Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
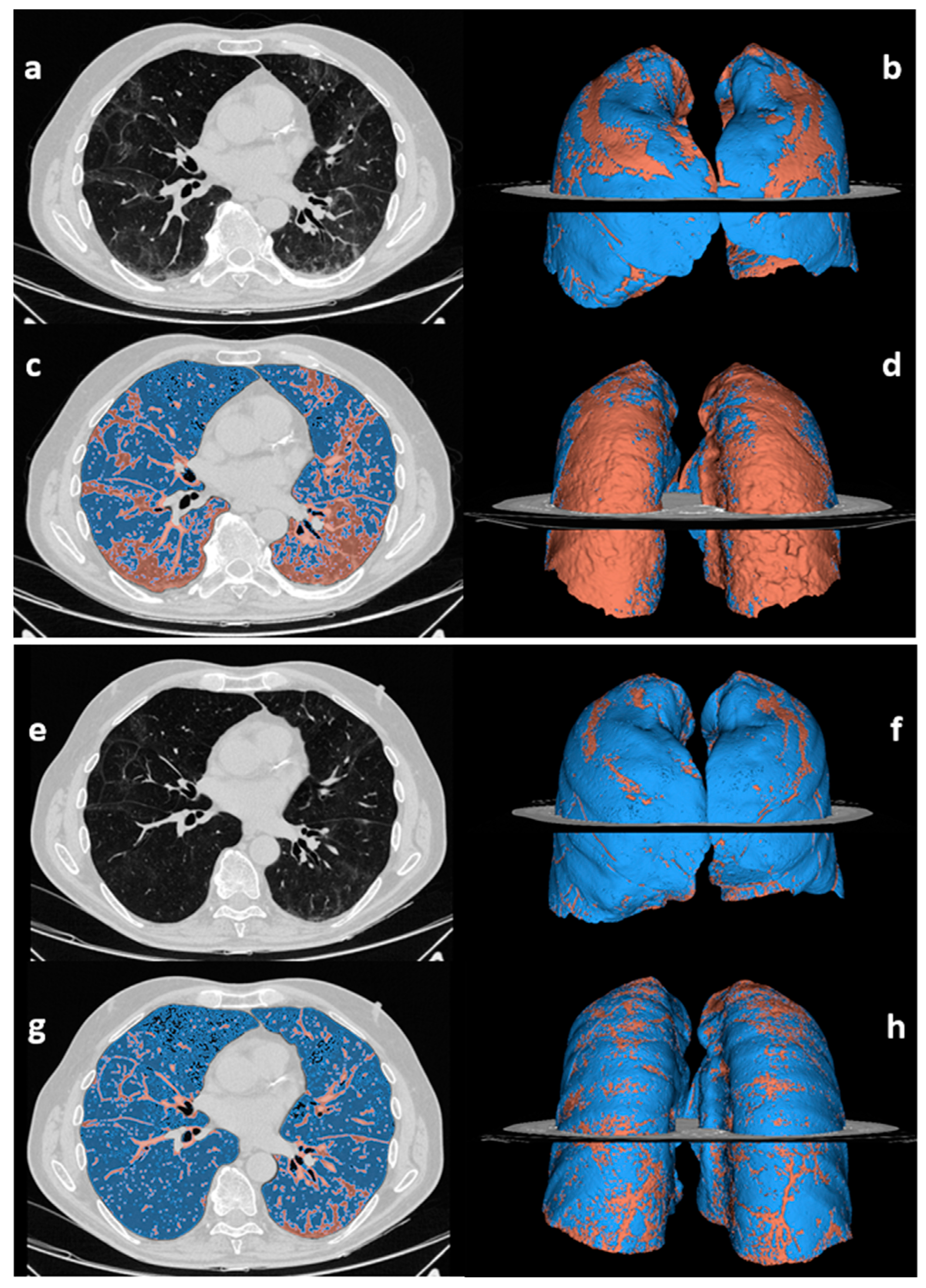
2.2. CT Image Acquisition, Qualitative and Quantitative Analysis
2.3. Post-Discharge Pulmonary Assessment
2.4. Statistical Analysis
3. Results
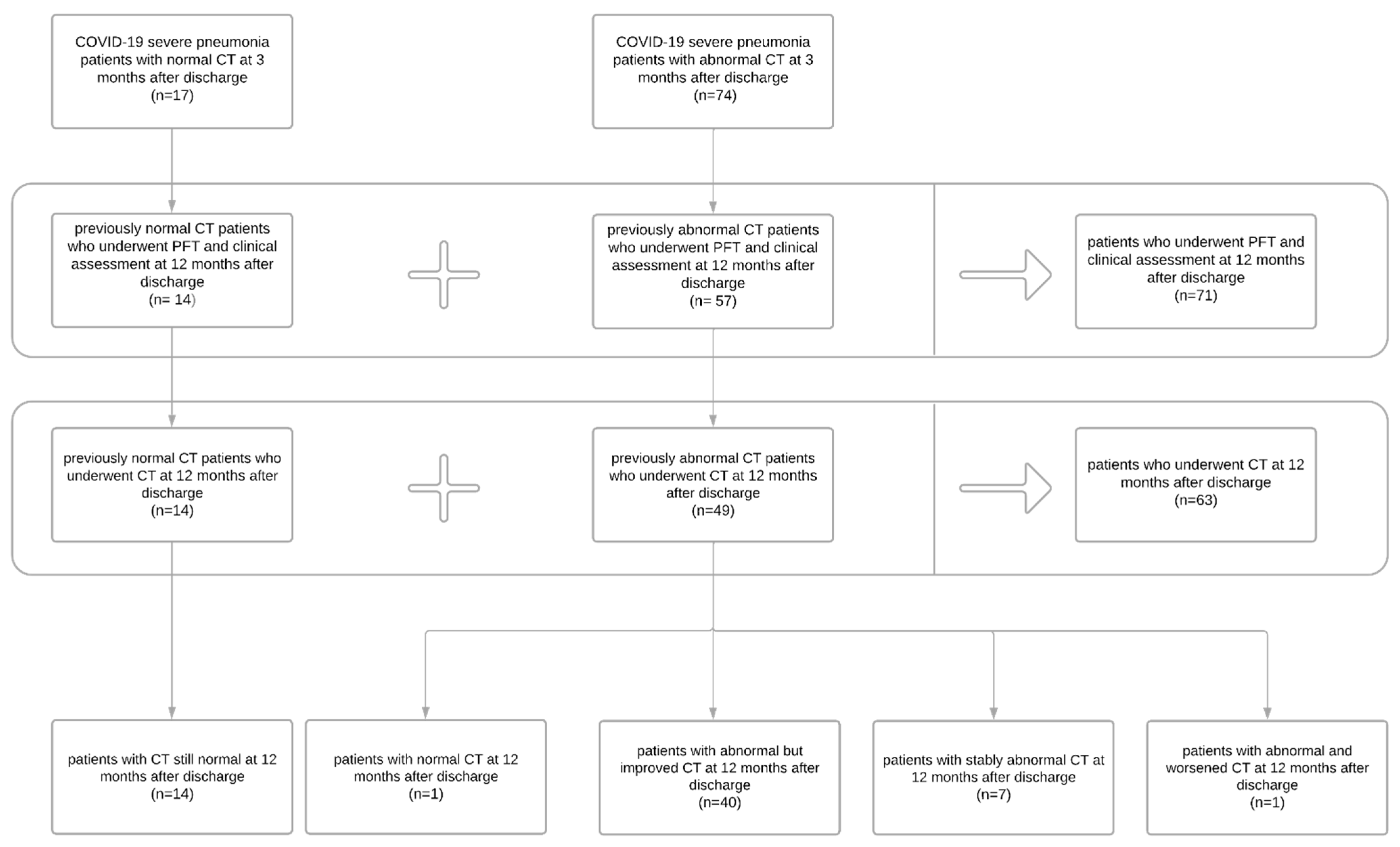
3.1. Patient Population
3.2. Clinical Assessment, Laboratory Data, and Pulmonary Function
3.3. Patients’ Reported Outcomes
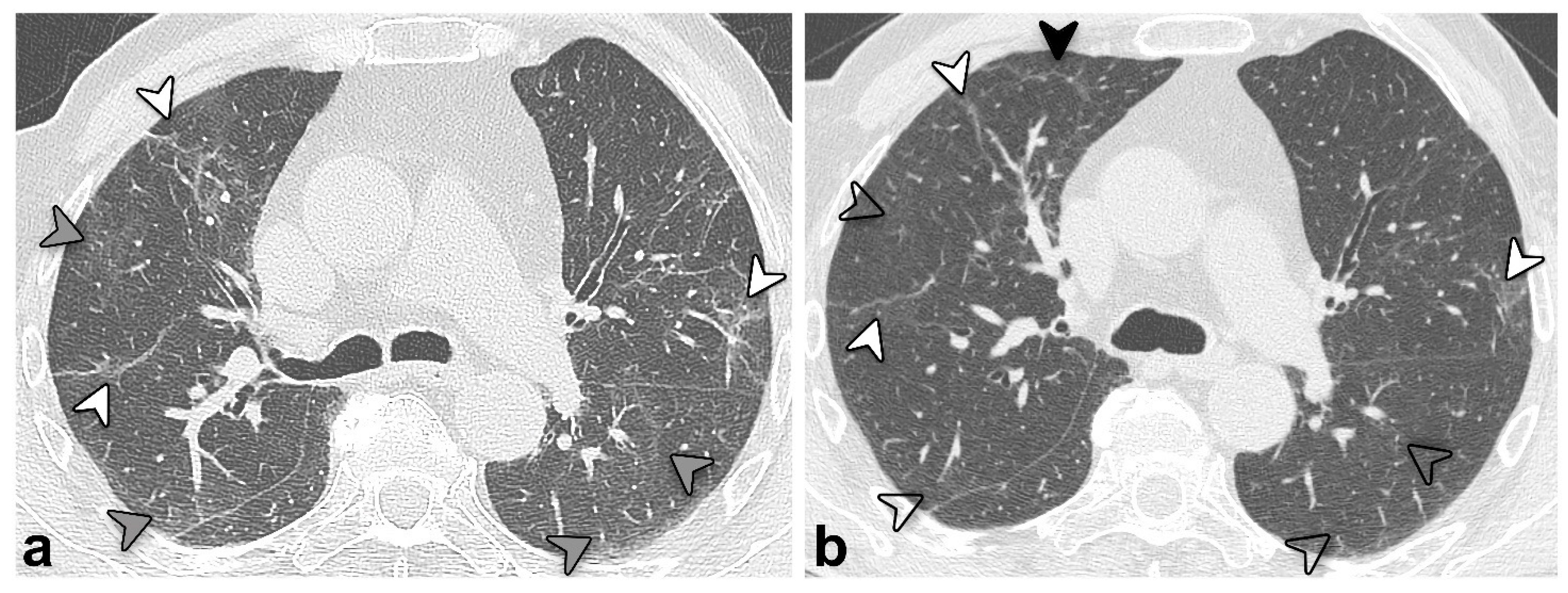
3.4. Chest CT Findings and Quantitative Analysis Results
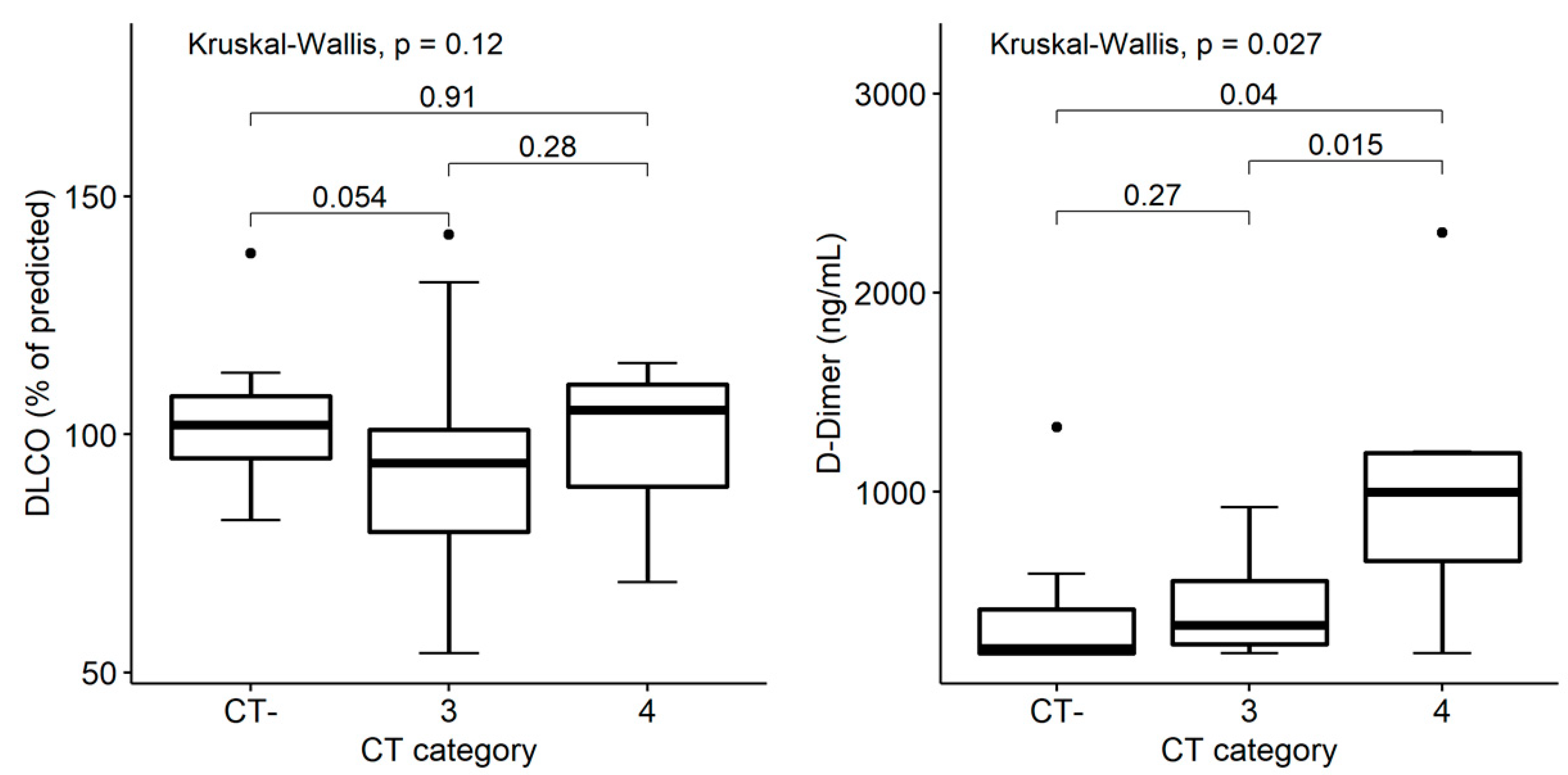
3.5. Structural and Functional Findings’ Association
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| DLCO | diffusing capacity for carbon monoxide |
| FEV1 | forced expiratory volume in the 1st second |
| FVC | vital capacity |
| GGO | ground-glass opacity |
| KCO | carbon monoxide transfer coefficient |
| mMRC | modified Medical Research Council |
| PFT | pulmonary function test |
| TLC | total lung capacity |
| VA | alveolar volume |
References
- WHO COVID-19 Dashboard. Geneva: World Health Organization. 2020. Available online: https://covid19.who.int/ (accessed on 3 October 2022).
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Tsagkaris, C.; Bilal, M.; Aktar, I.; Aboufandi, Y.; Tas, A.; Aborode, A.T.; Suvvari, T.K.; Ahmad, S.; Shkodina, A.; Phadke, R.; et al. Cytokine storm and neuropathological alterations in patients with neurological manifestations of COVID-19. Curr. Alzheimer Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Baratella, E.; Roman-Pognuz, E.; Zerbato, V.; Minelli, P.; Cavallaro, M.F.M.; Cova, M.A.; Luzzati, R.; Lucangelo, U.; Sanson, G.; Friso, F.; et al. Potential links between COVID-19-associated pulmonary aspergillosis and bronchiectasis as detected by high resolution computed tomography. Front. Biosci. 2021, 26, 1607–1612. [Google Scholar] [CrossRef]
- Marando, M.; Fusi-Schmidhauser, T.; Tamburello, A.; Gauthier, L.G.; Rigamonti, E.; Argentieri, G.; Puligheddu, C.; Pagnamenta, A.; Valenti, A.; Pons, M.; et al. 1-year radiological, functional and quality-of-life outcomes in patients with SARS-CoV-2 pneumonia—A prospective observational study. NPJ Prim. Care Respir. Med. 2022, 32, 8. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Prediletto, I.; Sintonen, H.; Scaramuzzo, G.; Allegri, D.; Colombo, D.; Tonetti, T.; Zani, G.; Capozzi, C.; et al. Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir. Med. 2021, 189, 106665. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID -19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Steinbeis, F.; Thibeault, C.; Doellinger, F.; Ring, R.M.; Mittermaier, M.; Ruwwe-Glösenkamp, C.; Alius, F.; Knape, P.; Meyer, H.-J.; Lippert, L.J.; et al. Severity of respiratory failure and computed chest tomography in acute COVID-19 correlates with pulmonary function and respiratory symptoms after infection with SARS-CoV-2: An observational longitudinal study over 12 months. Respir. Med. 2022, 191, 106709. [Google Scholar] [CrossRef]
- Yan, X.; Huang, H.; Wang, C.; Jin, Z.; Zhang, Z.; He, J.; Yin, S.; Fan, M.; Huang, J.; Chen, F.; et al. Follow-up study of pulmonary function among COVID-19 survivors 1 year after recovery. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Liu, M.; Lv, F.; Huang, Y.; Xiao, K. Follow-Up Study of the Chest CT Characteristics of COVID-19 Survivors Seven Months after Recovery. Front. Med. 2021, 8, 636298. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Parry, A.H.; Wani, A.H.; Shah, N.N.; Jehangir, M. Medium-term chest computed tomography (CT) follow-up of COVID-19 pneumonia patients after recovery to assess the rate of resolution and determine the potential predictors of persistent lung changes. Egypt. J. Radiol. Nucl. Med. 2021, 52, 55. [Google Scholar] [CrossRef]
- Balbi, M.; Conti, C.; Imeri, G.; Caroli, A.; Surace, A.; Corsi, A.; Mercanzin, E.; Arrigoni, A.; Villa, G.; Di Marco, F.; et al. Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur. J. Radiol. 2021, 138, 109676. [Google Scholar] [CrossRef]
- Besutti, G.; Monelli, F.; Schirò, S.; Milone, F.; Ottone, M.; Spaggiari, L.; Facciolongo, N.; Salvarani, C.; Croci, S.; Pattacini, P.; et al. Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study. Tomography 2022, 8, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. eClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Pan, F.; Yang, L.; Liang, B.; Ye, T.; Li, L.; Li, L.; Liu, D.; Wang, J.; Hesketh, R.L.; Zheng, C. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19. Radiology 2022, 302, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Tonkin, J.; Devaraj, A.; Philip, K.E.J.; Orton, C.M.; Desai, S.R.; Shah, P.L. CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge. Radiology 2022, 303, 444–454. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, C.; Yu, L.; Guo, W.; Feng, X.; Su, J.; Xu, T.; Ren, C.; Shi, D.; Wu, W.; et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-year pulmonary impairment after severe COVID-19: A prospective, multicenter follow-up study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef]
- Lorent, N.; Weygaerde, Y.V.; Claeys, E.; Fajardo, I.G.C.; De Vos, N.; De Wever, W.; Salhi, B.; Gyselinck, I.; Bosteels, C.; Lambrecht, B.N.; et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022, 8, 00004-2022. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Belletti, A.; Palumbo, D.; Calvi, M.R.; Guzzo, F.; Fominskiy, E.V.; Ortalda, A.; Nardelli, P.; Ripa, M.; Redaelli, M.B.; et al. One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Tao, M.; Shang, L.; Liu, Y.; Pan, G.; Jin, Y.; Wang, L.; Hu, S.; Li, J.; Zhang, M.; et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front. Med. 2021, 8, 717194. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Devaraj, A.; Desai, S.R. Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties. Radiology 2021, 299, E216–E218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance; 27 May 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- EuroQol Research Foundation. EQ-5D-3L User Guide. 2018. Available online: https://euroqol.org/publications/user-guides (accessed on 3 October 2022).
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Quirk, F.; Baveystock, C.M.; Littlejohns, P. A Self-complete Measure of Health Status for Chronic Airflow Limitation: The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 1992, 145, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.M.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Graham, B.L.; Cooper, B.; Thompson, B.R.; Carter, K.W.; Francis, R.W.; Hall, G. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur. Respir. J. 2017, 50, 1700010. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Tammeling, G.J.; Cotes, J.E.; Pedersen, O.F.; Peslin, R.; Yernault, J.-C. Lung volumes and forced ventilatory flows. Eur. Respir. J. 1993, 6 (Suppl. 16), 5–40. [Google Scholar] [CrossRef] [PubMed]
- sgrq-c. Available online: https://www.sgul.ac.uk/research/research-operations/research-administration/st-georges-respiratory-questionnaire/sgrq-c (accessed on 3 October 2022).
- Scalone, L.; Cortesi, P.A.; Ciampichini, R.; Cesana, G.; Mantovani, L.G. Health Related Quality of Life norm data of the Italian general population: Results using the EQ-5D-3L and EQ-5D-5L instruments. Epidemiol. Biostat. Public Health 2015, 12, e11457. [Google Scholar] [CrossRef]
- Chirico, F.; Nucera, G.; Szarpak, L. COVID-19 mortality in Italy: The first wave was more severe and deadly, but only in Lombardy region. J. Infect. 2021, 83, e16. [Google Scholar] [CrossRef]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Pan, F.; Li, L.; Yang, L.; Zheng, D.; Wang, J.; Liang, B. The pulmonary sequalae in discharged patients with COVID-19: A short-term observational study. Respir. Res. 2020, 21, 125. [Google Scholar] [CrossRef]
- Pan, Y.; Guan, H.; Zhou, S.; Wang, Y.; Li, Q.; Zhu, T.; Hu, Q.; Xia, L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): A study of 63 patients in Wuhan, China. Eur. Radiol. 2020, 30, 3306–3309. [Google Scholar] [CrossRef]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Zanini, U.; Bodini, B.D.; Corbetta, E.; Bini, F. Systemic consequences of COVID-19 infection in patients requiring mechanical ventilation: A 12-month prospective study. Infection 2022, 142, 1–3. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Karoli, R.; Gupta, N.; Shakya, S. Follow-up Study of Pulmonary Function, Exercise Capacity and Radiological Changes after Recovery from Moderate to Severe COVID Pneumonia without Mechanical Ventilation. J. Assoc. Physicians India 2022, 69, 11–12. [Google Scholar] [PubMed]
| No. | 71 |
|---|---|
| Age (years, range) | 66 [59–73] |
| Sex, F | 26 (37) |
| Smoking history (never/former/current) | 33 (46)/38 (54)/0 (0) |
| Comorbidities | |
| Any | 57 (81) |
| >2 | 19 (27) |
| Arterial hypertension | 42 (59) |
| Cardiovascular disease * | 14 (20) |
| Obesity ** | 20 (28) |
| Diabetes | 12 (17) |
| Dyslipidemia | 24 (34) |
| Chronic renal failure | 1 (1) |
| Neoplasia (active history) | 1 (1) |
| Rheumatic pathology | 5 (7) |
| Immunodepression | 3 (4) |
| Epilepsy | 1 (1) |
| Cirrhosis | 0 (0) |
| 3-Month FU | 12-Month FU | p | |
|---|---|---|---|
| Follow-up duration *, days | 105 [90–129] | 393 [379–409] | |
| Laboratory data | |||
| D-dimer (ng/mL) ** | 411 [244–749] | 335 [209–583] | 0.103 |
| CRP (mg/dL) | 0.2 [0.1–0.4] | --- | --- |
| WBC (109/L) | 6.55 [5.62–7.98] | 6.24 [5.27–7.57] | 0.029 |
| Lymphocytes (WBC %) | 32.9 [28.2–39.3] | 32.6 [27.8–38.4] | 0.498 |
| Lymphocytes (109/L) | 2.10 [1.71–2.82] | 2.04 [1.58–2.54] | 0.022 |
| <1—no./total no. (%) | 4/69 (6) | 6/71 (8) | 0.480 |
| Neutrophils (WBC %) | 55.5 [48.1–61.8] | 54.3 [48.5–59.4] | 0.195 |
| Neutrophils (109/L) | 3.61 [2.94–4.48] | 3.19 [2.49–4.37] | 0.021 |
| Symptoms | |||
| Asthenia | 31 (44) | 23 (32) | 0.201 |
| Dyspnea | 48 (68) | 41 (58) | 0.230 |
| mMRC dyspnea scale (0/1/2/3), no. (%) | 19 (27)/40 (56)/12 (17)/0 (0) | 25 (35)/33 (46)/10 (14)/3 (4) | 0.500 |
| Chest pain | 4 (6) | 4 (6) | 1.000 |
| Cough | 10 (14) | 13 (18) | 0.579 |
| Sputum | 2 (3) | 9 (13) | 0.046 |
| Patients’ reported outcomes | |||
| EQ5D—Mobility (1/2/3) | --- | 48 (70)/21 (30)/0 (0) | --- |
| EQ5D—Self-care (1/2/3) | --- | 65 (93)/4 (6)/1 (1) | --- |
| EQ5D—Activity(1/2/3) | --- | 50 (71)/20 (29)/0 (0) | --- |
| EQ5D—Pain (1/2/3) | --- | 39 (57)/27 (39)3 (4) | --- |
| EQ5D—Anxiety (1/2/3) | --- | 51 (73)/17 (24)/2 (3) | --- |
| EQ5D—Visual Analog Scale | --- | 80 [68–88] | --- |
| HADS—Anxiety | --- | 3 [1–5] | --- |
| >10—no./total no. (%) | 2/66 (3) | ||
| HADS—Depression | --- | 2 [1–4] | --- |
| >10—no./total no. (%) | 2/66 (3) | ||
| SGRQ—Symptoms | --- | 18.27 [7.80–35.34] | --- |
| SGRQ—Activity | --- | 47.58 [18.48–59.46] | --- |
| SGRQ—Impact | --- | 8.85 [0.00–20.86] | --- |
| SGRQ—Total | --- | 21.46 [11.34–33.90] | --- |
| Pulmonary function tests | |||
| FVC % predicted | 89 [79–99] | 97 [86–106] | <0.001 |
| <LLN—no. (%) | 11 (15) | 3 (4) | 0.027 |
| FEV1 % predicted | 91 [83–102] | 97 [88–107] | <0.001 |
| <LLN—no. (%) | 10 (14) | 3 (4) | 0.046 |
| FEV1/FVC % predicted | 104 [99–108] | 100 [97–105] | <0.001 |
| <LLN—no. (%) | 3 (4) | 2 (3) | 1.000 |
| VA % predicted | 92 [85–102] | 95 [84–103] | 0.079 |
| <LLN—no. (%) | 14 (20) | 7/68 (10) | 0.077 |
| DLCO % predicted | 85 [71–104] | 95 [82–105] | <0.001 |
| <LLN—no. (%) | 24 (34) | 5/68 (7) | <0.001 |
| KCO % predicted | 95 [81–103] | 101 [87–112] | <0.001 |
| <LLN—no. (%) | 11 (15) | 3/68 (4) | 0.013 |
| TLC % predicted | --- | 96 [83–104] | --- |
| <LLN—no. (%) | --- | 8/69 (12) | --- |
| 3-Month FU | 12-Month FU | p | |
|---|---|---|---|
| No. of abnormal chest CT scans | 49/63 (78%) | 48/63 (76%) * | 1.000 |
| % of compromised lung volume | 14.4 [10.6–21.2] | 12.3 [9.2–15.9] | <0.001 |
| Type of CT pattern | 0.038 | ||
| GGO | 8 (16) | 2 (4) | |
| Reticular | 31 (63) | 38 (79) | |
| Combined | 10 (21) | 8 (17) | |
| Consolidation | 2 (4) | 2 (4) | 1.000 |
| Architectural distortion | 45 (92) | 46 (96) | 1.000 |
| Bronchial dilatation | 44 (90) | 42 (88) | 0.683 |
| Emphysema | 7 (14) | 9 (19) | 0.617 |
| Cavitation | 1 (2) | 0 (0) | NA ** |
| Pleural effusion | 1 (2) | 1 (2) | 1.000 |
| Distribution | |||
| Bilateral | 47 (96) | 48 (100) | NA ** |
| Axial distribution (central/peripheral/neither) | 1 (2)/18 (37)/30 (61) | 0 (0)/28 (58)/20 (42) | NA ** |
| Cranio-caudal distribution (superior/medium/inferior/none) | 2 (4)/1 (2)/7 (14)/39 (80) | 1 (2)/6 (13)/12 (25)/29 (60) | 0.047 |
| RUL | 48 (98) | 45 (94) | 0.479 |
| ML | 46 (94) | 45 (94) | 1.000 |
| RLL | 46 (94) | 46 (96) | 1.000 |
| LUL | 43 (88) | 40 (83) | 0.617 |
| Lingula | 44 (90) | 43 (90) | 1.000 |
| LLL | 46 (94) | 47 (98) | 0.480 |
| Improved (Group 3), n = 40/47 | Stably Abnormal (Group 4), n = 7/47 | |||
|---|---|---|---|---|
| 3 Months | 12 Months | 3 Months | 12 Months | |
| Compromised lung volume % | 15.6 [11.0–21.7] | 12.0 [9.2–14.3] | 12.1 [10.9–20.5] | 16.6 [11.8–19.7] |
| Type of CT pattern | ||||
| GGO | 6 (15) | 2 (5) | 1 (14) | 0 (0) |
| Reticular | 28 (70) | 34 (85) | 2 (29) | 3 (43) |
| Combined GGO- reticular | 6 (15) | 4 (10) | 4 (57) | 4 (57) |
| Consolidation | 2 (5) | 2 (5) | 0 (0) | 0 (0) |
| Architectural distortion | 38 (95) | 38 (95) | 6 (86) | 7 (100) |
| Bronchial dilatation | 38 (95) | 35 (88) | 5 (71) | 6 (86) |
| Emphysema | 5 (13) | 6 (15) | 2 (29) | 3 (43) |
| Cavitation | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Pleural effusion | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| Distribution | ||||
| Bilateral | 39 (98) | 40 (100) | 6 (86) | 7 (100) |
| Axial distribution (central/peripheral/ neither) | 1 (3)/13 (32)/26 (65) | 0 (9)/24 (60)/16 (49) | 0 (0)/4 (57)/3 (43) | 0 (0)/3 (43)/4 (57) |
| Cranio-caudal distribution (superior/medium/inferior/none) | 1 (3)/0 (9)/7 (17)/32 (80) | 1 (3)/5 (12)/11 (2723(58) | 1 (14)/1 (14)/0 (0)/5 (72) | 0 (0)/1 (14)/1 (14)/5 (72) |
| RUL | 40 (100) | 38 (95) | 6 (86) | 6 (86) |
| ML | 38 (95) | 37 (93) | 6 (86) | 7 (100) |
| RLL | 39 (98) | 39 (98) | 5 (71) | 6 (86) |
| LUL | 36 (90) | 33 (83) | 5 (71) | 6 (86) |
| Lingula | 37 (93) | 33 (90) | 6 (86) | 6 (86) |
| LLL | 39 (98) | 40 (100) | 5 (71) | 6 (86) |
| CT- | CT+ | p | |
|---|---|---|---|
| n | 15 | 48 | |
| Laboratory data | |||
| D-dimer (ng/mL) ** | 213 [190–410] | 446 [260–596] | 0.136 |
| WBC (109/L) | 5.50 [4.52–6.63] | 6.27 [5.37–7.57] | 0.162 |
| Lymphocytes (WBC %) | 32.9 [31.0–39.5] | 32.0 [25.4–38.4] | 0.303 |
| Lymphocytes (109/L) | 2.06 [1.68–2.56] | 2.03 [1.47–2.53] | 0.810 |
| <1—no./total no. (%) | 1/14 (7) | 4/47 (9) | 1.000 |
| Neutrophils (WBC %) | 52.9 [49.0–57.5] | 56.1 [47.7–62.3] | 0.451 |
| Neutrophils (109/L) | 2.89 [2.36–3.17] | 3.34 [2.55–4.33] | 0.107 |
| Symptoms | |||
| Asthenia | 3 (21) | 16 (34) | 0.516 |
| Dyspnea | 10 (71) | 25 (53) | 0.356 |
| mMRC dyspnea scale (0/1/2/3), no. (%) | 4 (29)/6 (43)/3 (21)/1 (7) | 18 (38)/22 (47)/5 (11)/2 (4) | 0.564 |
| Chest pain | 1 (7) | 2 (4) | 0.550 |
| Cough | 4 (29) | 7 (14) | 0.256 |
| Sputum | 2/13 (15) | 5 (10) | 0.639 |
| Pulmonary function tests | |||
| FVC % predicted | 102 [91–111] | 96 [86–107] | 0.359 |
| <LLN—no. (%) | 1/14 (7) | 0/47 (0) | 0.230 |
| FEV1 % predicted | 100 [92–110] | 97 [88–107] | 0.619 |
| <LLN—no. (%) | 1/14 (7) | 0/47 (0) | 0.230 |
| FEV1/FVC % predicted | 100 [93–104] | 100 [98–106] | 0.375 |
| <LLN—no. (%) | 1/14 (7) | 0/47 (0) | 0.230 |
| VA % predicted | 103 [86–114] | 96 [87–102] | 0.245 |
| <LLN—no. (%) | 2/14 (14) | 3/45 (7) | 0.583 |
| DLCO % predicted | 102 [95–108] | 95 [80–104] | 0.085 |
| <LLN—no. (%) | 0/14 (0) | 4/45 (9) | 0.564 |
| KCO % predicted | 102 [91–114] | 99 [86–112] | 0.487 |
| <LLN—no. (%) | 0/14 (0) | 3/45 (7) | 1.000 |
| TLC % predicted | 105 [99–114] | 95 [82–102] | 0.005 |
| <LLN—no. (%) | 0/14 (0) | 7/45 (15) | 0.184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsi, A.; Caroli, A.; Bonaffini, P.A.; Conti, C.; Arrigoni, A.; Mercanzin, E.; Imeri, G.; Anelli, M.; Balbi, M.; Pace, M.; et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022, 8, 2588-2603. https://doi.org/10.3390/tomography8050216
Corsi A, Caroli A, Bonaffini PA, Conti C, Arrigoni A, Mercanzin E, Imeri G, Anelli M, Balbi M, Pace M, et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography. 2022; 8(5):2588-2603. https://doi.org/10.3390/tomography8050216
Chicago/Turabian StyleCorsi, Andrea, Anna Caroli, Pietro Andrea Bonaffini, Caterina Conti, Alberto Arrigoni, Elisa Mercanzin, Gianluca Imeri, Marisa Anelli, Maurizio Balbi, Marina Pace, and et al. 2022. "Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge" Tomography 8, no. 5: 2588-2603. https://doi.org/10.3390/tomography8050216
APA StyleCorsi, A., Caroli, A., Bonaffini, P. A., Conti, C., Arrigoni, A., Mercanzin, E., Imeri, G., Anelli, M., Balbi, M., Pace, M., Zanoletti, A., Capelli, M., Di Marco, F., & Sironi, S. (2022). Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography, 8(5), 2588-2603. https://doi.org/10.3390/tomography8050216







