Visualizing Bioabsorbable Spacer Effectiveness by Confirming the Distal-Tail of Carbon-Ion Beams: First-In-Human Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Carbon-Ion Radiotherapy
2.2. Auto-Activation Positron Emission Tomography
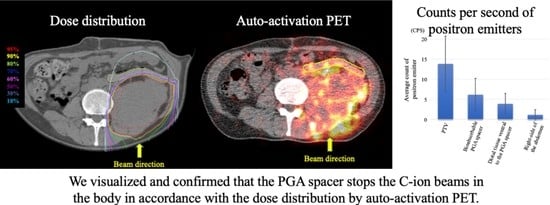
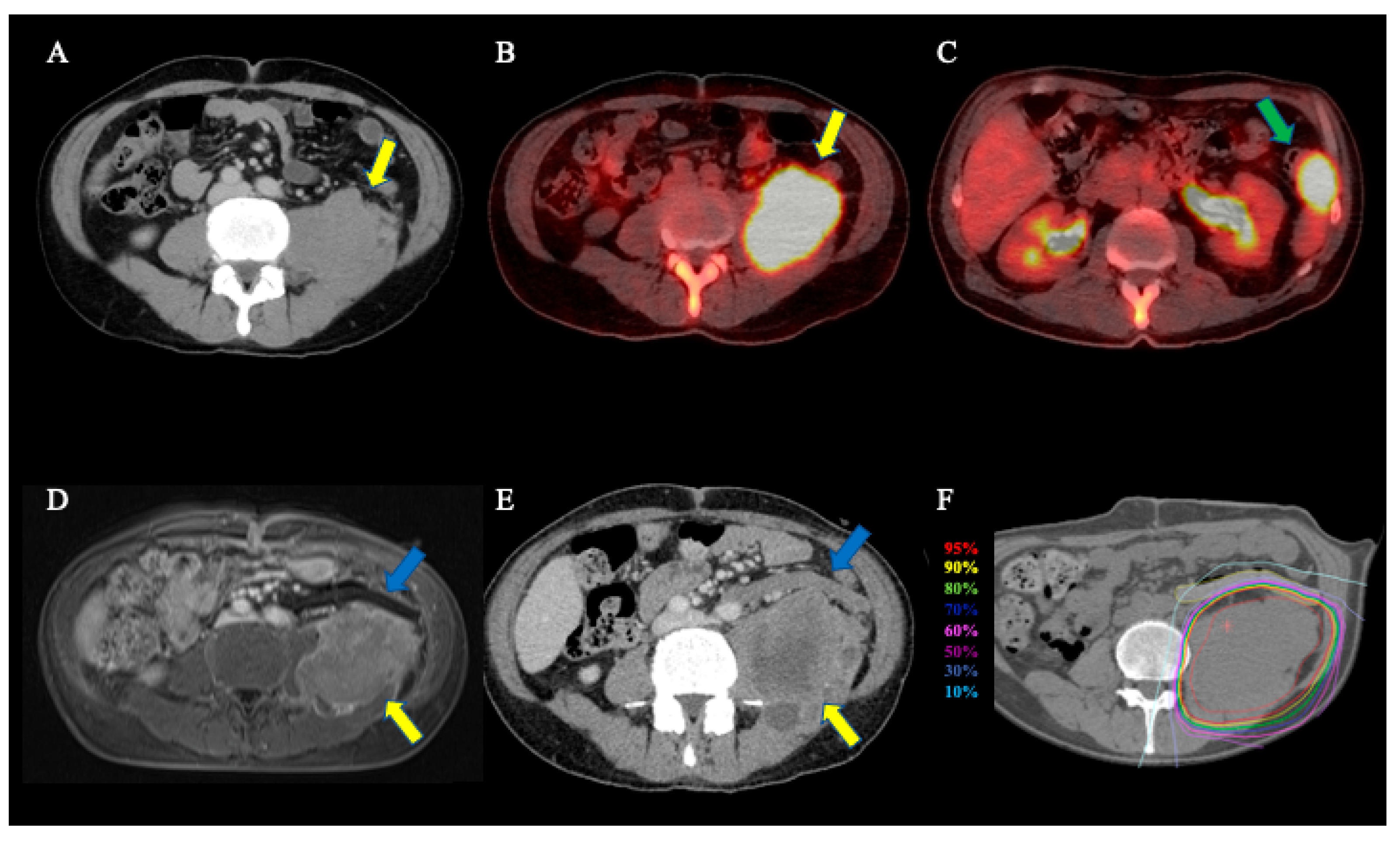
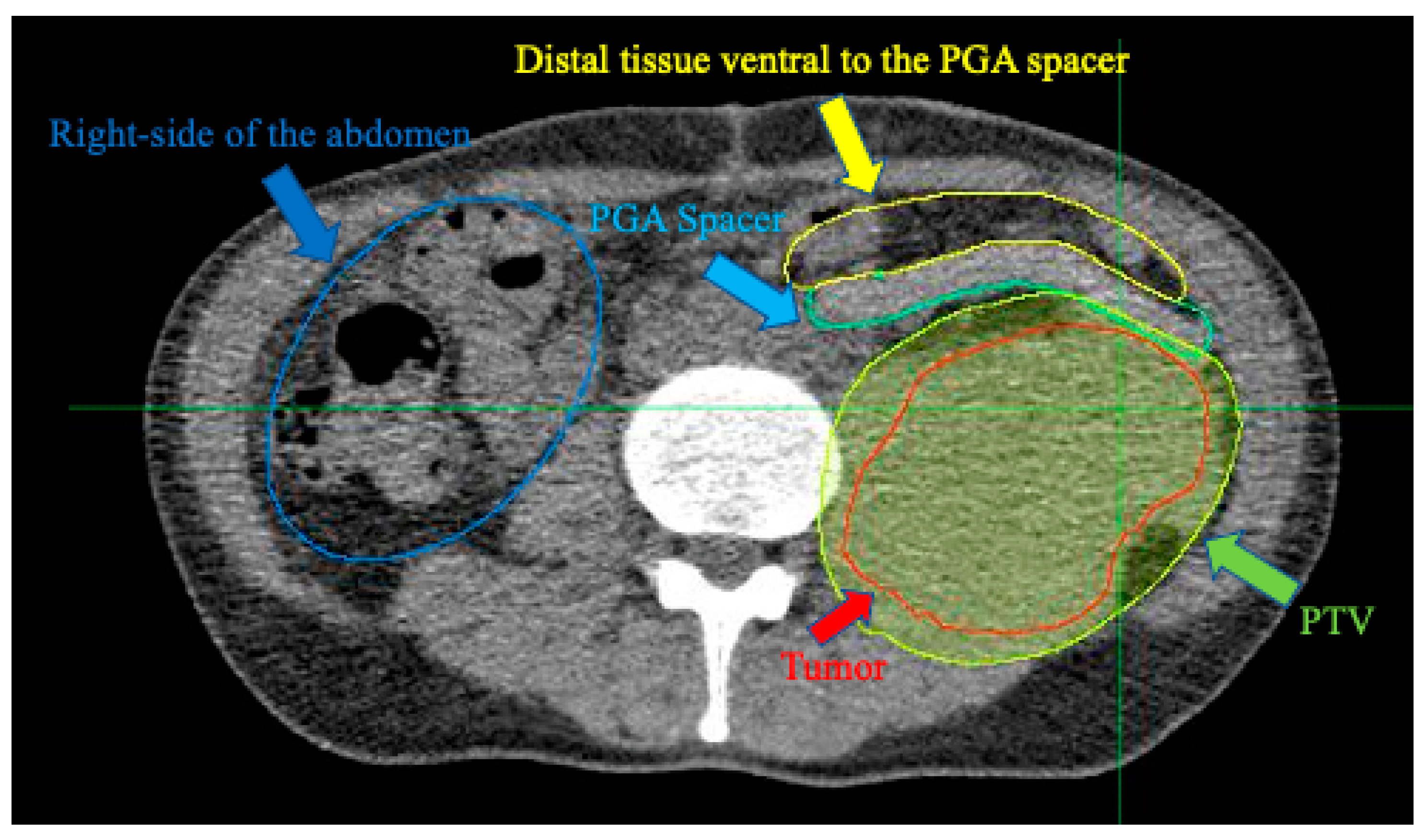
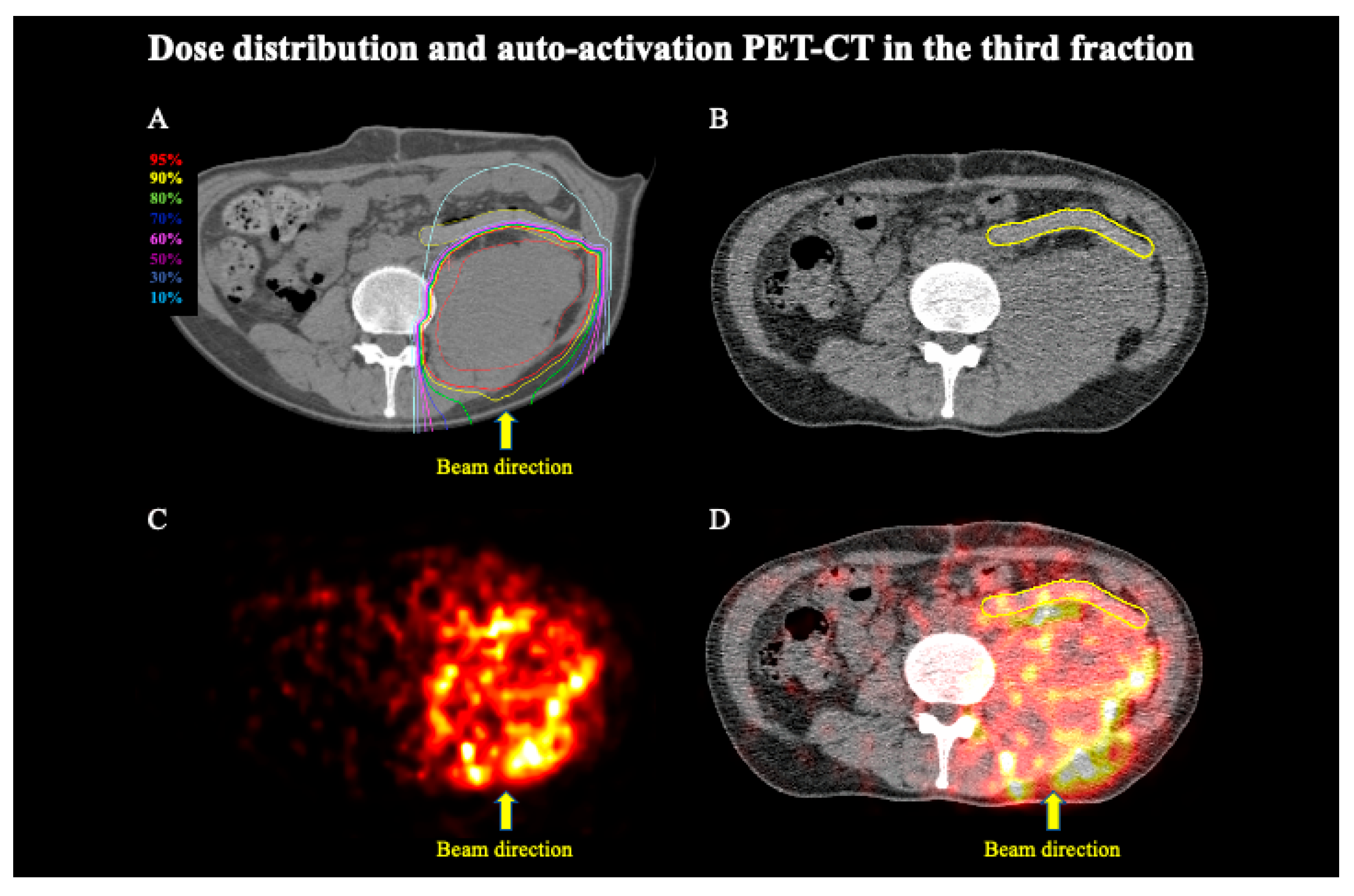
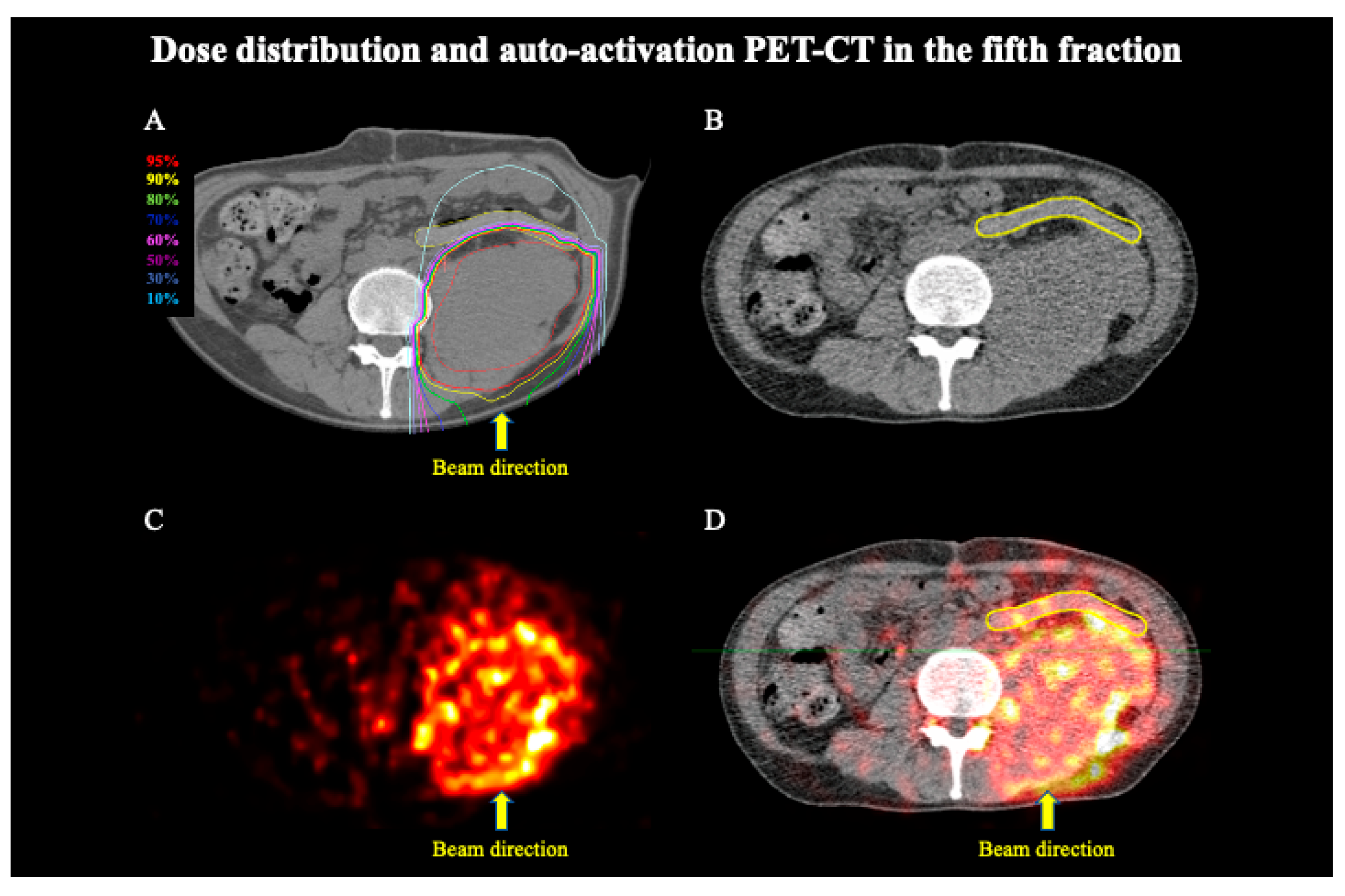
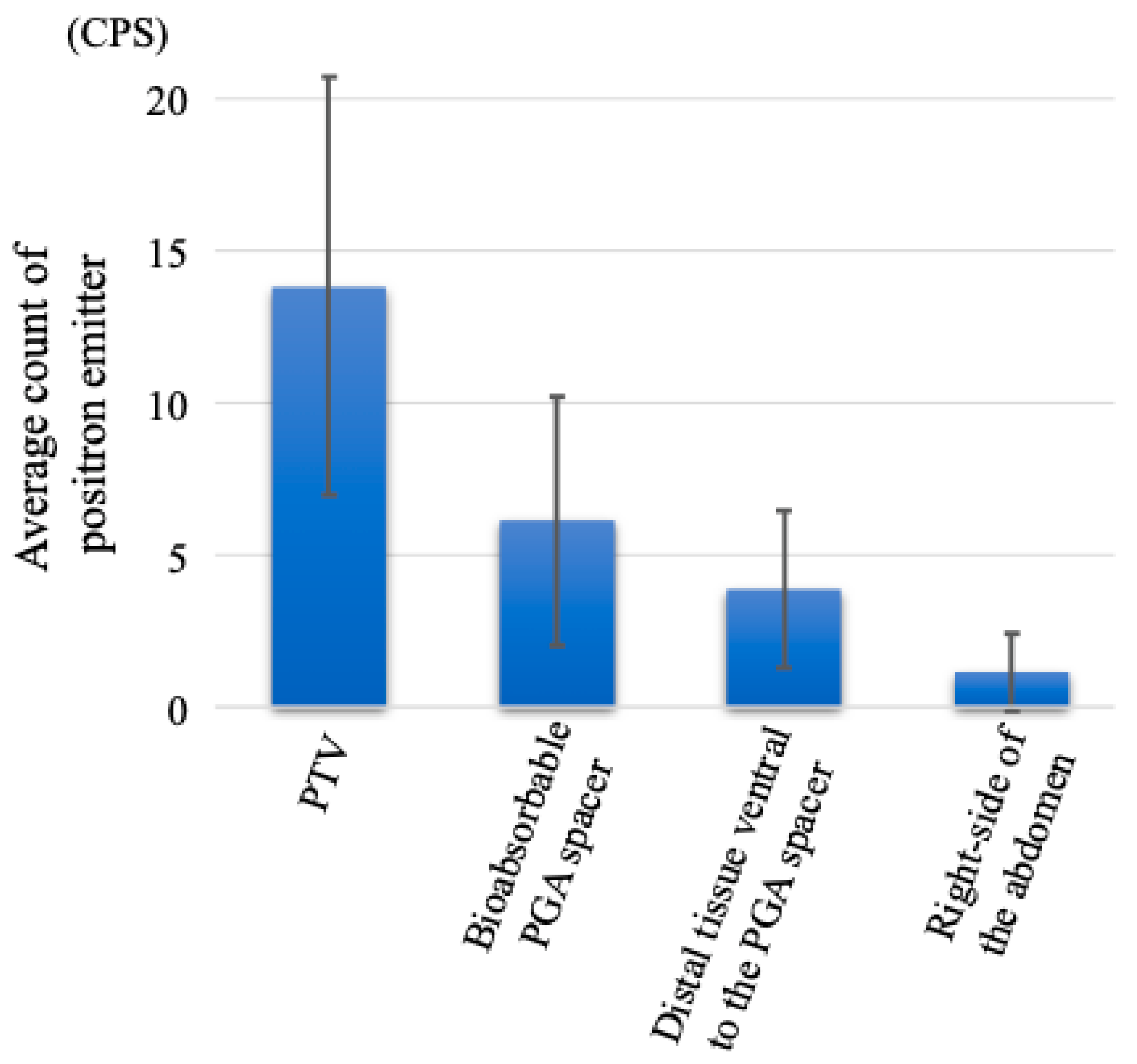
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibuya, K.; Ohno, T.; Katoh, H.; Okamoto, M.; Shiba, S.; Koyama, Y.; Kakizaki, S.; Shirabe, K.; Nakano, T. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother. Oncol. 2019, 132, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, N.; Ando, K.; Murata, K.; Wakatsuki, M.; Noda, S.E.; Irie, D.; Tsuji, H.; Shozu, M.; Ohno, T. Multi-Institutional Retrospective Analysis of Carbon-Ion Radiotherapy for Patients with Locally Advanced Adenocarcinoma of the Uterine Cervix. Cancers 2021, 13, 2713. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, O.; Imai, R.; Kamada, T.; Nitta, Y.; Araki, N.; Working Group for Bone and Soft Tissue Sarcoma. Carbon ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget 2018, 9, 22976–22985. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Nemoto, K.; et al. Multicenter Study of Carbon-Ion Radiation Therapy for Mucosal Melanoma of the Head and Neck: Subanalysis of the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study (1402 HN). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Okamoto, M.; Shioyama, Y.; Ohno, T.; Nakano, T.; Nemoto, K.; Isozaki, Y.; Kawashiro, S.; Tsuji, H.; et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1404 Rectum. Radiother. Oncol. 2019, 132, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Suzuki, Y.; Ohno, T.; Kato, S.; Suzuki, M.; Morita, S.; Sato, S.; Oka, K.; Tsujii, H. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin. Cancer Res. 2006, 12, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Saitoh, J.; Kobayashi, D.; Shibuya, K.; Koyama, Y.; Shimada, H.; Shirai, K.; Ohno, T.; Nakano, T. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat. Oncol. 2015, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Demizu, Y.; Yamashita, T.; Komatsu, S.; Akasaka, H.; Miyawaki, D.; Yoshida, K.; Wang, T.; Okimoto, T.; Fukumoto, T. First-In-Human Phase 1 Study of a Nonwoven Fabric Bioabsorbable Spacer for Particle Therapy: Space-Making Particle Therapy (SMPT). Adv. Radiat. Oncol. 2019, 4, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Andrea, P.; Barbara, V.; Denis, P.; Rosaria, F.M.; Piero, F.; Viviana, V.; Alberto, I.; Mario, C.; Brugnatelli, S.; et al. Surgical spacer placement prior carbon ion radiotherapy (CIRT): An effective feasible strategy to improve the treatment for sacral chordoma. World J. Surg. Oncol. 2016, 14, 211. [Google Scholar] [PubMed]
- Shiba, S.; Okamoto, M.; Tashiro, M.; Ogawa, H.; Osone, K.; Yanagawa, T.; Kohama, I.; Okazaki, S.; Miyasaka, Y.; Osu, N.; et al. Rectal dose-sparing effect with bioabsorbable spacer placement in carbon ion radiotherapy for sacral chordoma: Dosimetric comparison of a simulation study. J. Radiat. Res. 2021, 62, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, I.; Kusano, Y.; Kano, K.; Shima, S.; Tsuchida, K.; Takakusagi, Y.; Mizoguchi, N.; Kamada, T.; Yoshida, D.; Katoh, H. Three cases of retroperitoneal sarcoma in which bioabsorbable spacers (bioabsorbable polyglycolic acid spacers) were inserted prior to carbon ion radiotherapy. J. Radiat. Res. 2022, 63, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Komatsu, S.; Hori, Y.; Murakami, M.; Hishikawa, Y.; Ku, Y. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J. Surg. Oncol. 2010, 101, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Unholtz, D.; Sommerer, F.; Kurz, C.; Haberer, T.; Herfarth, K.; Welzel, T.; Combs, S.E.; Debus, J.; Parodi, K. Implementation and initial clinical experience of offline PET/CT-based verification of scanned carbon ion treatment. Radiother. Oncol. 2013, 107, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, R.K.; Sakai, M.; Kada, W.; Torikai, K.; Kikuchi, M.; Arakawa, K.; Torikoshi, M.; Nakano, T. Annihilation gamma imaging for carbon ion beam range monitoring using Si/CdTe Compton camera. Phys. Med. Biol. 2019, 64, 055003. [Google Scholar] [CrossRef] [PubMed]
- Ammar, C.; Frey, K.; Bauer, J.; Melzig, C.; Chiblak, S.; Hildebrandt, M.; Unholtz, D.; Kurz, C.; Brons, S.; Debus, J.; et al. Comparing the biological washout of β+-activity induced in mice brain after 12C-ion and proton irradiation. Phys. Med. Biol. 2014, 59, 7229–7244. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.; Gospodarowicz, M.; Wittekind, C. International Union Against Cancer (UICC): TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Inaniwa, T.; Kanematsu, N.; Matsufuji, N.; Kanai, T.; Shirai, T.; Noda, K.; Tsuji, H.; Kamada, T.; Tsujii, H. Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys. Med. Biol. 2015, 60, 3271–3286. [Google Scholar] [CrossRef] [PubMed]
- Noda, K. Beam Delivery Method for Carbon-ion Radiotherapy with the Heavy-ion Medical Accelerator in Chiba. Int. J. Part. Ther. 2016, 2, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, W.; Lai, S.; Shi, L.; Chen, J. In Vivo 3-D Dose Verification Using PET/CT Images after Carbon-Ion Radiation Therapy. Front. Oncol. 2021, 11, 621394. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Sakai, M.; Okamoto, M.; Ohno, T. Visualisation of Range Shortening in Carbon Ion Beams and Washout of Positron Emitter: First-in-Human Report. In Vivo 2021, 35, 3521–3526. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiba, S.; Okamoto, M.; Sakai, M.; Ohno, T. Visualizing Bioabsorbable Spacer Effectiveness by Confirming the Distal-Tail of Carbon-Ion Beams: First-In-Human Report. Tomography 2022, 8, 2339-2346. https://doi.org/10.3390/tomography8050195
Shiba S, Okamoto M, Sakai M, Ohno T. Visualizing Bioabsorbable Spacer Effectiveness by Confirming the Distal-Tail of Carbon-Ion Beams: First-In-Human Report. Tomography. 2022; 8(5):2339-2346. https://doi.org/10.3390/tomography8050195
Chicago/Turabian StyleShiba, Shintaro, Masahiko Okamoto, Makoto Sakai, and Tatsuya Ohno. 2022. "Visualizing Bioabsorbable Spacer Effectiveness by Confirming the Distal-Tail of Carbon-Ion Beams: First-In-Human Report" Tomography 8, no. 5: 2339-2346. https://doi.org/10.3390/tomography8050195
APA StyleShiba, S., Okamoto, M., Sakai, M., & Ohno, T. (2022). Visualizing Bioabsorbable Spacer Effectiveness by Confirming the Distal-Tail of Carbon-Ion Beams: First-In-Human Report. Tomography, 8(5), 2339-2346. https://doi.org/10.3390/tomography8050195