18F-FES PET/CT Improves the Detection of Intraorbital Metastases in Estrogen-Receptor-Positive Breast Cancer: Two Representative Cases and Review of the Literature
Abstract
:1. Introduction
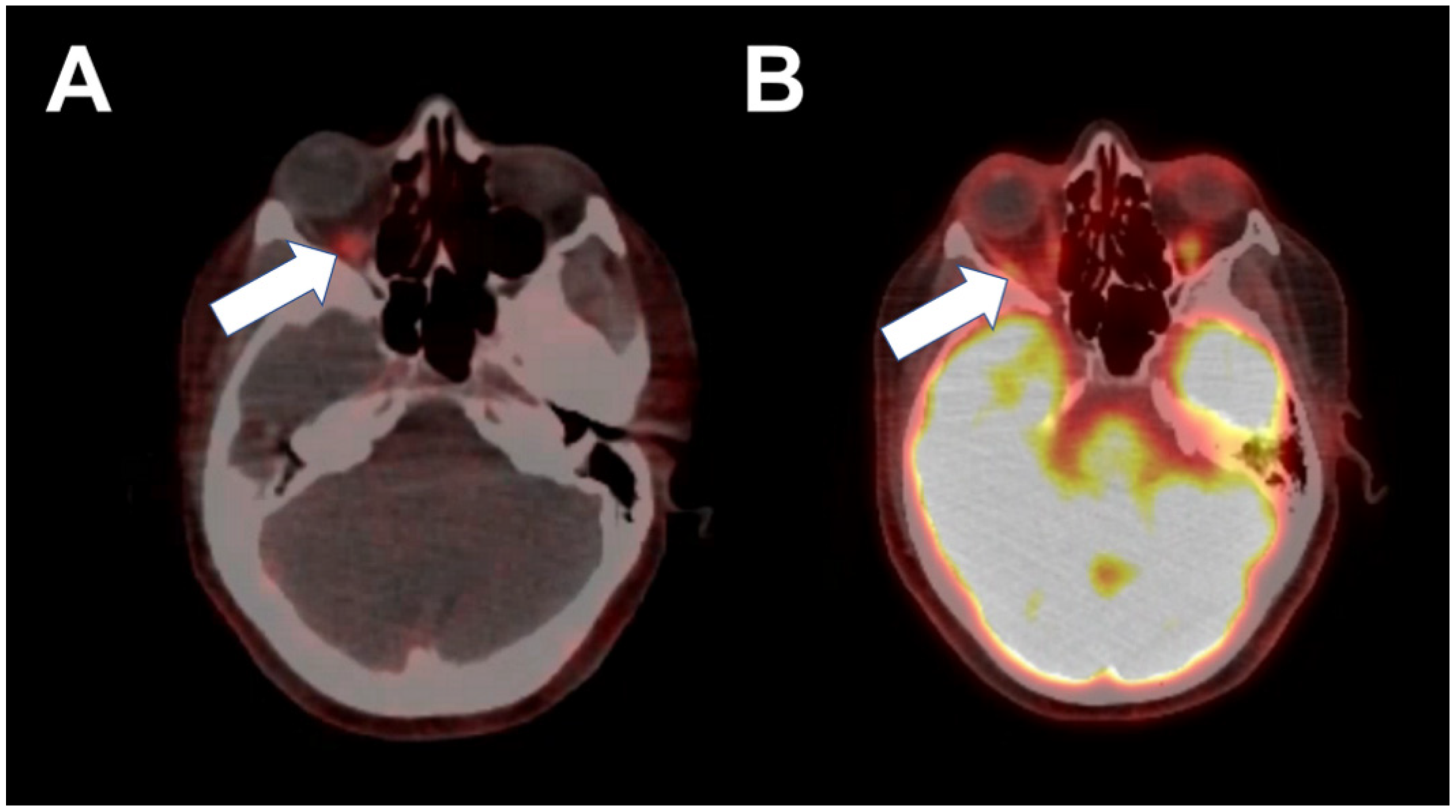
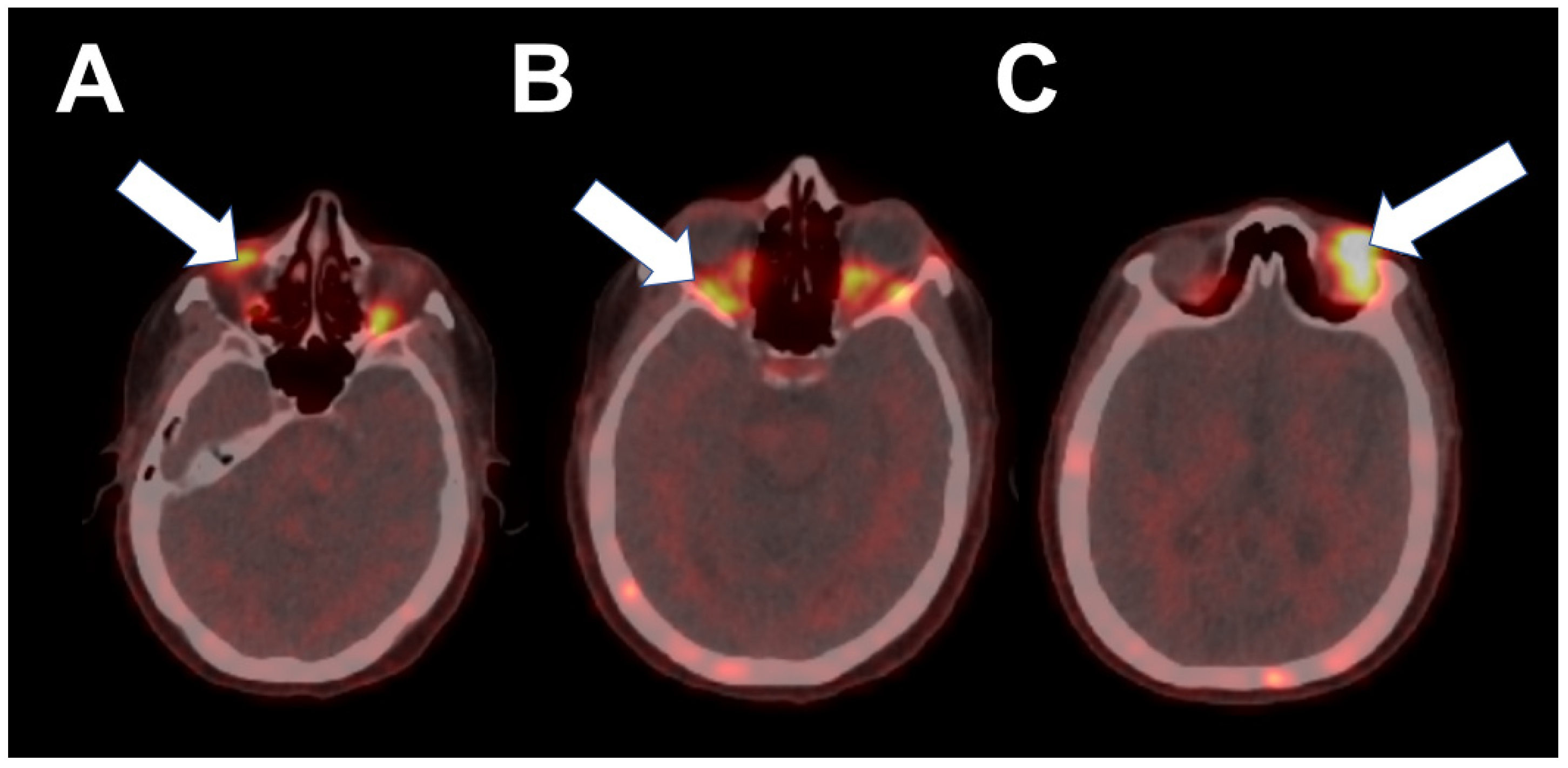
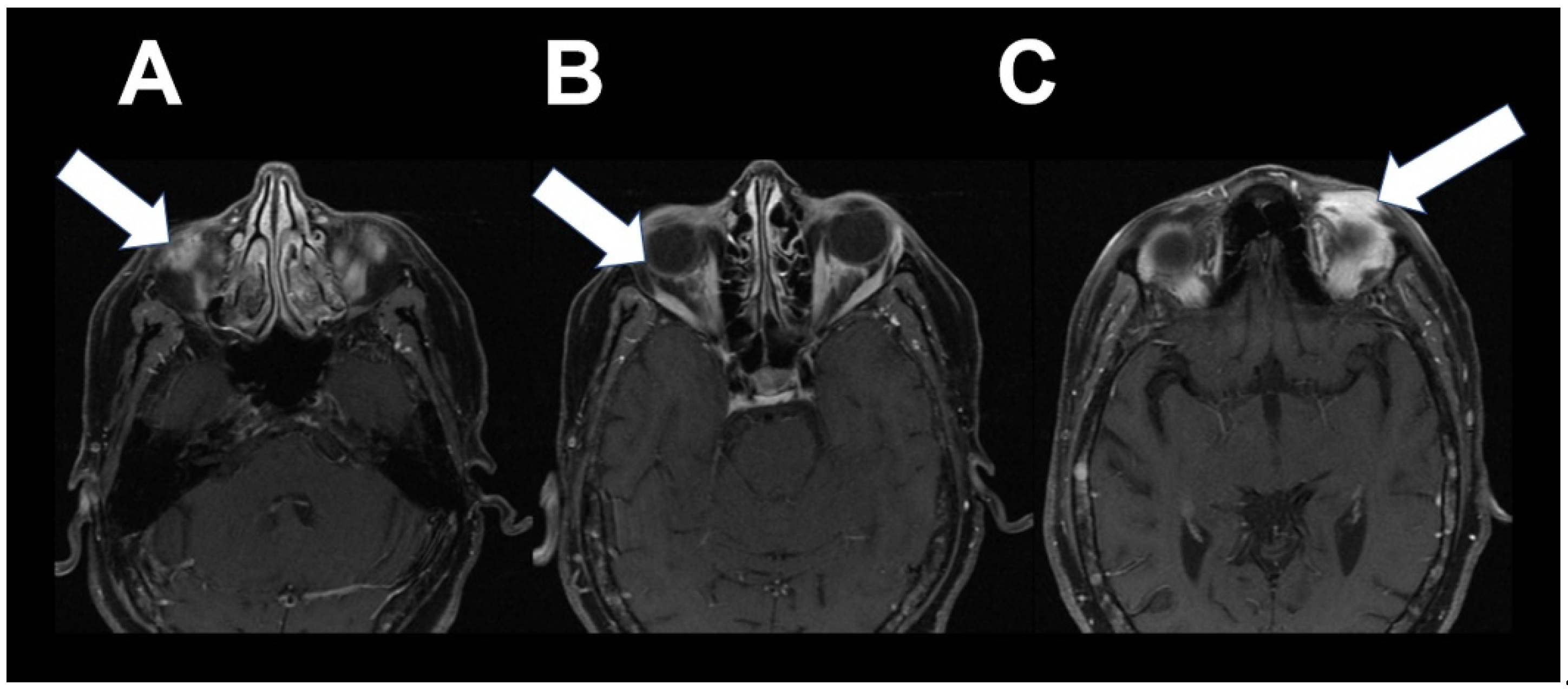
2. Case 1
3. Case 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antosz, Z.S.; Walocha, J.; Porȩba, R.; Sioma-Markowska, U. Sudden loss of vision due to breast cancer metastasis to the eyeball. Neuroendocr. Lett. 2014, 35, 249–251. [Google Scholar]
- Shields, J.A.; Shields, C.L.; Brotman, H.K.; Carvalho, C.; Perez, N.; Eagle, R.C. Cancer metastatic to the orbit: The 2000 Robert M. Curts lecture. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Vlachostergios, P.J.; Voutsadakis, I.A.; Papandreou, C.N. Orbital metastasis of breast carcinoma. Breast Cancer Basic Clin. Res. 2009, 3, 91–97. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Esmaeli, B. Metastatic tumors of the orbit and ocular adnexa. Curr. Opin. Ophthalmol. 2007, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J. Systemic Therapy for estrogen receptor–positive, HER2-negative breast cancer. N. Engl. J. Med. 2020, 383, 2557–2570. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Jhaveri, K.; Chandarlapaty, S.; Hatzoglou, V.; Riedl, C.C.; Lewis, J.S. Head-to-Head Evaluation of 18F-FES and 18F-FDG PET/CT in Metastatic Invasive Lobular Breast Cancer. J. Nucl. Med. 2021, 62, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.M.; Stekhova, S.A.; Link, J.M.; Gralow, J.R.; Livingston, R.B.; Ellis, G.K.; Petra, P.H.; Peterson, L.M.; Schubert, E.K.; Dunnwald, L.K.; et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J. Clin. Oncol. 2006, 24, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.S.; Ailianou, A.; Dulguerov, N.; Becker, C.D.; Ratib, O.; Becker, M. FDG-PET/CT pitfalls in oncological head and neck imaging. Insights Imaging 2014, 5, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Yang, Z.; Sun, Y.; Zhang, J.; Zheng, C.; Wang, L.; Zhang, Y.; Xue, J.; Yao, Z.; Pan, H.; et al. A preliminary study of 18F-FES PET/CT in predicting metastatic breast cancer in patients receiving docetaxel or fulvestrant with docetaxel. Sci. Rep. 2017, 7, 6584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, S.Y.; Ahn, S.H.; Kim, S.B.; Han, S.; Lee, S.H.; Oh, S.J.; Lee, S.J.; Kim, H.J.; Ko, B.S.; Lee, J.W.; et al. Diagnostic accuracy and safety of 16α-[ 18 F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: A prospective cohort study. Lancet Oncol. 2019, 20, 546–555. [Google Scholar] [CrossRef]
- Peterson, L.M.; Kurland, B.F.; Schubert, E.K.; Link, J.M.; Gadi, V.K.; Specht, J.M.; Eary, J.F.; Porter, P.; Shankar, L.K.; Mankoff, D.A.; et al. A phase 2 study of 16α-[18F]-fluoro-17β-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol. Imaging Biol. 2014, 16, 431–440. [Google Scholar] [CrossRef]
- Chae, S.Y.; Son, H.J.; Lee, D.Y.; Shin, E.; Oh, J.S.; Seo, S.Y.; Baek, S.; Kim, J.Y.; Na, S.J.; Moon, D.H. Comparison of diagnostic sensitivity of [18F]fluoroestradiol and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for breast cancer recurrence in patients with a history of estrogen receptor-positive primary breast cancer. EJNMMI Res. 2020, 10, 54. [Google Scholar] [CrossRef]
- Salem, K.; Kumar, M.; Kloepping, K.C.; Michel, C.J.; Yan, Y.; Fowler, A.M. Determination of binding affinity of molecular imaging agents for steroid hormone receptors in breast cancer. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 119–126. [Google Scholar] [PubMed]
- Linden, H.M.; Kurland, B.F.; Peterson, L.M.; Schubert, E.K.; Gralow, J.R.; Specht, J.M.; Ellis, G.K.; Lawton, T.J.; Livingston, R.B.; Petra, P.H.; et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin. Cancer Res. 2011, 17, 4799–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venema, C.M.; Mammatas, L.H.; Schröder, C.P.; Van Kruchten, M.; Apollonio, G.; Glaudemans, A.W.J.M.; Bongaerts, A.H.H.; Hoekstra, O.S.; Verheul, H.M.W.; Boven, E.; et al. Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J. Nucl. Med. 2017, 58, 1906–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seimbille, Y.; Rousseau, J.; Bénard, F.; Morin, C.; Ali, H.; Avvakumov, G.; Hammond, G.L.; van Lier, J.E. 18F-labeled difluoroestradiols: Preparation and preclinical evaluation as estrogen receptor-binding radiopharmaceuticals. Steroids 2002, 67, 765–775. [Google Scholar] [CrossRef]
- Huang, B.; Omoto, Y.; Iwase, H.; Yamashita, H.; Toyama, T.; Coombes, R.C.; Filipovic, A.; Warner, M.; Gustafsson, J.-Å. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 1933–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodapati, S.; Abraham, P.; Chen, A.; Guilbault, D.; McDonald, M.; Matro, J.; Shatsky, R.; Obrzut, S. 18F-FES PET/CT Improves the Detection of Intraorbital Metastases in Estrogen-Receptor-Positive Breast Cancer: Two Representative Cases and Review of the Literature. Tomography 2022, 8, 1060-1065. https://doi.org/10.3390/tomography8020086
Bodapati S, Abraham P, Chen A, Guilbault D, McDonald M, Matro J, Shatsky R, Obrzut S. 18F-FES PET/CT Improves the Detection of Intraorbital Metastases in Estrogen-Receptor-Positive Breast Cancer: Two Representative Cases and Review of the Literature. Tomography. 2022; 8(2):1060-1065. https://doi.org/10.3390/tomography8020086
Chicago/Turabian StyleBodapati, Sandhya, Peter Abraham, Angela Chen, Denise Guilbault, Marin McDonald, Jennifer Matro, Rebecca Shatsky, and Sebastian Obrzut. 2022. "18F-FES PET/CT Improves the Detection of Intraorbital Metastases in Estrogen-Receptor-Positive Breast Cancer: Two Representative Cases and Review of the Literature" Tomography 8, no. 2: 1060-1065. https://doi.org/10.3390/tomography8020086
APA StyleBodapati, S., Abraham, P., Chen, A., Guilbault, D., McDonald, M., Matro, J., Shatsky, R., & Obrzut, S. (2022). 18F-FES PET/CT Improves the Detection of Intraorbital Metastases in Estrogen-Receptor-Positive Breast Cancer: Two Representative Cases and Review of the Literature. Tomography, 8(2), 1060-1065. https://doi.org/10.3390/tomography8020086





