Vascular Choroidal Alterations in Uncomplicated Third-Trimester Pregnancy
Abstract
1. Introduction
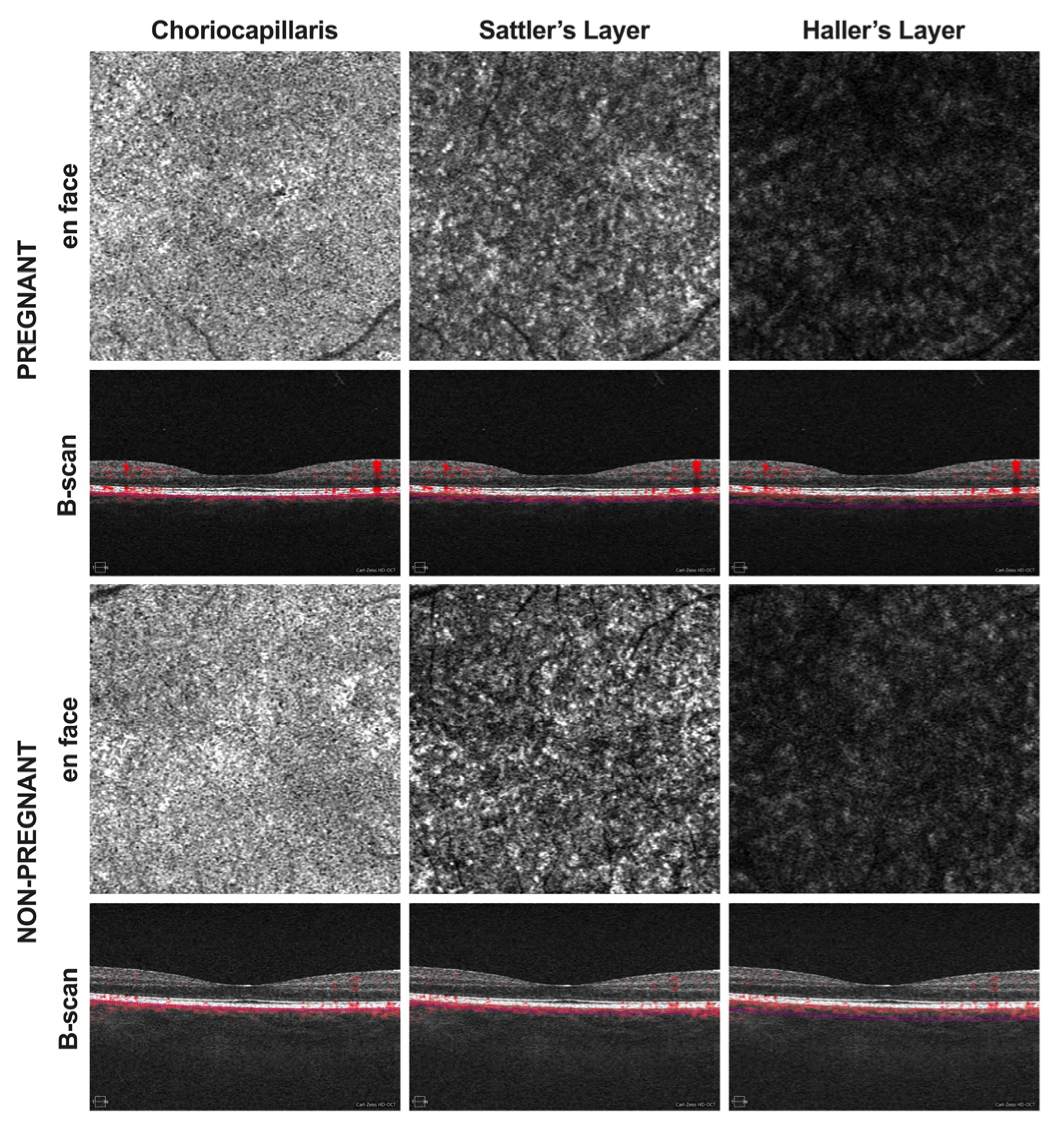
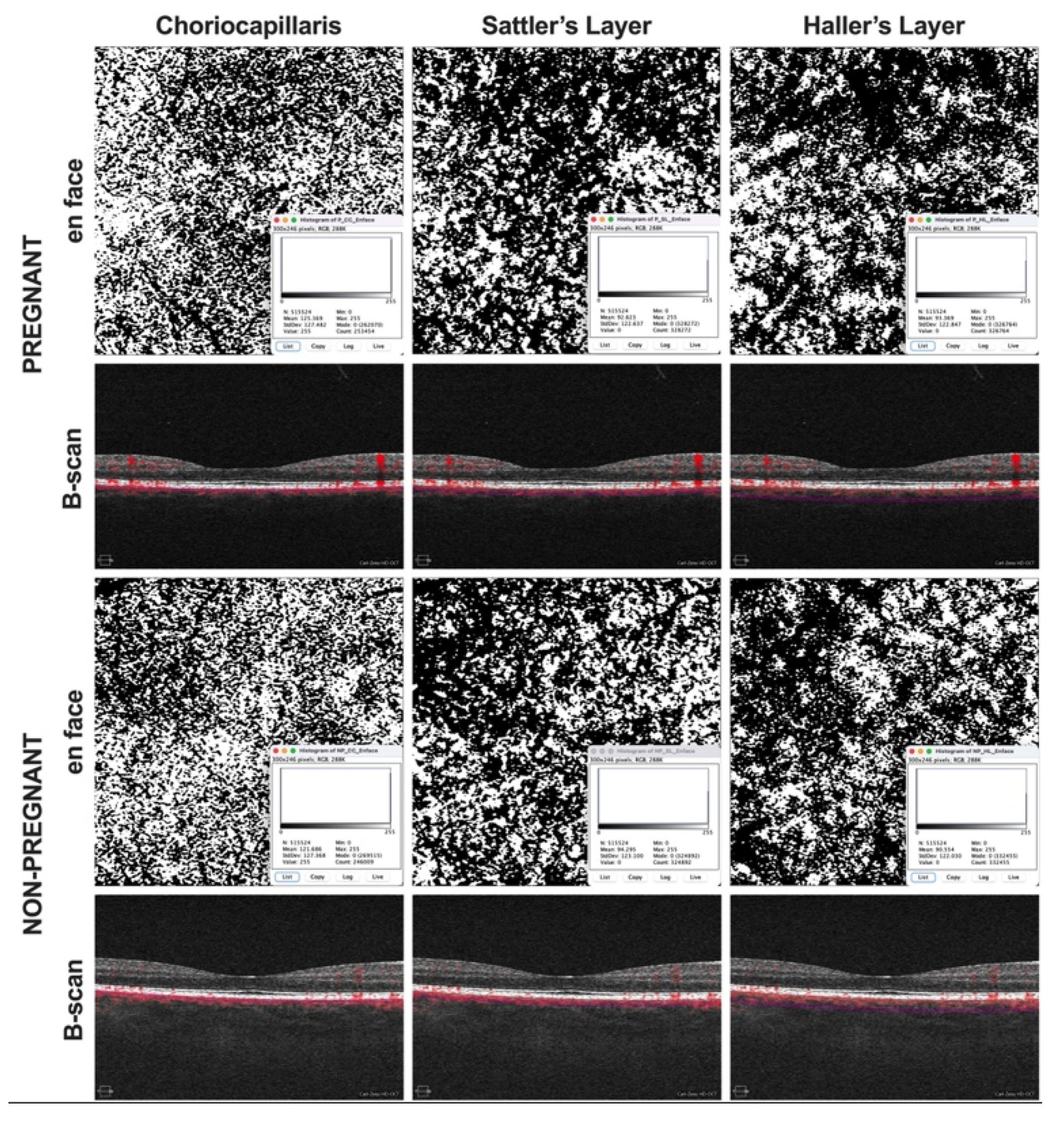
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Hear. Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Ko, N.L.; Mandalà, M. Plasticity of the Maternal Vasculature During Pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Schock, H.; Zeleniuch-Jacquotte, A.; Lundin, E.; Grankvist, K.; Lakso, H.; Idahl, A.; Lehtinen, M.; Surcel, H.-M.; Fortner, R.T. Hormone concentrations throughout uncomplicated pregnancies: A longitudinal study. BMC Pregnancy Childbirth 2016, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Newman, E.A.; Chan-Ling, T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012, 31, 377–406. [Google Scholar] [CrossRef]
- Mackensen, F.; Paulus, W.E.; Max, R.; Ness, T. Ocular Changes During Pregnancy. Dtsch. Ärzteblatt Int. 2014, 111, 567–576. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Lauermann, J.L.; Sochurek, J.A.M.; Plöttner, P.; Alten, F.; Kasten, M.; Prasuhn, J.; Brüggemann, N.; Ranjbar, M. Applicability of optical coherence tomography angiography (OCTA) imaging in Parkinson’s disease. Sci. Rep. 2021, 11, 5520. [Google Scholar] [CrossRef]
- Laviers, H.; Zambarakji, H. Enhanced depth imaging-OCT of the choroid: A review of the current literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1871–1883. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Siegfried, F.; Rommel, F.; Rothe, M.; Brinkmann, M.P.; Sochurek, J.A.M.; Freitag, J.; Grisanti, S.; Ranjbar, M. Evaluating diurnal changes in choroidal sublayer perfusion using optical coherence tomography angiography. Acta Ophthalmol. 2019, 97, e1062–e1068. [Google Scholar] [CrossRef] [PubMed]
- Barteselli, G.; Chhablani, J.; El-Emam, S.; Wang, H.; Chuang, J.; Kozak, I.; Cheng, L.; Bartsch, D.-U.; Freeman, W.R. Choroidal Volume Variations with Age, Axial Length, and Sex in Healthy Subjects: A Three-Dimensional Analysis. Ophthalmology 2012, 119, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Roskal-Wałek, J.; Laudańska-Olszewska, I.; Biskup, M.; Gierada, M.; Odrobina, D. Choroidal Thickness in Women with Uncomplicated Pregnancy: Literature Review. BioMed Res. Int. 2017, 2017, 5694235. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Taweebanjongsin, W.; Gaw, S.L.; Rabina, G.; Sadda, S.R.; Tsui, I. Evaluation of the Choroid in Women with Uncomplicated Pregnancy. Transl. Vis. Sci. Technol. 2020, 9, 24. [Google Scholar] [CrossRef]
- Ranjbar, M.; Rothe, M.; Klapa, S.; Lange, T.; Prasuhn, M.; Grisanti, S.; Riemekasten, G.; Humrich, J.Y. Evaluation of choroidal substructure perfusion in patients affected by systemic sclerosis: An optical coherence tomography angiography study. Scand. J. Rheumatol. 2019, 49, 141–145. [Google Scholar] [CrossRef]
- Rommel, F.; Siegfried, F.; Sochurek, J.A.M.; Rothe, M.; Brinkmann, M.P.; Kurz, M.; Prasuhn, M.; Grisanti, S.; Ranjbar, M. Mapping diurnal variations in choroidal sublayer perfusion in patients with idiopathic epiretinal membrane: An optical coherence tomography angiography study. Int. J. Retin. Vitr. 2019, 5, 12. [Google Scholar] [CrossRef]
- Borrelli, E.; Parravano, M.; Sacconi, R.; Costanzo, E.; Querques, L.; Vella, G.; Bandello, F.; Querques, G. Guidelines on Optical Coherence Tomography Angiography Imaging: 2020 Focused Update. Ophthalmol. Ther. 2020, 9, 697–707. [Google Scholar] [CrossRef]
- Campbell, I.; Coudrillier, B.; Ethier, C.R. Biomechanics of the Posterior Eye: A Critical Role in Health and Disease. J. Biomech. Eng. 2014, 136, 021005. [Google Scholar] [CrossRef]
- Ferrara, M.; Lugano, G.; Sandinha, M.T.; Kearns, V.R.; Geraghty, B.; Steel, D.H.W. Biomechanical properties of retina and choroid: A comprehensive review of techniques and translational relevance. Eye 2021, 35, 1818–1832. [Google Scholar] [CrossRef]
- Azuma, K.; Okubo, A.; Suzuki, T.; Igarashi, N.; Nomura, Y.; Soga, H.; Murata, H.; Fujino, R.; Ogawa, A.; Matsui, H.; et al. Assessment of the choroidal structure in pregnant women in the first trimester. Sci. Rep. 2021, 11, 4629. [Google Scholar] [CrossRef]
- Centofanti, M.; Migliardi, R.; Bonini, S.; Manni, G.; Bucci, M.; Pesavento, C.; Amin, C.; Harris, A. Pulsatile Ocular Blood Flow during Pregnancy. Eur. J. Ophthalmol. 2002, 12, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Sayin, N.; Pirhan, D.; Vural, A.D.; Araz-Ersan, H.B.; Tekirdag, A.I.; Yildirim, G.Y.; Gulac, B.; Yilmaz, G. Evaluation of Subfoveal Choroidal Thickness in Pregnant Women Using Enhanced Depth Imaging Optical Coherence Tomography. Curr. Eye Res. 2014, 39, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Dadaci, Z.; Alptekin, H.; Acir, N.O.; Borazan, M. Changes in choroidal thickness during pregnancy detected by enhanced depth imaging optical coherence tomography. Br. J. Ophthalmol. 2015, 99, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Scalabrin, S.; Becco, A.; Panzica, G. Gonadal Hormones and Retinal Disorders: A Review. Front. Endocrinol. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.C.; Faria, M.; Pettersen, H.; Sampaio, M.; Geber, S. Menstrual phase-related differences in the pulsatility index on the central retinal artery suggest an oestrogen vasodilatation effect that antagonizes with progesterone. Arch. Gynecol. Obstet. 2011, 283, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Rabiolo, A.; Gelormini, F.; Sacconi, R.; Cicinelli, M.V.; Triolo, G.; Bettin, P.; Nouri-Mahdavi, K.; Bandello, F.; Querques, G. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS ONE 2018, 13, e0205773. [Google Scholar] [CrossRef]
- Chanwimol, K.; Balasubramanian, S.; Nassisi, M.; Gaw, S.L.; Janzen, C.; Sarraf, D.; Sadda, S.R.; Tsui, I. Retinal Vascular Changes During Pregnancy Detected With Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2019, 60, 2726–2732. [Google Scholar] [CrossRef]
- Khalil, R.A. Sex Hormones as Potential Modulators of Vascular Function in Hypertension. Hypertension 2005, 46, 249–254. [Google Scholar] [CrossRef]
- Nesper, P.L.; Lee, H.E.; Fayed, A.E.; Schwartz, G.W.; Yu, F.; Fawzi, A.A. Hemodynamic Response of the Three Macular Capillary Plexuses in Dark Adaptation and Flicker Stimulation Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2019, 60, 694–703. [Google Scholar] [CrossRef]
- Nethery, E.; Brauer, M.; Janssen, P. Time–activity patterns of pregnant women and changes during the course of pregnancy. J. Expo. Sci. Environ. Epidemiol. 2008, 19, 317–324. [Google Scholar] [CrossRef]
| Pregnant Group | Control Group | p | |
|---|---|---|---|
| Eyes | 26 eyes (26 subjects) | 26 eyes (26 subjects) | / |
| Age (years) | 29 (22–35) | 29 (21–35) | 0.501 |
| Gestational age (weeks) | 35 (32–40) | / | / |
| MAP (mmHg) | 93 (80–112) | 89 (79–111) | 0.115 |
| AL (mm) | 23.23 (22.18–25.69) | 23.75 (22.25–25.64) | 0.067 |
| SE (D) | −0.25 (−2.630–1.630) | −0.25 (−2.750–1.875) | 0.300 |
| OCTA image quality (1–10) | 10 (8–10) | 10 (9–10) | 0.365 |
| BCVA (logMAR) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.423 |
| IOP (mmHg) | 15.5 (10–21) | 16 (12–20) | 0.114 |
| Pregnant Group | Control Group | p | |
|---|---|---|---|
| SFCT (µm) | 332 (211–469) | 371.5 (224–466) | 0.018 |
| CCP (%) | 46 (41–50) | 48 (41–52) | 0.039 |
| SLP (%) | 62 (50–66) | 61.5 (57–67) | 0.839 |
| HLP (%) | 63 (48–69) | 64 (58–68) | 0.612 |
| MAP | SFCT | CCP | SLP | HLP | ||
|---|---|---|---|---|---|---|
| MAP | cc | 1 | −0.209 | 0.291 | 0.044 | 0.430 |
| p | - | 0.305 | 0.150 | 0.830 | 0.028 | |
| SFCT | cc | −0.209 | 1 | −0.256 | −0.177 | −0.121 |
| p | 0.305 | - | 0.207 | 0.386 | 0.555 | |
| CCP | cc | 0.291 | −0.256 | 1 | −0.365 | 0.258 |
| p | 0.150 | 0.207 | - | 0.067 | 0.203 | |
| SLP | cc | 0.044 | −0.177 | −0.365 | 1 | 0.134 |
| p | 0.830 | 0.386 | 0.067 | - | 0.516 | |
| HLP | cc | 0.430 | −0.121 | 0.258 | 0.134 | 1 |
| p | 0.028 | 0.555 | 0.203 | 0.516 | - | |
| MAP | SFCT | CCP | SLP | HLP | ||
|---|---|---|---|---|---|---|
| MAP | cc | 1 | −0.009 | 0.175 | 0.272 | 0.054 |
| p | - | 0.965 | 0.392 | 0.178 | 0.792 | |
| SFCT | cc | −0.009 | 1 | 0.148 | 0.223 | 0.457 |
| p | 0.965 | - | 0.471 | 0.273 | 0.019 | |
| CCP | cc | 0.175 | 0.148 | 1 | 0.580 | 0.453 |
| p | 0.392 | 0.471 | - | 0.002 | 0.020 | |
| SLP | cc | 0.272 | 0.223 | 0.580 | 1 | 0.503 |
| p | 0.178 | 0.273 | 0.002 | - | 0.009 | |
| HLP | cc | 0.054 | 0.457 | 0.453 | 0.503 | 1 |
| p | 0.792 | 0.019 | 0.020 | 0.009 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochurek, J.A.M.; Gembicki, M.; Grisanti, S.; Ranjbar, M. Vascular Choroidal Alterations in Uncomplicated Third-Trimester Pregnancy. Tomography 2022, 8, 2609-2617. https://doi.org/10.3390/tomography8050218
Sochurek JAM, Gembicki M, Grisanti S, Ranjbar M. Vascular Choroidal Alterations in Uncomplicated Third-Trimester Pregnancy. Tomography. 2022; 8(5):2609-2617. https://doi.org/10.3390/tomography8050218
Chicago/Turabian StyleSochurek, Jan A. M., Michael Gembicki, Salvatore Grisanti, and Mahdy Ranjbar. 2022. "Vascular Choroidal Alterations in Uncomplicated Third-Trimester Pregnancy" Tomography 8, no. 5: 2609-2617. https://doi.org/10.3390/tomography8050218
APA StyleSochurek, J. A. M., Gembicki, M., Grisanti, S., & Ranjbar, M. (2022). Vascular Choroidal Alterations in Uncomplicated Third-Trimester Pregnancy. Tomography, 8(5), 2609-2617. https://doi.org/10.3390/tomography8050218






