Different Prognostic Values of Dual-Time-Point FDG PET/CT Imaging Features According to Treatment Modality in Patients with Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
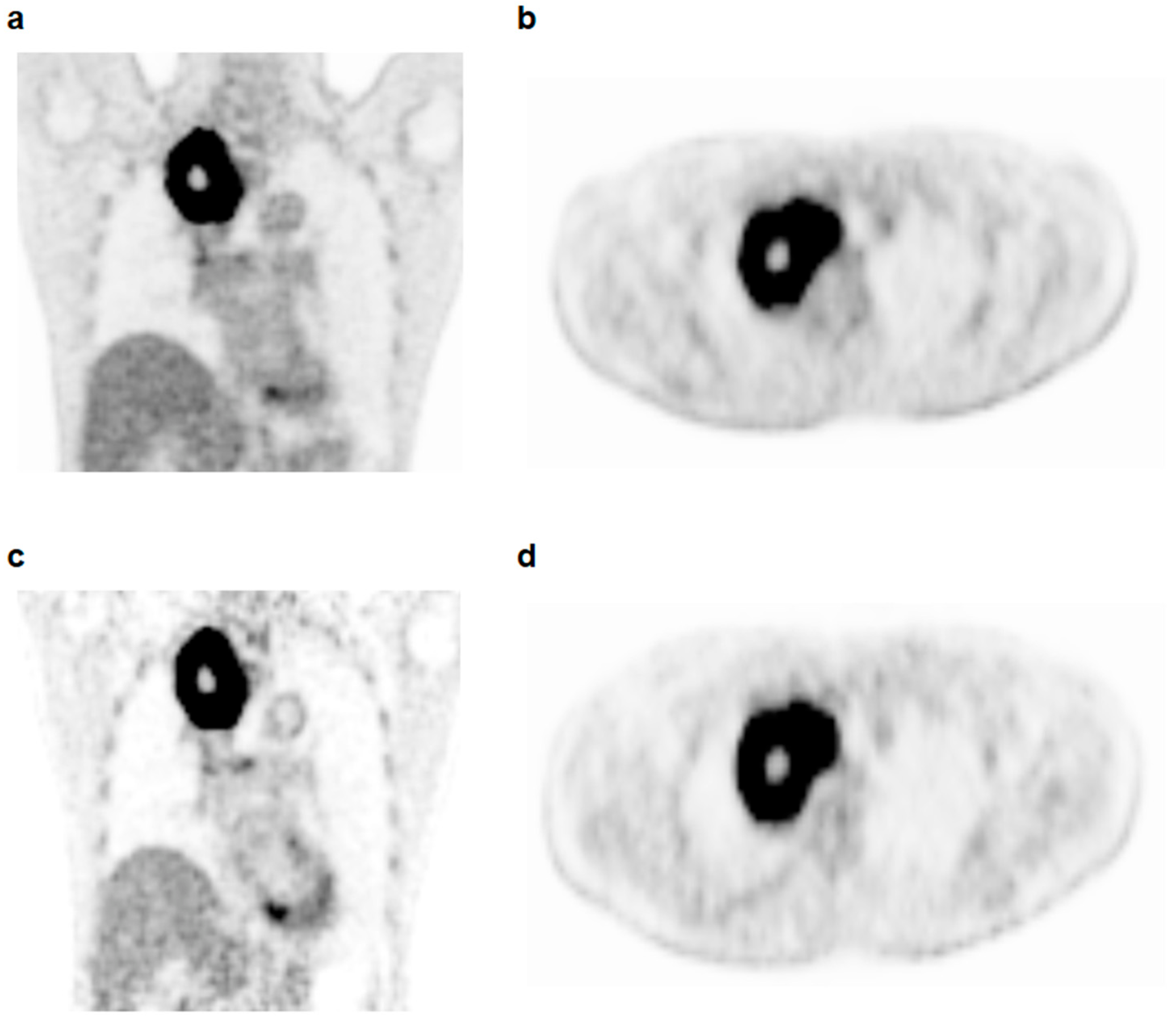
2.2. Dual-Time-Point FDG PET/CT
2.3. Imaging Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparisons of PET/CT Parameters
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, S.; Ma, Y.; Zhou, H.; Wu, X.; Han, J.; Hou, J.; Hao, L.; Spicer, J.D.; Koh, Y.W.; et al. Consistency of recommendations for the diagnosis and treatment of non-small cell lung cancer: A systematic review. Transl. Lung Cancer Res. 2021, 10, 2715–2732. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; McMurry, T.L.; Stukenborg, G.J.; Francescatti, A.B.; Amato-Martz, C.; Schumacher, J.R.; Chang, G.J.; Greenberg, C.C.; Winchester, D.P.; McKellar, D.P.; et al. Impact of age and comorbidity on treatment of non-small cell lung cancer recurrence following complete resection: A nationally representative cohort study. Lung Cancer 2016, 102, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Seung, S.J.; Hurry, M.; Walton, R.N.; Evans, W.K. Retrospective cohort study of unresectable stage III non-small-cell lung cancer in Canada. Curr. Oncol. 2020, 27, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Ball, D.; Jett, J.R.; Le Chevalier, T.; Lim, E.; Nicholson, A.G.; Shepherd, F.A. Non-small-cell lung cancer. Lancet 2011, 378, 1727–1740. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Ghaderi, N.; Jung, J.H.; Odde, D.J.; Peacock, J. Clinically validated model predicts the effect of intratumoral heterogeneity on overall survival for non-small cell lung cancer (NSCLC) patients. Comput. Methods Programs Biomed. 2021, 212, 106455. [Google Scholar] [CrossRef]
- Everitt, S.; Ball, D.; Hicks, R.J.; Callahan, J.; Plumridge, N.; Trinh, J.; Herschtal, A.; Kron, T.; Mac Manus, M. Prospective Study of Serial Imaging Comparing Fluorodeoxyglucose Positron Emission Tomography (PET) and Fluorothymidine PET During Radical Chemoradiation for Non-Small Cell Lung Cancer: Reduction of Detectable Proliferation Associated with Worse Survival. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 947–955. [Google Scholar] [CrossRef]
- Salem, A.; Mistry, H.; Backen, A.; Hodgson, C.; Koh, P.; Dean, E.; Priest, L.; Haslett, K.; Trigonis, I.; Jackson, A.; et al. Cell Death, Inflammation, Tumor Burden, and Proliferation Blood Biomarkers Predict Lung Cancer Radiotherapy Response and Correlate With Tumor Volume and Proliferation Imaging. Clin. Lung Cancer 2018, 19, 239–248. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Y.; Yang, B.; Wang, Y. A meta-analysis to evaluate the diagnostic value of dual-time-point F-fluorodeoxyglucose positron emission tomography/computed tomography for diagnosis of pulmonary nodules. J. Cancer Res. Ther. 2016, 12, 304–308. [Google Scholar] [CrossRef]
- Lee, J.W.; Seo, K.H.; Kim, E.S.; Lee, S.M. The role of (18)F-fluorodeoxyglucose uptake of bone marrow on PET/CT in predicting clinical outcomes in non-small cell lung cancer patients treated with chemoradiotherapy. Eur. Radiol. 2017, 27, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.E.; Huang, Y.J.; Ko, M.; Hsu, C.C.; Chen, C.F. Dual-time-point (18)F-FDG PET/CT in the diagnosis of solitary pulmonary lesions in a region with endemic granulomatous diseases. Ann. Nucl. Med. 2016, 30, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Chen, M.; Yin, Y.; Li, N.; Li, Y. Limited diagnostic value of Dual-Time-Point (18)F-FDG PET/CT imaging for classifying solitary pulmonary nodules in granuloma-endemic regions both at visual and quantitative analyses. Eur. J. Radiol. 2016, 85, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Jinguji, M.; Aoki, M.; Tani, A.; Sato, M.; Yoshiura, T. The clinical value of texture analysis of dual-time-point (18)F-FDG-PET/CT imaging to differentiate between (18)F-FDG-avid benign and malignant pulmonary lesions. Eur. Radiol. 2020, 30, 1759–1769. [Google Scholar] [CrossRef]
- Grisanti, F.; Zulueta, J.; Rosales, J.J.; Morales, M.I.; Sancho, L.; Lozano, M.D.; Mesa-Guzmán, M.; García-Velloso, M.J. Diagnostic accuracy of visual analysis versus dual time-point imaging with (18)F-FDG PET/CT for the characterization of indeterminate pulmonary nodules with low uptake. Rev. Esp. Med. Nucl. Imagen Mol. 2021, 40, 155–160. [Google Scholar] [CrossRef]
- Okazaki, E.; Seura, H.; Hasegawa, Y.; Okamura, T.; Fukuda, H. Prognostic Value of the Volumetric Parameters of Dual-Time-Point (18)F-FDG PET/CT in Non-Small Cell Lung Cancer Treated With Definitive Radiation Therapy. Am. J. Roentgenol. 2019, 213, 1366–1373. [Google Scholar] [CrossRef]
- Jin, F.; Zhu, H.; Fu, Z.; Kong, L.; Yu, J. Prognostic value of the standardized uptake value maximum change calculated by dual-time-point (18)F-fluorodeoxyglucose positron emission tomography imaging in patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2016, 9, 2993–2999. [Google Scholar] [CrossRef]
- Satoh, Y.; Nambu, A.; Onishi, H.; Sawada, E.; Tominaga, L.; Kuriyama, K.; Komiyama, T.; Marino, K.; Aoki, S.; Araya, M.; et al. Value of dual time point F-18 FDG-PET/CT imaging for the evaluation of prognosis and risk factors for recurrence in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Eur. J. Radiol. 2012, 81, 3530–3534. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.K.; Kim, I.J.; Kim, Y.D.; Lee, M.K. Limited prognostic value of dual time point F-18 FDG PET/CT in patients with early stage (stage I & II) non-small cell lung cancer (NSCLC). Radiother. Oncol. 2011, 98, 105–108. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, S.H.; Ahn, H.; Lee, S.M.; Jang, S.J. Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT. Cancers 2021, 13, 3563. [Google Scholar] [CrossRef] [PubMed]
- Nestle, U.; Kremp, S.; Schaefer-Schuler, A.; Sebastian-Welsch, C.; Hellwig, D.; Rübe, C.; Kirsch, C.M. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J. Nucl. Med. 2005, 46, 1342–1348. [Google Scholar] [PubMed]
- Maisonobe, J.A.; Garcia, C.A.; Necib, H.; Vanderlinden, B.; Hendlisz, A.; Flamen, P.; Buvat, I. Comparison of PET metabolic indices for the early assessment of tumour response in metastatic colorectal cancer patients treated by polychemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Mamede, M.H.; Wada, M.; Doi, R.; Hosotani, R.; Imamura, M.; Konishi, J. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J. Nucl. Med. 2002, 43, 173–180. [Google Scholar] [PubMed]
- Demura, Y.; Tsuchida, T.; Ishizaki, T.; Mizuno, S.; Totani, Y.; Ameshima, S.; Miyamori, I.; Sasaki, M.; Yonekura, Y. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J. Nucl. Med. 2003, 44, 540–548. [Google Scholar] [PubMed]
- Houseni, M.; Chamroonrat, W.; Zhuang, J.; Gopal, R.; Alavi, A.; Zhuang, H. Prognostic implication of dual-phase PET in adenocarcinoma of the lung. J. Nucl. Med. 2010, 51, 535–542. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Okita, R.; Saisho, S.; Yukawa, T.; Maeda, A.; Nojima, Y.; Nakata, M. Clinical significance of dual-time-point 18F-FDG PET imaging in resectable non-small cell lung cancer. Ann. Nucl. Med. 2015, 29, 854–860. [Google Scholar] [CrossRef]
- Chen, H.H.; Lee, B.F.; Su, W.C.; Lai, Y.H.; Chen, H.Y.; Guo, H.R.; Yao, W.J.; Chiu, N.T. The increment in standardized uptake value determined using dual-phase 18F-FDG PET is a promising prognostic factor in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1478–1485. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, T.F.; Chu, S.C.; Lin, C.B.; Wang, L.Y.; Lue, K.H.; Liu, S.H.; Chan, S.C. Incorporating radiomic feature of pretreatment 18F-FDG PET improves survival stratification in patients with EGFR-mutated lung adenocarcinoma. PLoS ONE 2020, 15, e0244502. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Lee, H.J.; Heo, N.H.; Lee, S.M. [(18)F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020, 10, 72. [Google Scholar] [CrossRef]
- Tabchi, S.; Kassouf, E.; Rassy, E.E.; Kourie, H.R.; Martin, J.; Campeau, M.P.; Tehfe, M.; Blais, N. Management of stage III non-small cell lung cancer. Semin. Oncol. 2017, 44, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef]
- Im, H.J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All Patients (n = 190) | Surgery Group (n = 121) | CRT Group (n = 69) | p-Value |
|---|---|---|---|---|
| Age (years) * | 67 (40–86) | 66 (42–85) | 70 (40–86) | 0.065 |
| Sex | 0.099 | |||
| Men | 135 (71.1%) | 81 (66.9%) | 54 (78.3%) | |
| Women | 55 (28.9%) | 40 (33.1%) | 15 (21.7%) | |
| Smoking history | 0.014 | |||
| Yes | 127 (67.2%) | 73 (60.8%) | 54 (78.3%) | |
| No | 62 (32.8%) | 47 (39.2%) | 15 (21.7%) | |
| Histopathology | 0.523 | |||
| Adenocarcinoma | 113 (59.5%) | 75 (62.0%) | 38 (55.1%) | |
| Squamous cell | 73 (38.4%) | 43 (35.5%) | 30 (43.5%) | |
| Others | 4 (2.1%) | 3 (2.5%) | 1 (1.4%) | |
| Tumor location | 0.911 | |||
| RUL/RML | 74 (38.9%) | 45 (37.2%) | 29 (42.0%) | |
| RLL | 45 (23.7%) | 30 (24.8%) | 15 (21.7%) | |
| LUL | 44 (23.2%) | 29 (24.0%) | 15 (21.7%) | |
| LLL | 27 (14.2%) | 17 (14.0%) | 10 (14.5%) | |
| T stage | <0.001 | |||
| T1–T2 | 143 (75.3%) | 110 (90.9%) | 33 (47.8%) | |
| T3–T4 | 47 (24.7%) | 11 (9.1%) | 36 (52.2%) | |
| N stage | <0.001 | |||
| N0 | 110 (57.9%) | 91 (75.2%) | 19 (27.5%) | |
| N1 | 27 (14.2%) | 23 (19.0%) | 4 (5.8%) | |
| N2-3 | 53 (27.9%) | 7 (5.8%) | 46 (66.7%) | |
| TNM stage | <0.001 | |||
| Stage I | 82 (43.2%) | 78 (64.5%) | 4 (5.8%) | |
| Stage II | 40 (21.1%) | 29 (24.0%) | 11 (15.9%) | |
| Stage III | 68 (35.8%) | 14 (11.6%) | 54 (78.3%) | |
| Treatment | ||||
| Wedge resection | 20 (10.5%) | 20 (16.5%) | - | |
| Lobectomy | 93 (48.9%) | 93 (76.9%) | - | |
| Bilobectomy/pneumonectomy | 8 (4.2%) | 8 (6.6%) | - | |
| Concurrent chemoradiation | 41 (21.6%) | - | 41 (59.4%) | |
| Chemotherapy alone | 17 (8.9%) | - | 17 (24.6%) | |
| Radiotherapy alone | 11 (5.8%) | - | 11 (15.9%) |
| PET Parameters | Early PET | Delayed PET | p-Value | No. of Patients with the Percent Change of the Parameter (ΔPET Parameter) > 0 (%) |
|---|---|---|---|---|
| Maximum SUV | 13.6 (2.6–50.3) | 18.3 (2.3–64.0) | <0.001 | 181 (95.3%) |
| Mean SUV | 8.0 (2.5–24.1) | 9.7 (2.0–31.1) | <0.001 | 158 (83.2%) |
| MTV | 5.0 (0.6–166.4) | 6.7 (0.6–179.4) | <0.001 | 173 (91.1%) |
| TLG | 37.7 (1.5–2090.0) | 51.2 (1.5–2796.9) | <0.001 | 189 (99.5%) |
| Skewness | 0.68 (−0.58–2.04) | 0.74 (−0.17–2.64) | 0.034 | 106 (55.8%) |
| Kurtosis | 2.69 (1.00–6.88) | 2.76 (1.45–12.08) | 0.123 | 97 (51.1%) |
| Entropy | 3.91 (0.59–5.21) | 4.12 (0.44–5.29) | <0.001 | 138 (72.6%) |
| Energy | 0.07 (0.01–0.76) | 0.05 (0.01–0.83) | <0.001 | 14 (7.4%) |
| Variables | Surgery Group | CRT Group | |||
|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | ||
| Age (1-year increase) | 0.366 | 1.018 (0.980–1.057) | 0.562 | 1.007 (0.984–1.029) | |
| Sex (women vs. men) | 0.084 | 1.997 (0.912–4.374) | 0.297 | 1.425 (0.733–2.773) | |
| Smoking history (no vs. yes) | 0.045 | 2.104 (1.017–4.352) | 0.239 | 1.466 (0.775–2.774) | |
| Histopathology (adenocarcinoma vs.) | Squamous cell | 0.375 | 0.960 (0.254–1.984) | 0.455 | 0.819 (0.485–1.383) |
| Others | 0.417 | 1.842 (0.421–8.055) | 0.427 | 1.112 (0.774–2824) | |
| T stage (T1–T2 vs.) | T3–T4 | 0.048 | 2.62 (1.006–6.819) | 0.049 | 1.724 (1.003–2.965) |
| N stage (N0 vs.) | N1 | 0.003 | 2.913 (1.428–5.923) | 0.006 | 2.538 (1.320–4.882) |
| N2–N3 | 0.022 | 3.499 (1.195–10.251) | 0.005 | 5.149 (1.612–16.445) | |
| TNM stage (stage I vs.) | Stage II | 0.013 | 2.493 (1.209–5.141) | 0.225 | 2.656 (0.549–2.845) |
| Stage III | 0.003 | 3.963 (1.621–9.691) | 0.010 | 4.591 (1.470–19.708) | |
| Early PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.003 | 1.044 (1.015–1.073) | 0.007 | 1.048 (1.013–1.084) |
| Mean SUV | 0.002 | 1.105 (1.039–1.176) | 0.006 | 1.105 (1.028–1.187) | |
| MTV | <0.001 | 1.040 (1.021–1.059) | 0.006 | 1.010 (1.003–1.017) | |
| TLG | <0.001 | 1.003 (1.002–1.005) | 0.004 | 1.001 (1.000–1.001) | |
| Skewness | 0.331 | 0.679 (0.311–1.482) | 0.737 | 1.160 (0.488–2.759) | |
| Kurtosis | 0.612 | 1.083 (0.797–1.471) | 0.433 | 1.127 (0.836–1.519) | |
| Entropy | <0.001 | 1.846 (1.295–2.632) | 0.235 | 1.223 (0.877–1.707) | |
| Energy * | 0.017 | 0.604 (0.400–0.913) | 0.067 | 0.556 (0.311–1.331) | |
| Delayed PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.002 | 1.035 (1.012–1.058) | 0.002 | 1.042 (1.016–1.069) |
| Mean SUV | 0.004 | 1.072 (1.023–1.125) | 0.006 | 1.077 (1.021–1.137) | |
| MTV | <0.001 | 1.034 (1.012–1.051) | 0.228 | 1.004 (0.998–1.010) | |
| TLG | <0.001 | 1.003 (1.001–1.004) | 0.168 | 1.000 (0.999–1.001) | |
| Skewness | 0.514 | 0.750 (0.316–1.778) | 0.759 | 1.136 (0.504–2.561) | |
| Kurtosis | 0.139 | 1.187 (0.946–1.491) | 0.317 | 1.175 (0.857–1.612) | |
| Entropy | 0.005 | 1.766 (1.851–2.631) | 0.990 | 0.999 (0.708–1.412) | |
| Energy * | 0.008 | 0.374 (0.181–0.776) | 0.032 | 0.385 (0.161–0.923) | |
| ΔPET parameters (for 1.0 increase in the parameter value) | ΔMaximum SUV | 0.294 | 1.006 (0.995–1.018) | 0.333 | 1.018 (0.989–1.035) |
| ΔMean SUV | 0.300 | 1.001 (0.995–1.005) | 0.384 | 1.028 (0.981–1.055) | |
| ΔMTV | 0.389 | 0.999 (0.997–1.002) | 0.002 | 0.989 (0.983–0.996) | |
| ΔTLG | 0.508 | 0.999 (0.997–1.001) | 0.002 | 0.989 (0.982–0.996) | |
| ΔSkewness | 0.239 | 0.999 (0.998–1.001) | 0.543 | 0.999 (0.994–1.003) | |
| ΔKurtosis | 0.841 | 1.000 (0.993–1.008) | 0.686 | 0.998 (0.989–1.007) | |
| ΔEntropy | 0.161 | 0.993 (0.983–1.003) | 0.033 | 0.982 (0.965–0.999) | |
| ΔEnergy | 0.946 | 1.000 (0.987–1.015) | 0.783 | 0.996 (0.970–1.023) | |
| Variables | p-Value | Hazard Ratio (95% Confidence Interval) | |
|---|---|---|---|
| Early PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.260 | - |
| Mean SUV | 0.191 | - | |
| MTV | 0.022 | 1.025 (1.008–1.049) | |
| TLG | 0.024 | 1.002 (1.001–1.003) | |
| Entropy | 0.039 | 1.559 (1.076–2.259) | |
| Energy * | 0.177 | - | |
| Delayed PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.117 | - |
| Mean SUV | 0.149 | - | |
| MTV | 0.016 | 1.024 (1.005–1.044) | |
| TLG | 0.008 | 1.002 (1.001–1.004) | |
| Entropy | 0.040 | 1.436 (1.002–2.171) | |
| Energy * | 0.115 | - | |
| Variables | p-Value | Hazard Ratio (95% Confidence Interval) | |
|---|---|---|---|
| Early PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.051 | - |
| Mean SUV | 0.087 | - | |
| MTV | 0.073 | - | |
| TLG | 0.029 | 1.001 (1.000–1.001) | |
| Delayed PET parameters (for 1.0 increase in the parameter value) | Maximum SUV | 0.025 | 1.123 (1.015–1.242) |
| Mean SUV | 0.190 | - | |
| Energy * | 0.492 | - | |
| ΔPET parameters (for 1.0 increase in the parameter value) | ΔMTV | 0.010 | 0.991 (0.984–0.998) |
| ΔTLG | 0.007 | 0.991 (0.984–0.998) | |
| ΔEntropy | 0.289 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.J.; Lee, J.W.; Lee, J.-H.; Jo, I.Y.; Lee, S.M. Different Prognostic Values of Dual-Time-Point FDG PET/CT Imaging Features According to Treatment Modality in Patients with Non-Small Cell Lung Cancer. Tomography 2022, 8, 1066-1078. https://doi.org/10.3390/tomography8020087
Jang SJ, Lee JW, Lee J-H, Jo IY, Lee SM. Different Prognostic Values of Dual-Time-Point FDG PET/CT Imaging Features According to Treatment Modality in Patients with Non-Small Cell Lung Cancer. Tomography. 2022; 8(2):1066-1078. https://doi.org/10.3390/tomography8020087
Chicago/Turabian StyleJang, Su Jin, Jeong Won Lee, Ji-Hyun Lee, In Young Jo, and Sang Mi Lee. 2022. "Different Prognostic Values of Dual-Time-Point FDG PET/CT Imaging Features According to Treatment Modality in Patients with Non-Small Cell Lung Cancer" Tomography 8, no. 2: 1066-1078. https://doi.org/10.3390/tomography8020087
APA StyleJang, S. J., Lee, J. W., Lee, J.-H., Jo, I. Y., & Lee, S. M. (2022). Different Prognostic Values of Dual-Time-Point FDG PET/CT Imaging Features According to Treatment Modality in Patients with Non-Small Cell Lung Cancer. Tomography, 8(2), 1066-1078. https://doi.org/10.3390/tomography8020087






