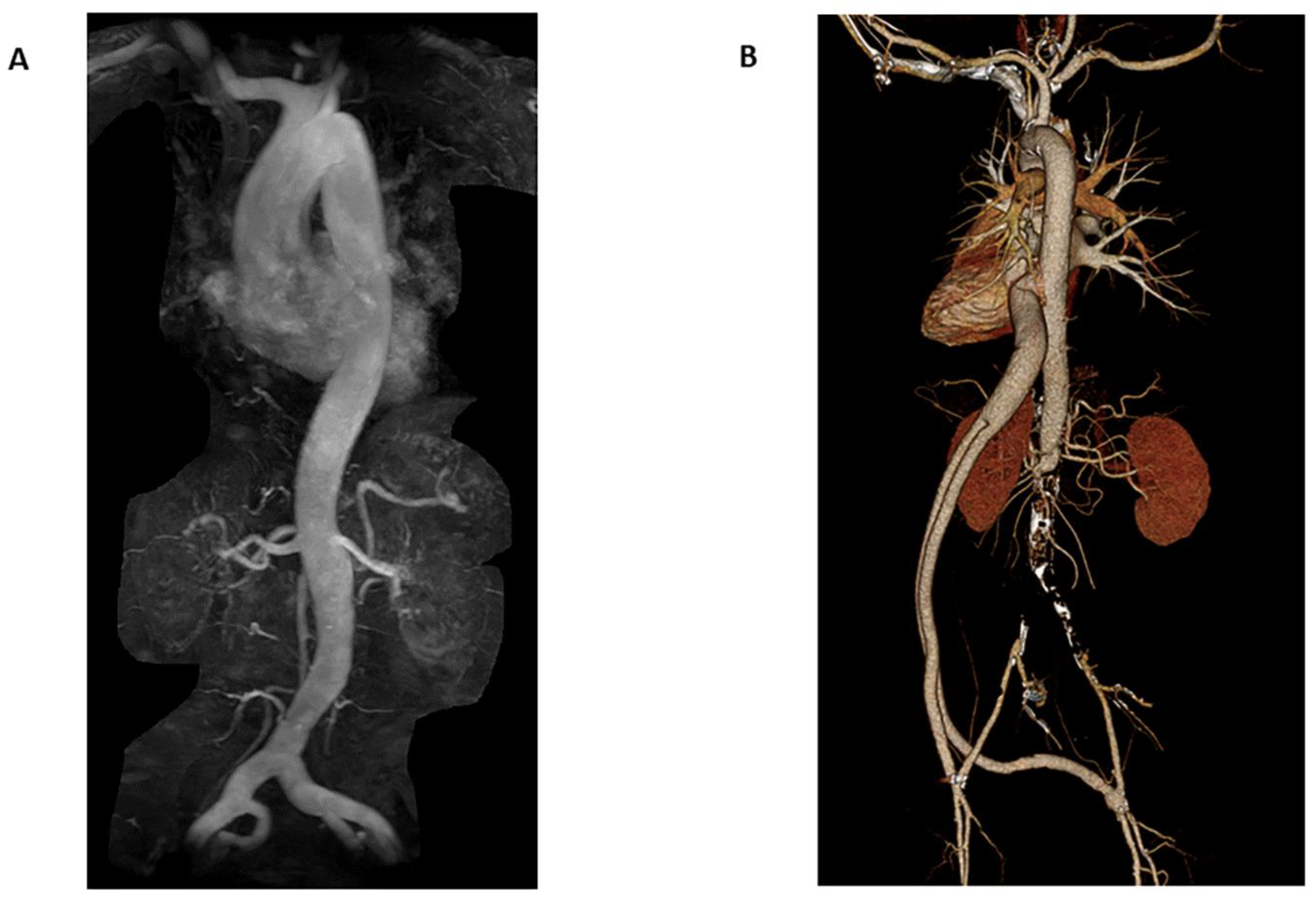
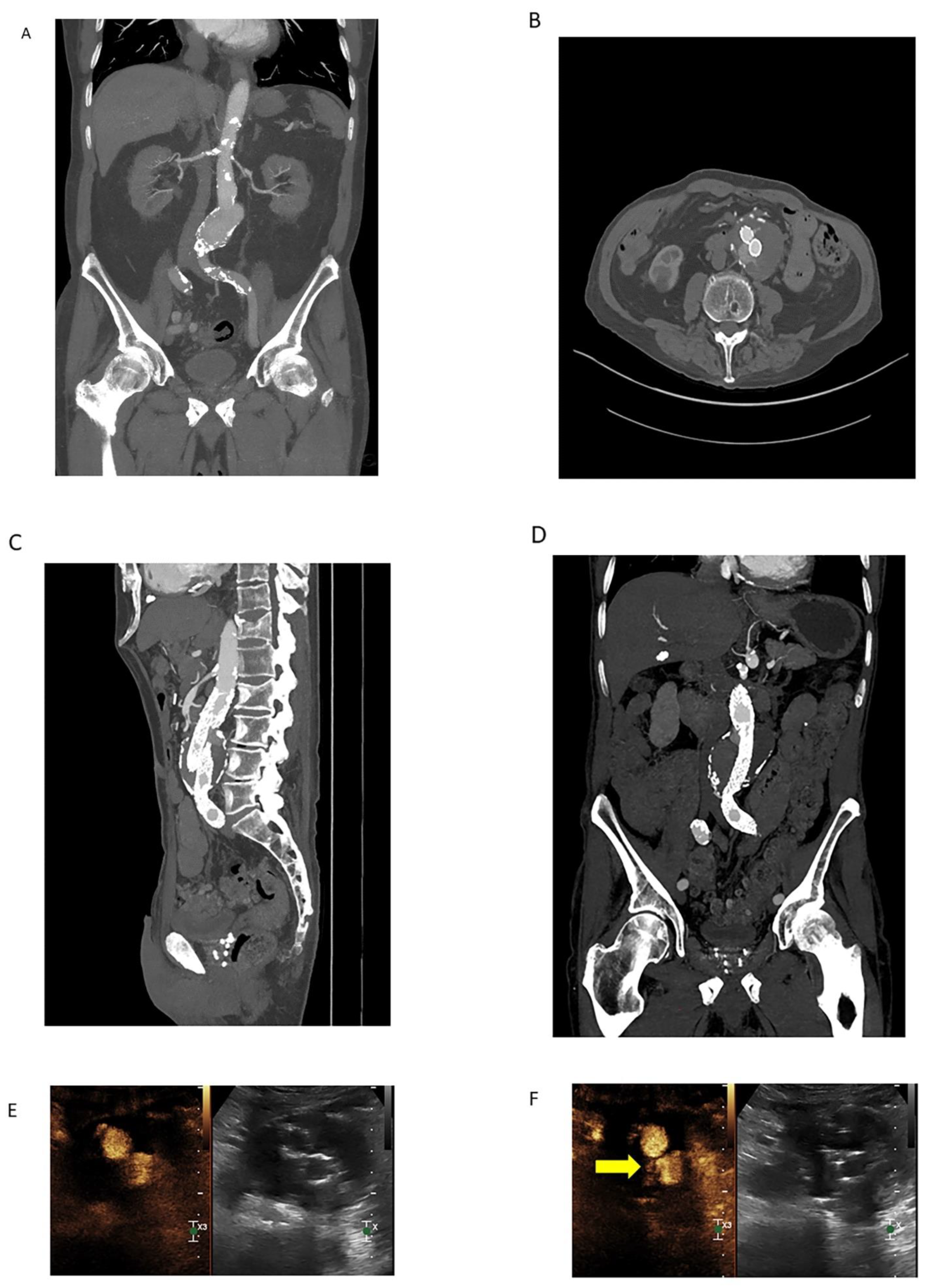
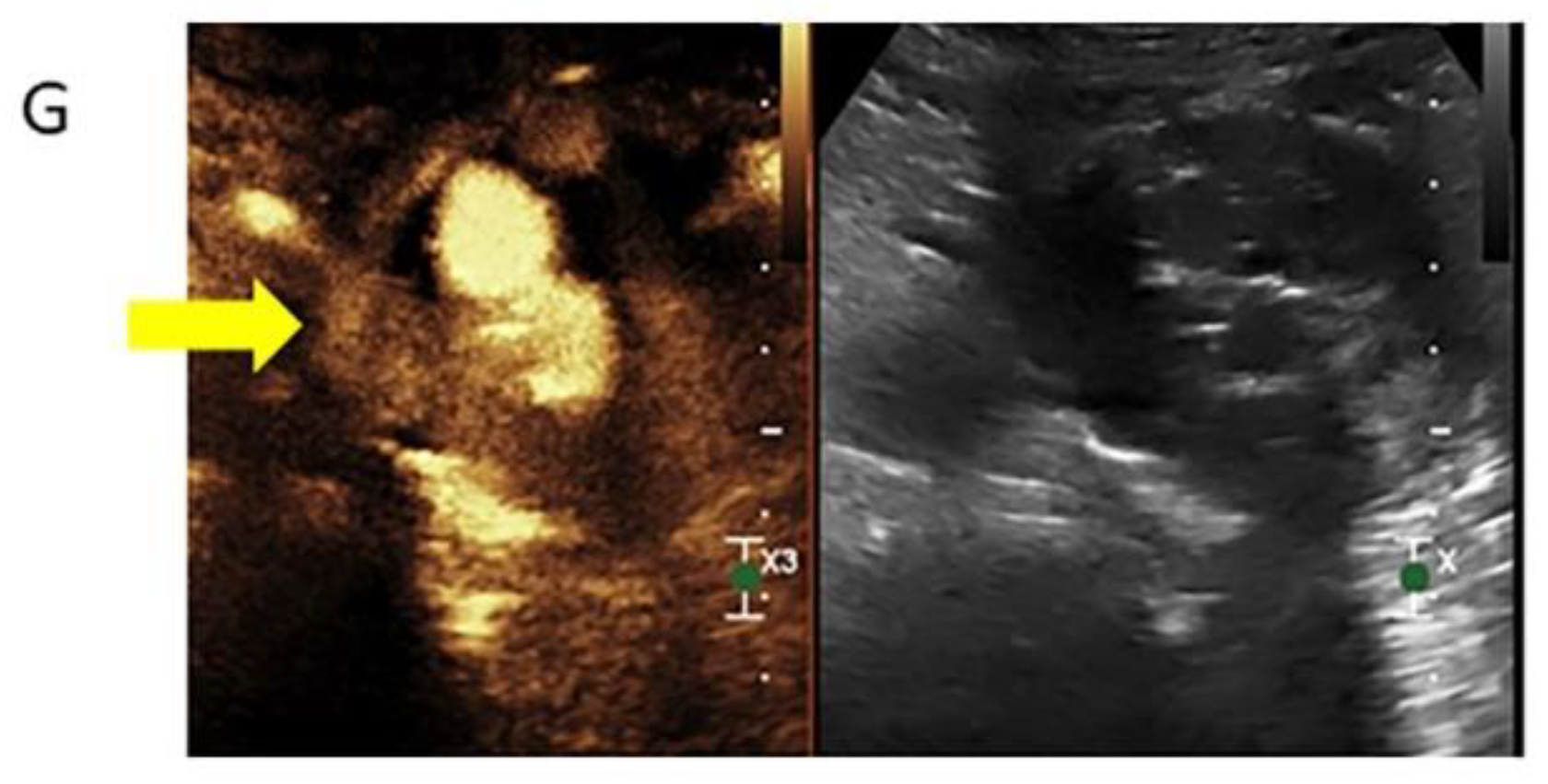
| Computed Tomography Radiation Reduction Methodologies | |
|---|---|
| Image Acquisition Techniques |
|
| Image Reconstruction Techniques |
|
| Artificial Intelligence |
|
| Dual-source CT |
|
| Dual-energy CT |
|
| Non-Ionizing Radiation Imaging Modalities | |
| Magnetic Resonance Angiography |
|
| Cardiac MR/MRA |
|
| Low-field MRI |
|
| Contrast-Enhanced Ultrasound |
|
| Other Methods of Radiation Reduction | |
| Alternating Imaging Modalities |
|
| Protocol Driven Radiation Dose Reduction |
|
| Low Frame Rate Fluoroscopy |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summerlin, D.; Willis, J.; Boggs, R.; Johnson, L.M.; Porter, K.K. Radiation Dose Reduction Opportunities in Vascular Imaging. Tomography 2022, 8, 2618-2638. https://doi.org/10.3390/tomography8050219
Summerlin D, Willis J, Boggs R, Johnson LM, Porter KK. Radiation Dose Reduction Opportunities in Vascular Imaging. Tomography. 2022; 8(5):2618-2638. https://doi.org/10.3390/tomography8050219
Chicago/Turabian StyleSummerlin, David, Joseph Willis, Robert Boggs, Loretta M. Johnson, and Kristin K. Porter. 2022. "Radiation Dose Reduction Opportunities in Vascular Imaging" Tomography 8, no. 5: 2618-2638. https://doi.org/10.3390/tomography8050219
APA StyleSummerlin, D., Willis, J., Boggs, R., Johnson, L. M., & Porter, K. K. (2022). Radiation Dose Reduction Opportunities in Vascular Imaging. Tomography, 8(5), 2618-2638. https://doi.org/10.3390/tomography8050219






