Full-Thickness Craniodural Metastasis with Leptomeningeal Infiltration of Salivary Origin: A Radiological Lesson and a Technical Remark
Abstract
1. Introduction
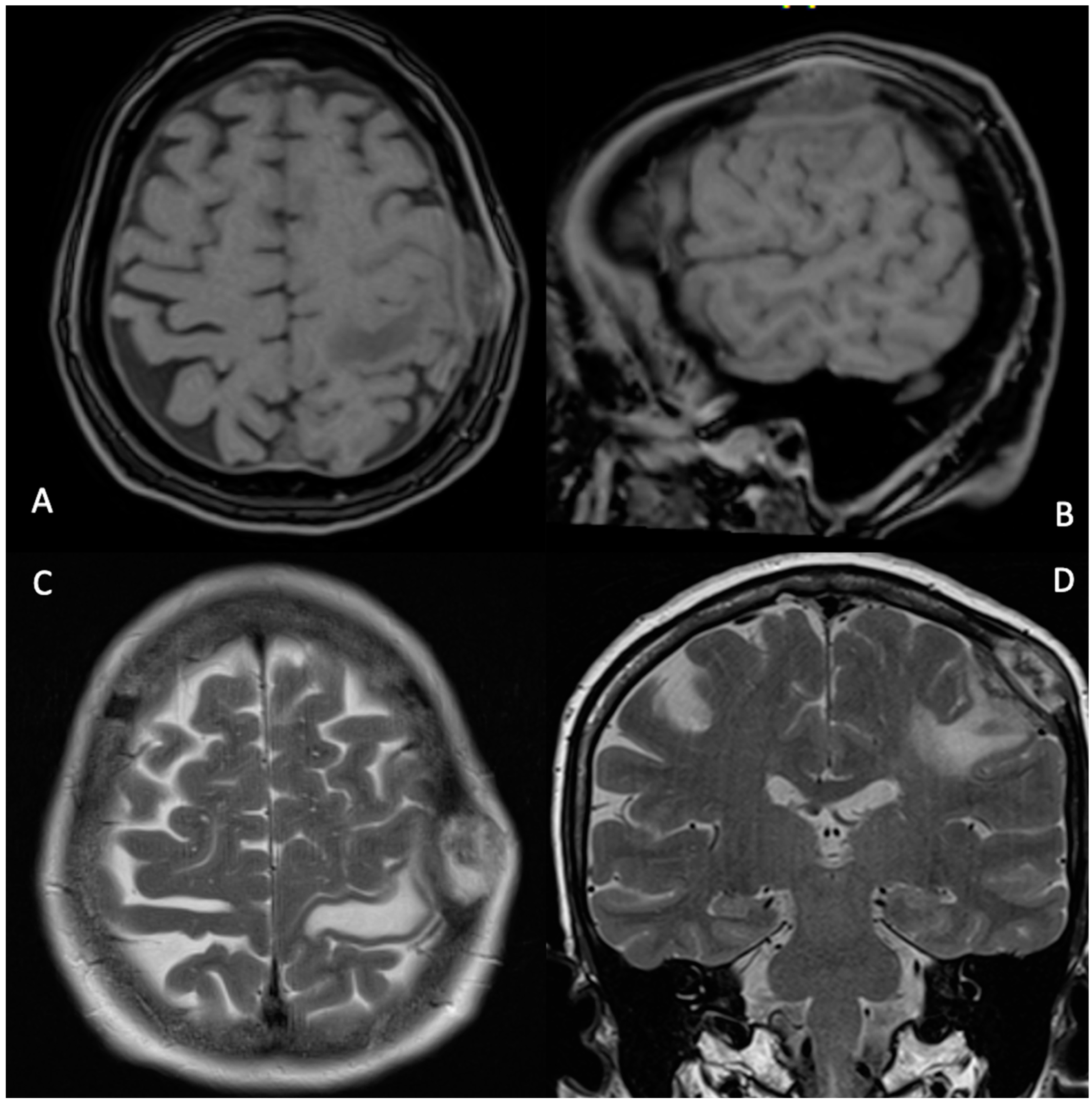
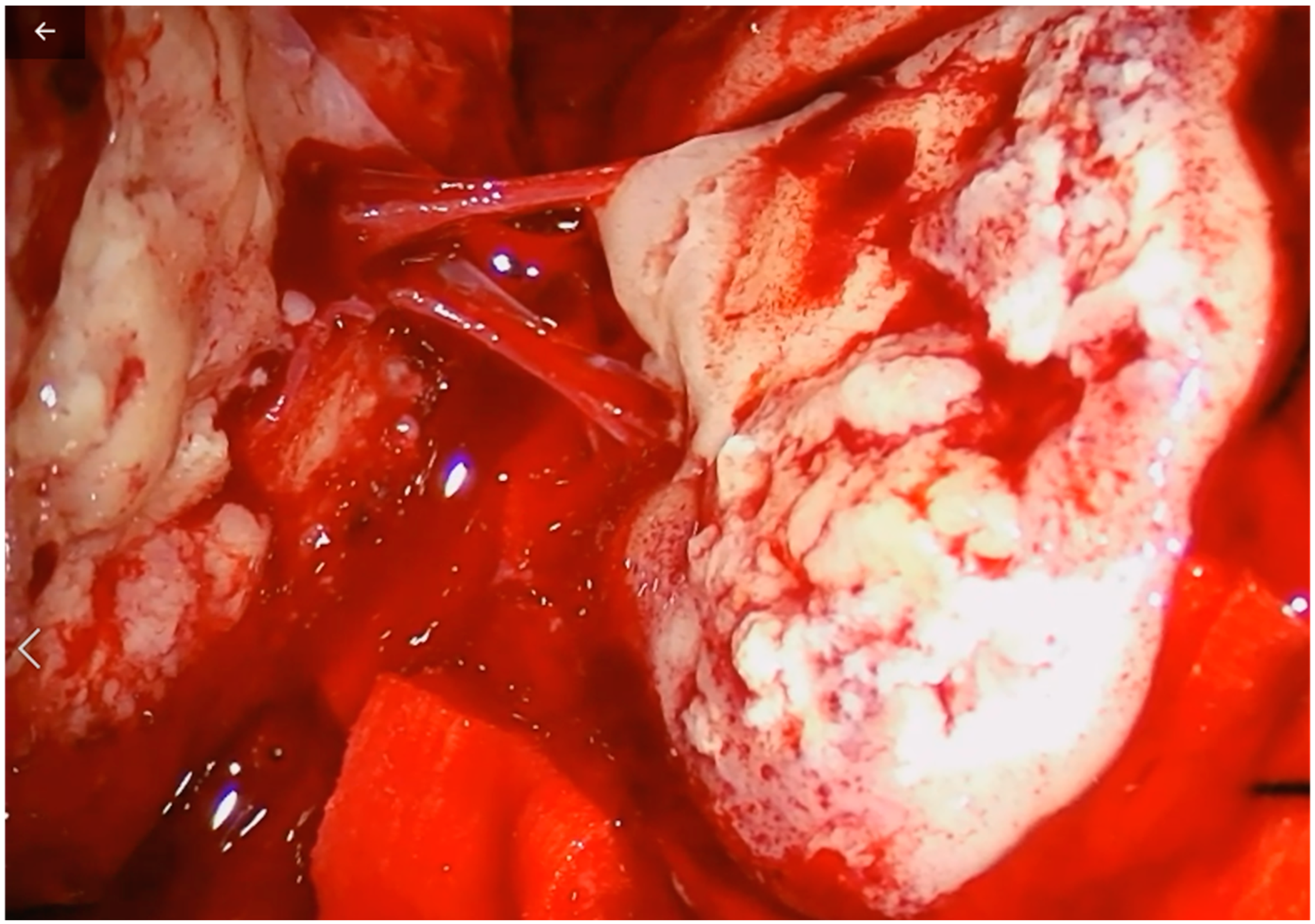
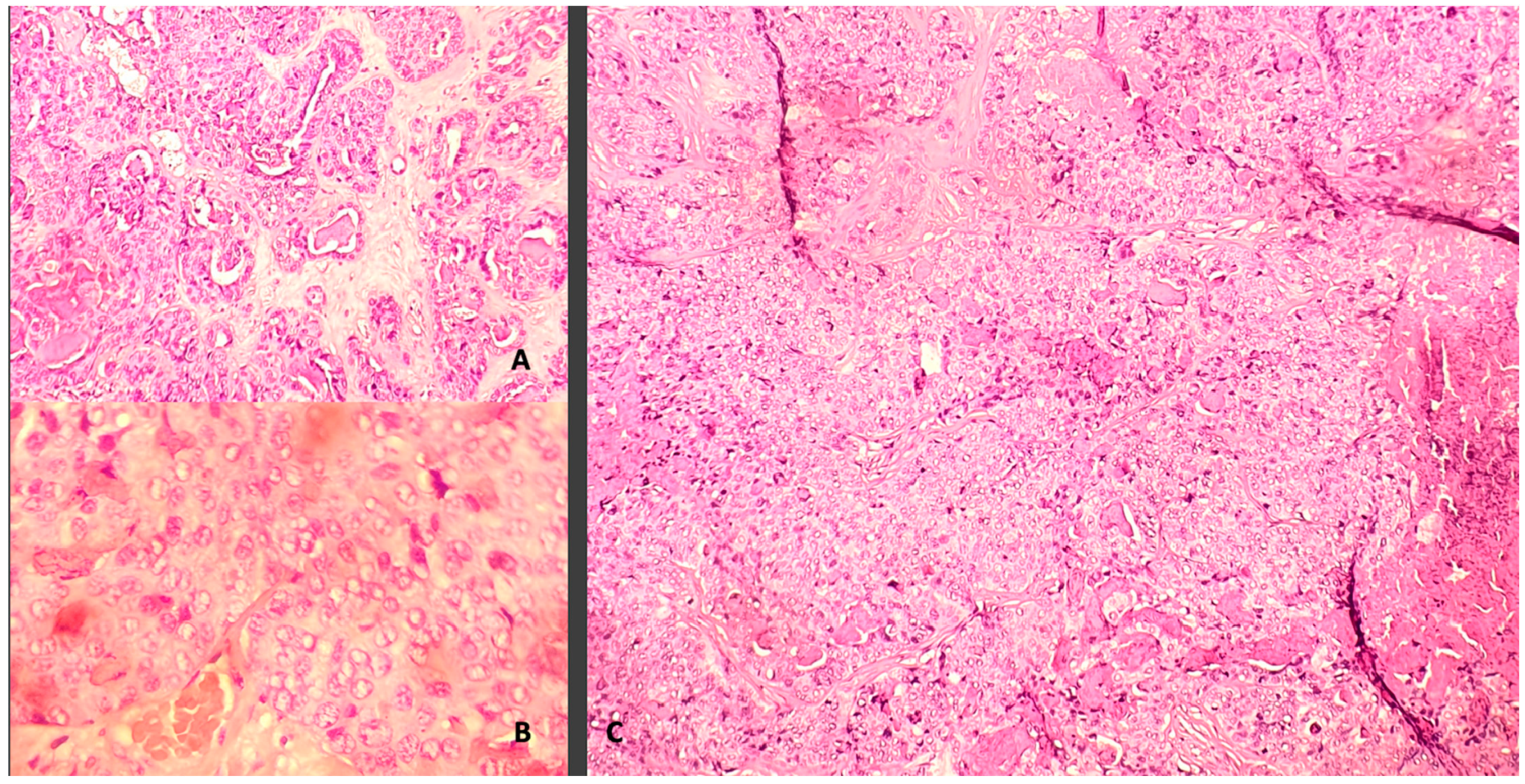
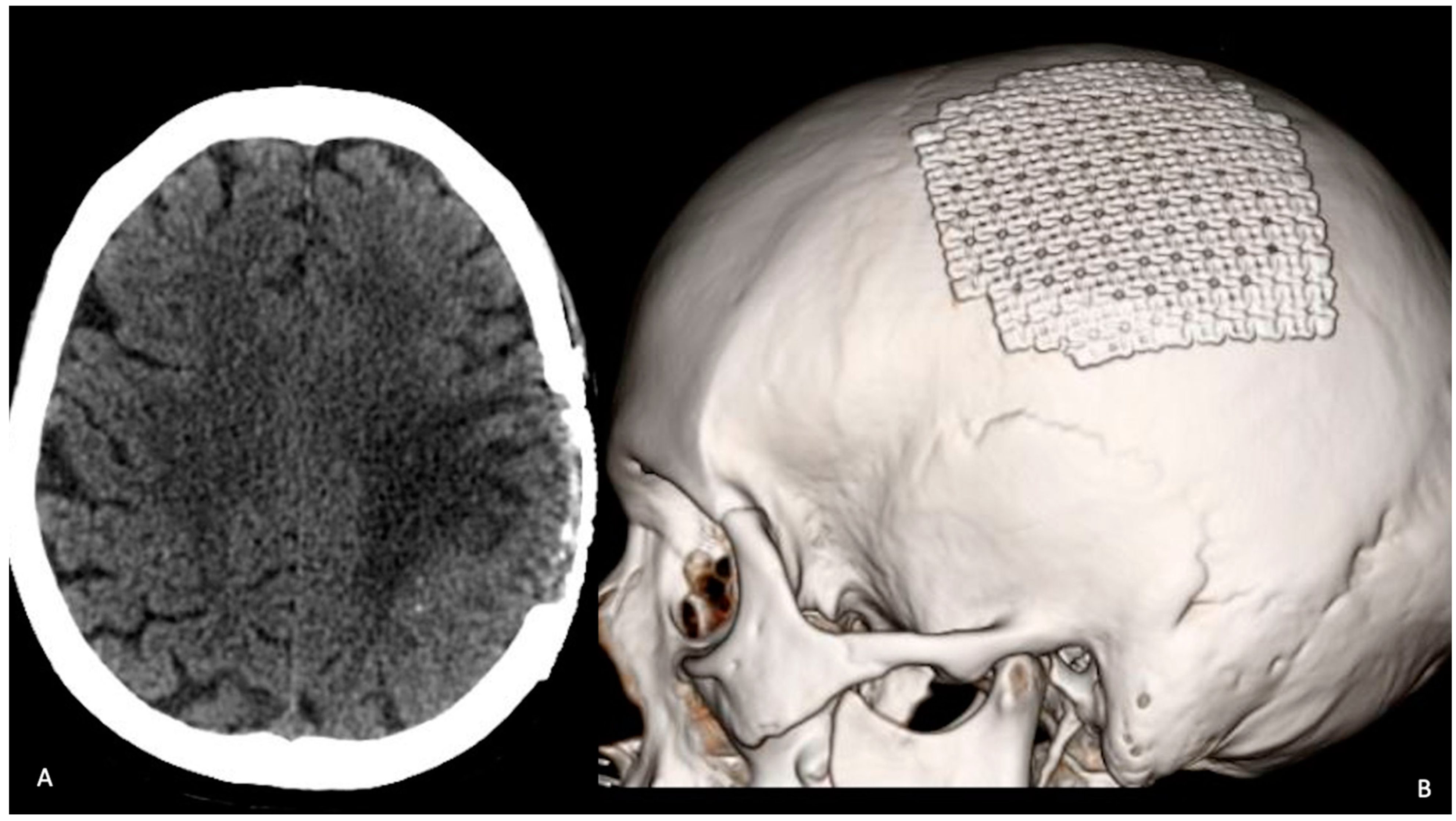
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kotecha, R.; Angelov, L.; Barnett, G.H.; Reddy, C.A.; Suh, J.H.; Murphy, E.S.; Neyman, G.; Chao, S.T. Calvarial and skull base metastases: Expanding the clinical utility of Gamma Knife surgery. J. Neurosurg. 2014, 121, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Maroldi, R.; Ambrosi, C.; Farina, D. Metastatic disease of the brain: Extra-axial metastases (skull, dura, leptomeningeal) and tumour spread. Eur. Radiol. 2004, 15, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G. Neurology in Clinical Practice: The Neurological Disorders; Butterworth-Heinemann: Philadelphia, PA, USA, 2004; p. 1455. [Google Scholar]
- Laigle-Donadey, F.; Taillibert, S.; Mokhtari, K.; Hildebrand, J.; Delattre, J.Y. Dural metastases. J. Neuro-Oncol. 2005, 75, 57–61. [Google Scholar] [CrossRef]
- Mitsuya, K.; Nakasu, Y.; Horiguchi, S.; Harada, H.; Nishimura, T.; Yuen, S.; Asakura, K.; Endo, M. Metastatic skull tumors: MRI features and a new conventional classification. J. Neuro-Oncol. 2010, 104, 239–245. [Google Scholar] [CrossRef]
- Lee, N.; Millender, L.E.; Larson, D.A.; Wara, W.M.; McDermott, M.W.; Kaplan, M.J.; Sneed, P.K. Gamma knife radiosurgery for recurrent salivary gland malignancies involving the base of skull. Head Neck 2002, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.G.; Goodkin, R.; Laramore, G.E. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck 2007, 30, 492–496. [Google Scholar] [CrossRef]
- Alsanie, I.; Rajab, S.; Cottom, H.; Adegun, O.; Agarwal, R.; Jay, A.; Graham, L.; James, J.; Barrett, A.W.; van Heerden, W.; et al. Distribution and Frequency of Salivary Gland Tumours: An International Multicenter Study. Head Neck Pathol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Gangemi, M.; Giamundo, A.; Mariniello, G.; Colella, A.; Vergara, P.; Del Basso De Caro, M.L. Intracranial extension of salivary gland tumors. Clin. Neuropathol. 2010, 29, 9–13. [Google Scholar] [CrossRef]
- Constans, J.P.; Ronjean, J. Les metastases cérébrales en carcinologie. Neurochirurgie 1974, 20 (Suppl. S2), 20–58. [Google Scholar]
- Ellis, G.L.; Corio, R.L. Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer 1983, 52, 542–549. [Google Scholar] [CrossRef]
- Spiro, R.H.; Huvos, A.G.; Strong, E.W. Acinic cell carcinoma of the salivary origin: A clinico pathologic study of 67 cases. Cancer 1978, 41, 924–935. [Google Scholar] [CrossRef]
- Tran, L.; Sadeghi, A.; Hanson, D. Major salivary gland tumors: Treatment results and prognostic factor. Laryngoscope 1986, 10, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Ma, D.Q. Carcinoma of the salivary gland: A clinicopathologic study of 405 cases. Semin. Surg. Oncol. 1987, 3, 240–244. [Google Scholar] [CrossRef]
- Van der Wal, J.E.; Beeking, A.G.; Snow, G.B.; van der Waal, I. Distant metastases of adenoid cystic carcinomaof the salivary glands and the value of diagnostic examinations during follow-up. Head Neck 2002, 24, 779–783. [Google Scholar] [CrossRef]
- Kumar, P.P.; Patil, A.A.; Ogren, F.P.; Johansson, S.L.; Reeves, M.A. Intracranial metastasis from parotid and facial skin tumors. Mechanism, diagnosis, and treatment. J. Natl. Med. Assoc. 1993, 85, 369–374. [Google Scholar] [PubMed]
- Blond, S.; Caparros-Lefebvre, D.; Defoort-Dhellemmes, S.; Parent, M.; Coche-Dequeant, B.; Rousseau, J.; Bradai, N.; Christiaens, J.L. Thalamocapsular metastasis of muco-epidermoid adenocarcinoma of the parotid gland. Neurochirurgie 1991, 37, 406–409. [Google Scholar]
- Hammond, M.A.; Hassenbuch, S.J.; Fuller, G.N.; Shi, W.; Leeds, N.E. Multiple brain metastases: A rare manifestation of adenoid cystic carcinoma of the parotidgland. J. Neurooncol. 1996, 27, 61–64. [Google Scholar] [CrossRef]
- Kazumoto, K.; Hayase, N.; Kurosumi, M.; Kishi, K.; Uki, J.; Takeda, F. Multiple brain metastases from adenoid cystic carcinoma of the parotid gland. Case report and review of the literature. Surg. Neurol. 1998, 50, 475–479. [Google Scholar] [CrossRef]
- Lesniak, M.S.; Tihan, T.; Olivi, A. Solitary central nervous system metastasis from acinic cell carcinoma of the parotid gland. J. Otolaryngol. 2002, 31, 38. [Google Scholar] [CrossRef]
- Pompili, A.; Carapella, C.M.; Cattani, F.; Fabi, A.; Giannarelli, D.; Giovannetti, M.; Mirri, A.; Occhipinti, E.; Telera, S.; Vidiri, A.; et al. Metastases to the cerebellum. Results and prognostic factors in a consecutive series of 44 operated patients. J. Neurooncol. 2008, 88, 331–337. [Google Scholar] [CrossRef]
- Sheedy, S.P.; Welker, K.M.; De Lone, D.R.; Gilbertson, D. CNS metastases of carcinoma ex pleomorphicadenoma of the parotid gland. Am. J. Neuroradiol. 2006, 27, 1483–1485. [Google Scholar] [PubMed]
- Watson, P.; Sutherland, G.; Diocee, M.; Sima, A. Acinic cell carcinoma metastatic to the brain: Case report and ultrastructural study. Head Neck 1987, 10, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Bumpous, J.M.; Maves, M.D.; Gomez, S.M.; Levy, B.K.; Johnson, F. Cavernous sinus involvement in head and neck cancer. Head Neck 1993, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, N.; Öksüzoğlu, B.; Vural, M.; Han, O.; Zengin, N. Case report: Cavernous sinus metastasis of the parotid carcinoma: A very unusual case. J. Neuro-Oncol. 2005, 73, 181–183. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Kitagawa, R.H.; Sherman, S.I.; Bruner, J.M. Adenocarcinoma of the salivary gland metastatic to the pituitary gland: Case report. Neurosurgery 2001, 48, 1161–1165. [Google Scholar]
- Cui, R.; Cheng, X.; Li, F.; Zhuang, H. Rare Cerebral and Pulmonary Metastases from Low-Grade Basal Cell Adenocarcinoma of the Parotid Gland. Clin. Nucl. Med. 2011, 36, 1124–1126. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Walcott, B.P.; Sheth, S.A.; Snuderl, M.; Patel, A.P.; Curry, W.T.; Nahed, B.V. Clinical features of brain metastasis from salivary gland tumors. J. Clin. Neurosci. 2013, 20, 1533–1537. [Google Scholar] [CrossRef][Green Version]
- Schwentner, I.; Obrist, P.; Thumfart, W.; Sprinzl, G. Distant metastasis of parotid gland tumors. Acta Oto-Laryngol. 2006, 126, 340–345. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesce, A.; Armocida, D.; Fiorentino, F.; Ciarlo, S.; La Pira, B.; Salvati, M.; Frati, A.; Pompucci, A.; Palmieri, M. Full-Thickness Craniodural Metastasis with Leptomeningeal Infiltration of Salivary Origin: A Radiological Lesson and a Technical Remark. Tomography 2022, 8, 2164-2170. https://doi.org/10.3390/tomography8050181
Pesce A, Armocida D, Fiorentino F, Ciarlo S, La Pira B, Salvati M, Frati A, Pompucci A, Palmieri M. Full-Thickness Craniodural Metastasis with Leptomeningeal Infiltration of Salivary Origin: A Radiological Lesson and a Technical Remark. Tomography. 2022; 8(5):2164-2170. https://doi.org/10.3390/tomography8050181
Chicago/Turabian StylePesce, Alessandro, Daniele Armocida, Francesco Fiorentino, Silvia Ciarlo, Biagia La Pira, Maurizio Salvati, Alessandro Frati, Angelo Pompucci, and Mauro Palmieri. 2022. "Full-Thickness Craniodural Metastasis with Leptomeningeal Infiltration of Salivary Origin: A Radiological Lesson and a Technical Remark" Tomography 8, no. 5: 2164-2170. https://doi.org/10.3390/tomography8050181
APA StylePesce, A., Armocida, D., Fiorentino, F., Ciarlo, S., La Pira, B., Salvati, M., Frati, A., Pompucci, A., & Palmieri, M. (2022). Full-Thickness Craniodural Metastasis with Leptomeningeal Infiltration of Salivary Origin: A Radiological Lesson and a Technical Remark. Tomography, 8(5), 2164-2170. https://doi.org/10.3390/tomography8050181





