Phantom Validation of a Conservation of Activity-Based Partial Volume Correction Method for Arterial Input Function in Dynamic PET Imaging
Abstract
1. Introduction
2. Methods
2.1. Partial Volume Effect Correction via Conservation of Activity
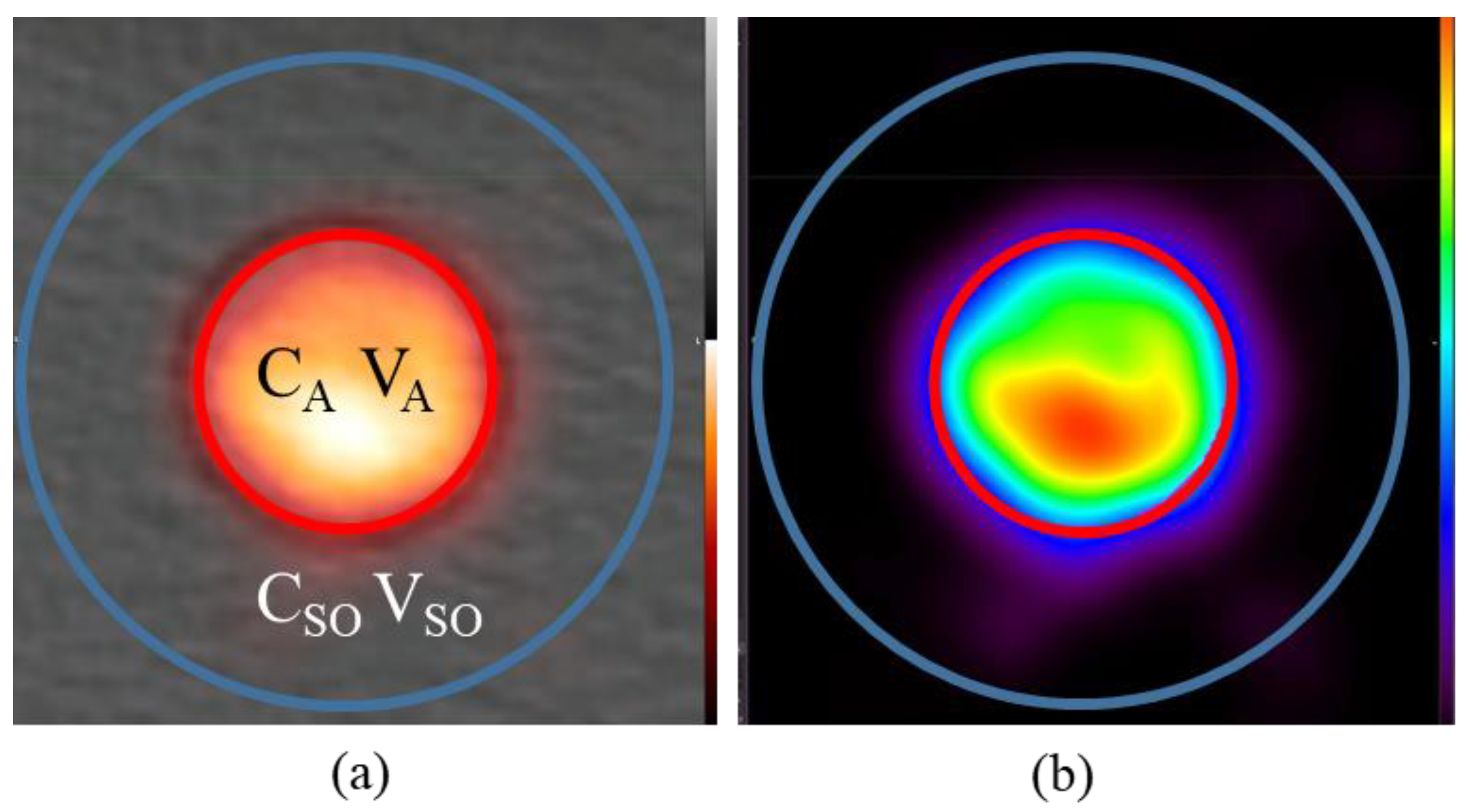
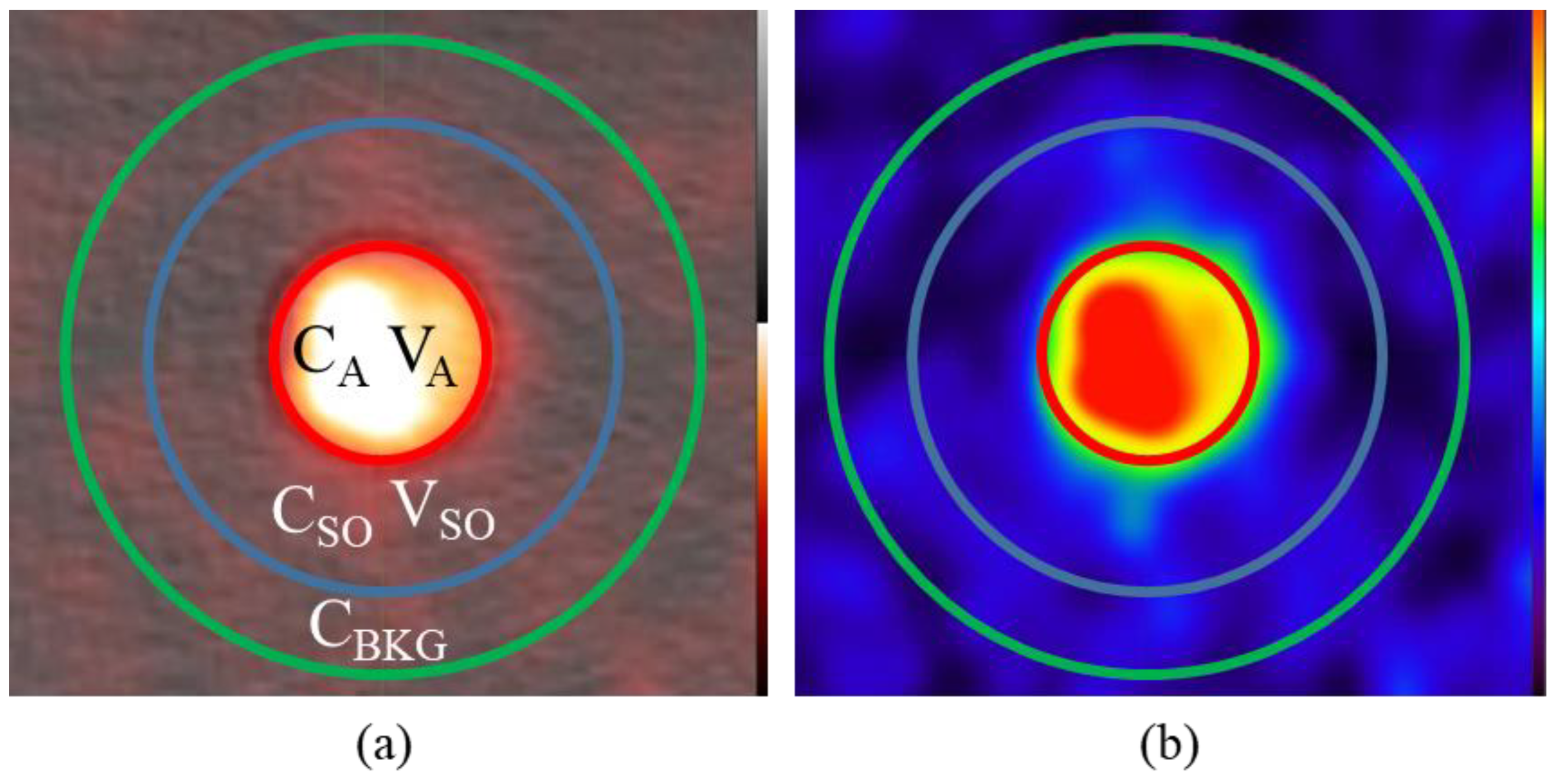
2.2. Phantom Design
2.3. Experimental Setup and Validation in CT
2.4. PET-CT Experiments
2.5. Image Analysis and Partial Volume Correction
2.6. Application of the Input Function Correction Model to Sample Patient Image Sets
3. Results
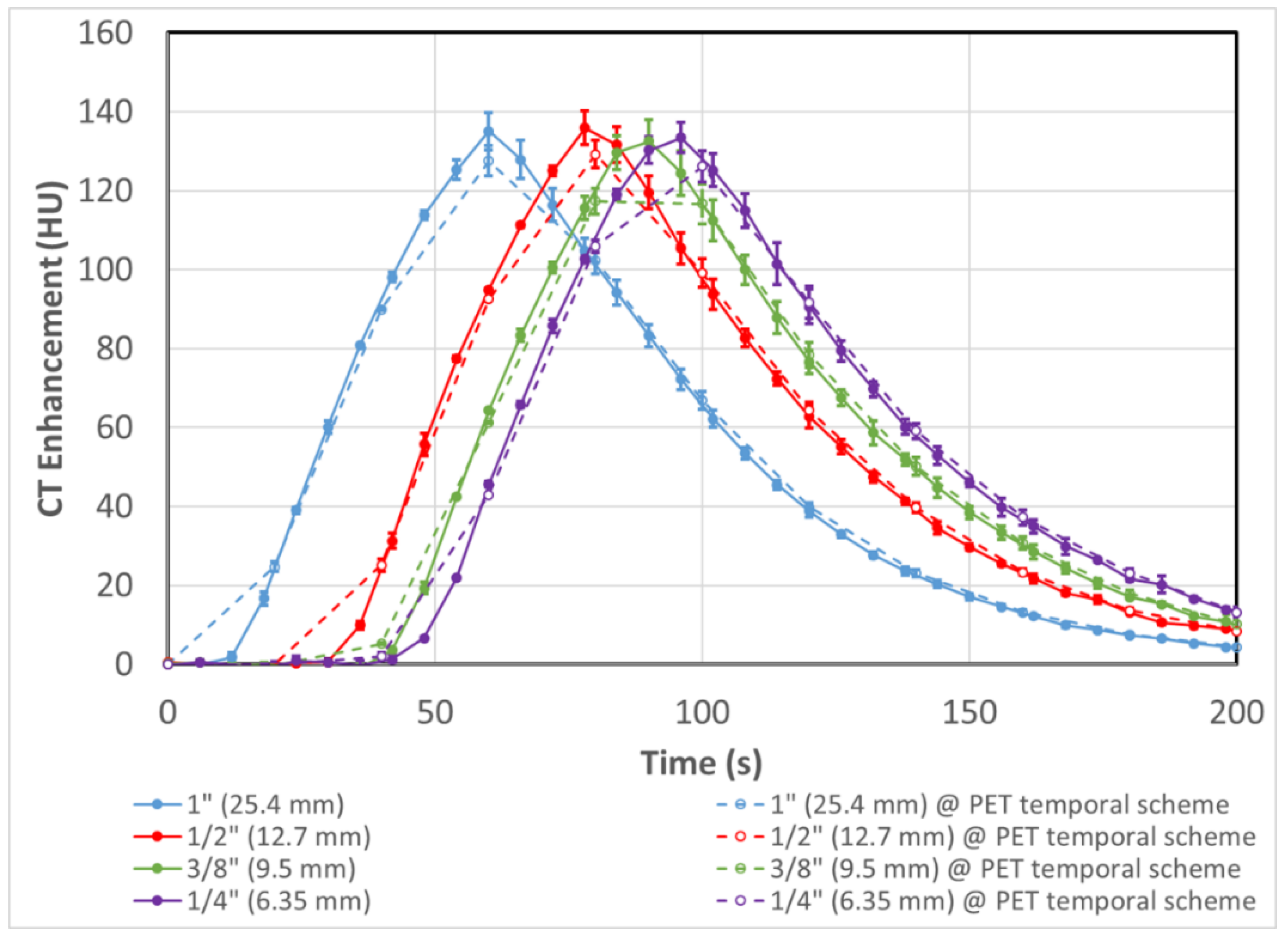
3.1. DCE-CT Results and Validation
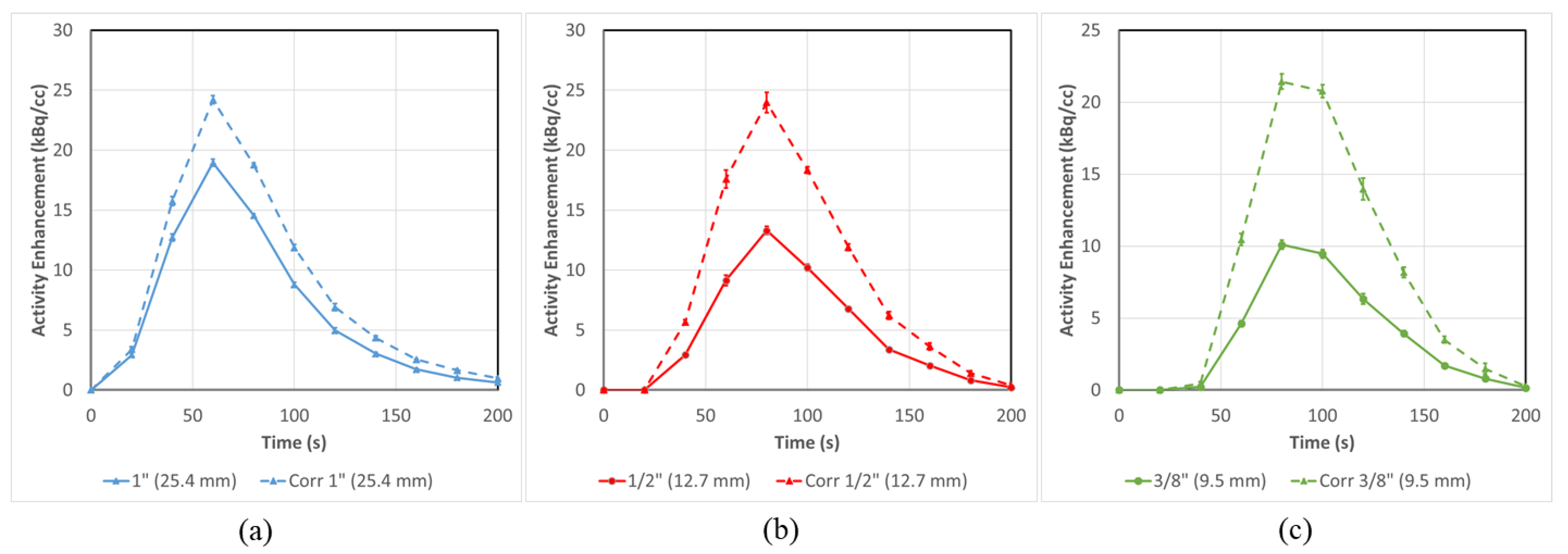
3.2. PET-CT Results and Corrections—Without Background
3.3. PET-CT Results and Corrections (with Background)
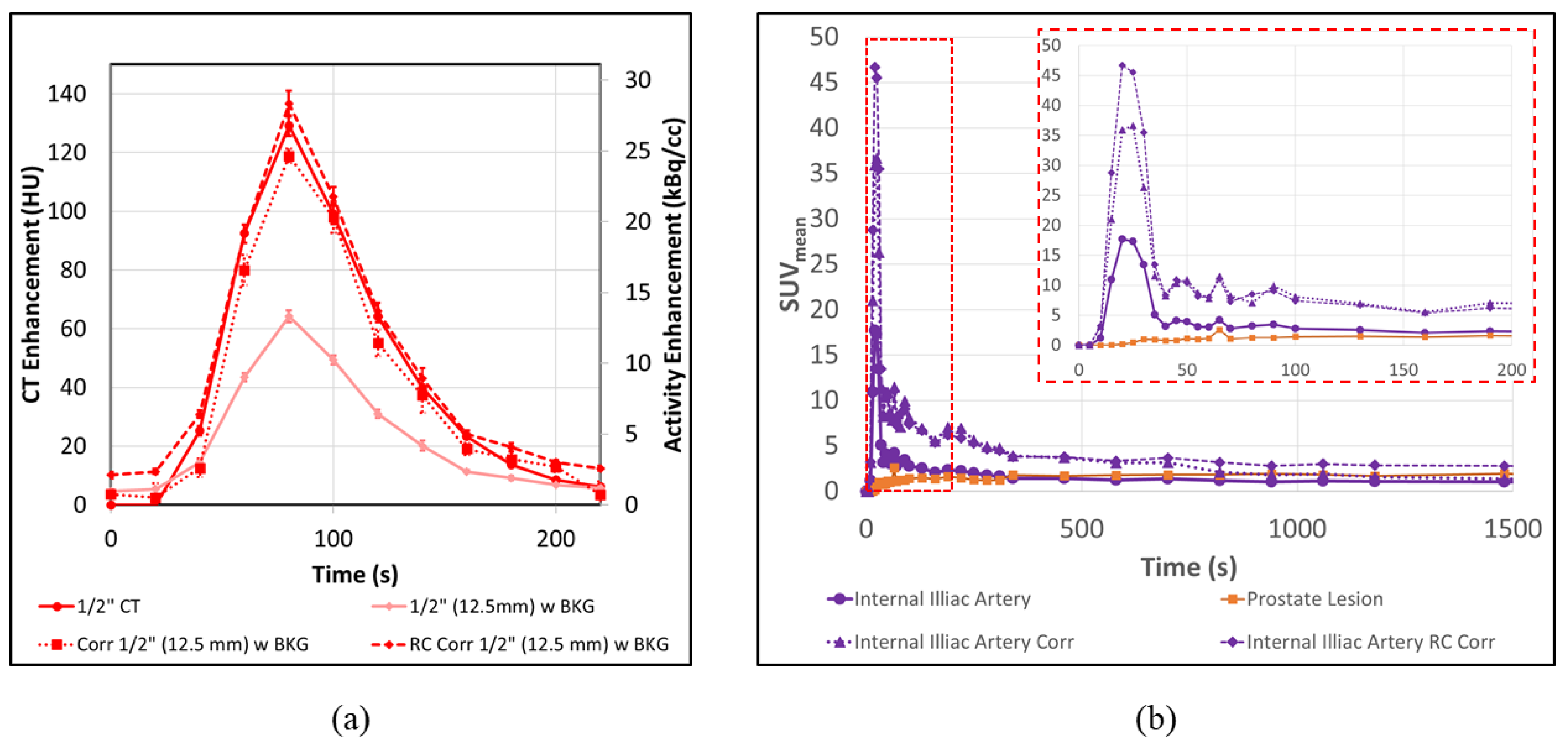
3.4. PET-CT Results Corrections for Artery/Vein in Close Proximity
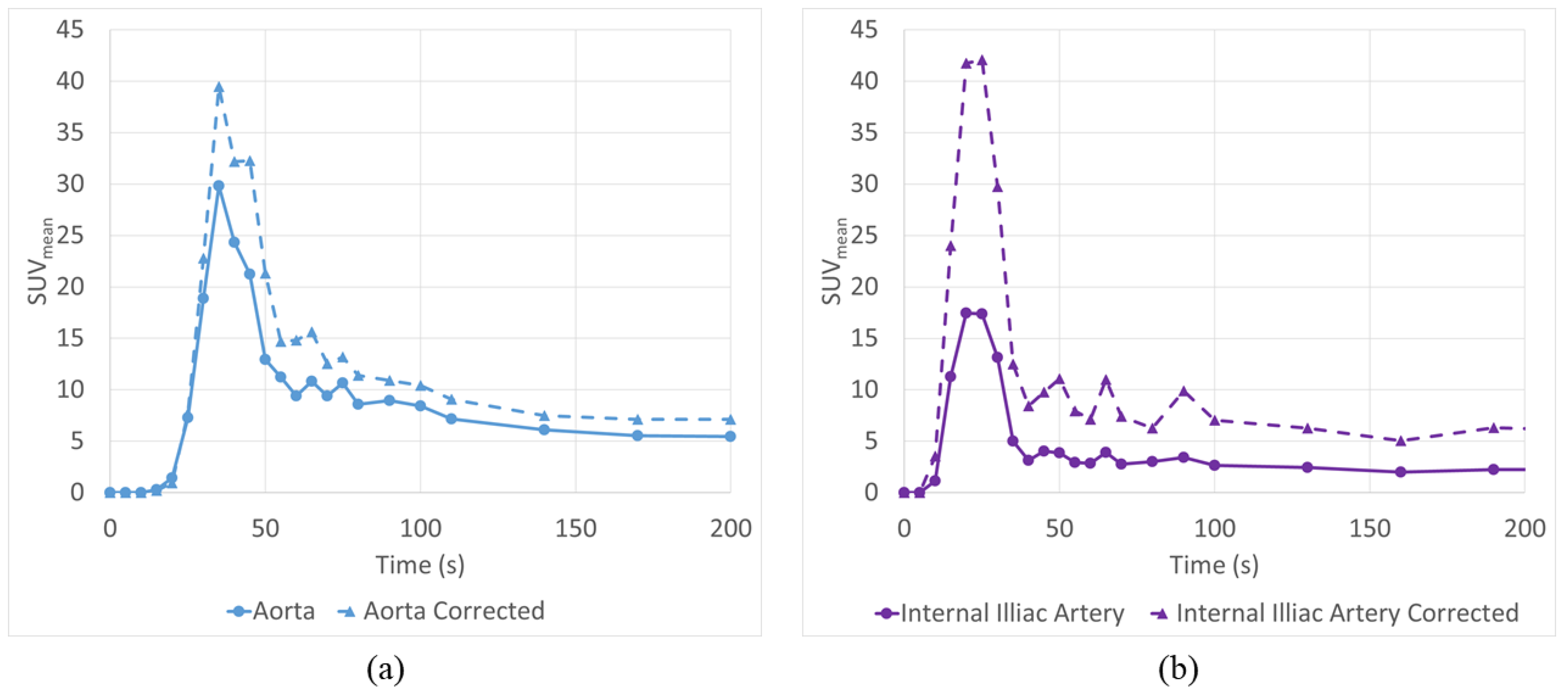
3.5. Application to Patient Scans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitrakopoulou-Strauss, A.; Pan, L.; Sachpekidis, C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: General considerations, current clinical applications, and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C] deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure and normal values in the conscious and anerstized albino rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Wienhard, K. Measurements of glucose consumption using [18F)fluorodeoxyglucose. Methods 2002, 27, 218–225. [Google Scholar] [CrossRef]
- Tomasi, G.; Turkheimer, F.; Aboagye, E. Importance of quantification for the analysis of PET data in oncology: Review of current methods and trends for the future. Mol. Imaging Biol. 2012, 4, 131–146. [Google Scholar] [CrossRef]
- Bettinardi, V.; Castiglioni, I.; De Bernardi, E.; Gilardi, M.C. PET quantification: Strategies for partial volume correction. Clin. Transl. Imaging 2014, 2, 199–218. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Solnes, L.; Vallabhajosula, B. A broad overview of positron emission tomography radiopharmaceuticals and clinical applications: What is new? Semin. Nucl. Med. 2014, 41, 246–264. [Google Scholar] [CrossRef]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-glucose: Validation of methos. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef]
- Carson, R.E. Tracer kinetic modeling in PET. In Positron Emission Tomography: Basic Science and Clinical Practice; Valk, P.E., Bailey, D.L., Townsend, D.W., Maisey, M.N., Eds.; Springer: London, UK, 2003; Chapter 6; Volume 78, pp. 147–179. [Google Scholar]
- Bentourkia, M.; Zaidi, H. Positron emission tomography. Tracer kinetic modeling in PET. PET Clin. 2007, 2, 267–277. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Eder, M.; Kopka, K.; Mier, W.; Hadaschik, B.A.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. 68Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1288–1299. [Google Scholar] [CrossRef]
- Koukouraki, S.; Strauss, L.G.; Georgoulias, V.; Eisenhut, M.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F] FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1115–1120. [Google Scholar] [CrossRef]
- Henze, M.; Dimitrakopoulou-Strauss, A.; Milker-Zabel, S.; Schuhmacher, J.; Strauss, L.G.; Doll, J.; Mäcke, H.R.; Eisenhut, M.; Debus, J.; Haberkorn, U. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J. Nucl. Med. 2005, 46, 763–769. [Google Scholar] [PubMed]
- Ilan, E.; Sandström, M.; Velikyan, I.; Sundin, A.; Eriksson, B.; Lubberink, M. Parametric net imflux rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: Quantitative accuracy and improved image contrast. J. Nucl. Med. 2017, 58, 744–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sachpekidis, C.; Afshar-Oromieh, A.; Kopka, K.; Strauss, D.S.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. 18F-PSMA-1007 multiparametric, dynamic PET/CT in biochemical relapse and progression of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, S.; Mamach, M.; Wilke, F.; von Klot, C.A.; Henkenberens, C.; Thackeray, J.T.; Sohns, J.M.; Geworski, L.; Ross, T.L.; Wester, H.J.; et al. Multiple time-point 68Ga-PSMA I&T PET/CT for characterization of primary prostate Cancer: Value of early dynamic and delayed imaging. Clin. Nucl. Med. 2017, 42, 286–293. [Google Scholar]
- Koopman, T.; Verburg, N.; Pouwels, P.J.; Wesseling, P.; Hoekstra, O.S.; De Witt Hamer, P.C.; Lammertsma, A.A.; Yaqub, M.; Wesseling, P. Quantitative parametric maps of O-(2-(18F)fluoroethyl)-L-tyrosine kinetics in diffuse gliomas. J. Cereb. Blood Flow Metab. 2020, 40, 895–903. [Google Scholar] [CrossRef]
- Kudomi, N.; Maeda, Y.; Hatakeyama, T.; Yamamoto, Y.; Nishiyama, Y. Fully parametric imaging with reversible tracer 18F-FLT within a reasonable time. Radiol. Phys. Technol. 2017, 10, 41–48. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Thieke, C.; Askoxylakis, V.; Nicolay, N.H.; Huber, P.E.; Thomas, M.; Dimitrakopoulou, G.; Debus, J.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Combined use of 18F-FDG and 18F-FMISO in unresectable non-small cell lung cancer patients planned for radiotherapy: A dynamic PET/CT study. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 127–142. [Google Scholar]
- Schwartz, J.; Grkovski, M.; Rimner, A.; Schroeder, H.; Zanzonico, P.B.; Carlin, S.D.; Staton, K.D.; Humm, J.L.; Nehmeh, S.A. Pharmacokinetic analysis of dynamic 18F-Fluoromisonidazole PET data in non-small cell lung cancer. J. Nucl. Med. 2017, 58, 911–919. [Google Scholar] [CrossRef]
- Grkovski, M.; Lee, N.Y.; Schroeder, H.; Carlin, S.D.; Beattie, B.J.; Riaz, N.; Leeman, J.E.; O’Donoghue, J.A.; Humm, J.L. Monitoring early response to chemoradiotherapy with 18F-MISO dynamic PET in head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1682–1691. [Google Scholar] [CrossRef]
- Michaud, L.; Beattie, B.J.; Akhurst, T.; Dunphy, M.; Zanzonico, P.; Finn, R.; Mauguen, A.; Schöder, H.; Weber, W.A.; Lassman, A.B.; et al. 18F-Fluciclovine (18F-FACBC) PET imaging of recurrent brain tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 10, 1007. [Google Scholar]
- Verwer, E.E.; Zegers, C.M.; van Elmpt, W.; Wierts, R.; Windhorst, A.D.; Mottaghy, F.M.; Lambin, P.; Boellaard, R. Pharmacokinetic modeling of a novel hypoxia PET tracer [18F]HX4 in patients with non-small cell lung cancer. EJNMMI Phys. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.T.; Cui, M.; Gao, J.; Wu, B.; Sha, W.; Huang, B. Image-derived arterial input function in dynamic positron emission tomography-computed tomography: A method using both positron emission tomographic and computed tomographic images. J. Comput. Assist. Tomogr. 2012, 36, 762–767. [Google Scholar] [CrossRef]
- Anazodo, U.; Kewin, M.; Finger, E.; Thiessen, J.; Hadway, J.; Butler, L.; Pavlosky, W.; Prato, F.; Thompson, T.; Lawrence, K.S. Preliminary evaluation of MRI-derived input function for quantitative measurement of glucose metabolism in an integrated PET-MRI. EJNMMI Phys. 2015, 2, A80. [Google Scholar] [CrossRef] [PubMed]
- Muzi, M.; O’Sullivan, F.; Mankoff, D.A.; Doot, R.K.; Pierce, L.A.; Kurland, B.F.; Linden, H.M.; Kinahan, P.E. Quantitative assessment of dynamic PET imaging data in cancer imaging. Magn. Reson. Imaging 2012, 30, 1203–1215. [Google Scholar] [CrossRef]
- Litton, J.E. Input function in PET brain studies using MRdefined arteries. J. Comput. Assist. Tomogr. 1997, 21, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Croteau, E.; Lavallée, E.; Labbe, S.M.; Hubert, L.; Pifferi, F.; Rousseau, J.A.; Cunnane, S.C.; Carpentier, A.C.; Lecomte, R.; Bénard, F. Image-derived input function in dynamic human PET/CT: Methodology and validation with 11C-acetate and 18F-fluorothioheptadecanoic acid in muscle and 18F-fluorodeoxyglucose in brain. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.A.; Chaudhry, M.A.; Wahl, R.L. Noise considerations for PET quantification using maximum and peak standardized uptake value. J. Nucl. Med. 2012, 53, 1041–1047. [Google Scholar] [CrossRef]
- Mourik, J.E.; Lubberink, M.; Klumpers, U.M.; Comans, E.F.; Lammertsma, A.A.; Boellaard, R. Partial volume corrected image derived input functions for dynamic PET brain studies: Methodology and validation for [11C]flumazenil. Neuroimage 2008, 39, 1041–1050. [Google Scholar] [CrossRef]
- Cysouw, M.C.F.; Kramer, G.M.; Hoekstra, O.S.; Frings, V.; de Langen, A.J.; Smit, E.F.; van den Eertwegh, A.J.M.; Oprea-Lager, D.E.; Boellaard, R. Accuracy and Precision of Partial-Volume Correction in Oncological PET/CT Studies. J. Nucl. Med. 2016, 57, 1642–1649. [Google Scholar] [CrossRef]
- Chen, K.; Bandy, D.; Reiman, E.; Huang, S.C.; Lawson, M.; Feng, D.; Yun, L.S.; Palant, A. Non invasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J. Cereb. Blood Flow Metab. 1998, 18, 716–723. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Chen, K.; Liow, J.S.; Fujita, M.; Innis, R.B. Image-derived input function for brain PET studies: Many challenges and few opportunities. J. Cereb. Blood Flow Metab. 2011, 31, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, B.; Keller, H.; Coolens, C. Development of a dynamic flow imaging phantom for dynamic contrast-enhanced CT. Med. Phys. 2011, 38, 4866–4880. [Google Scholar] [CrossRef] [PubMed]


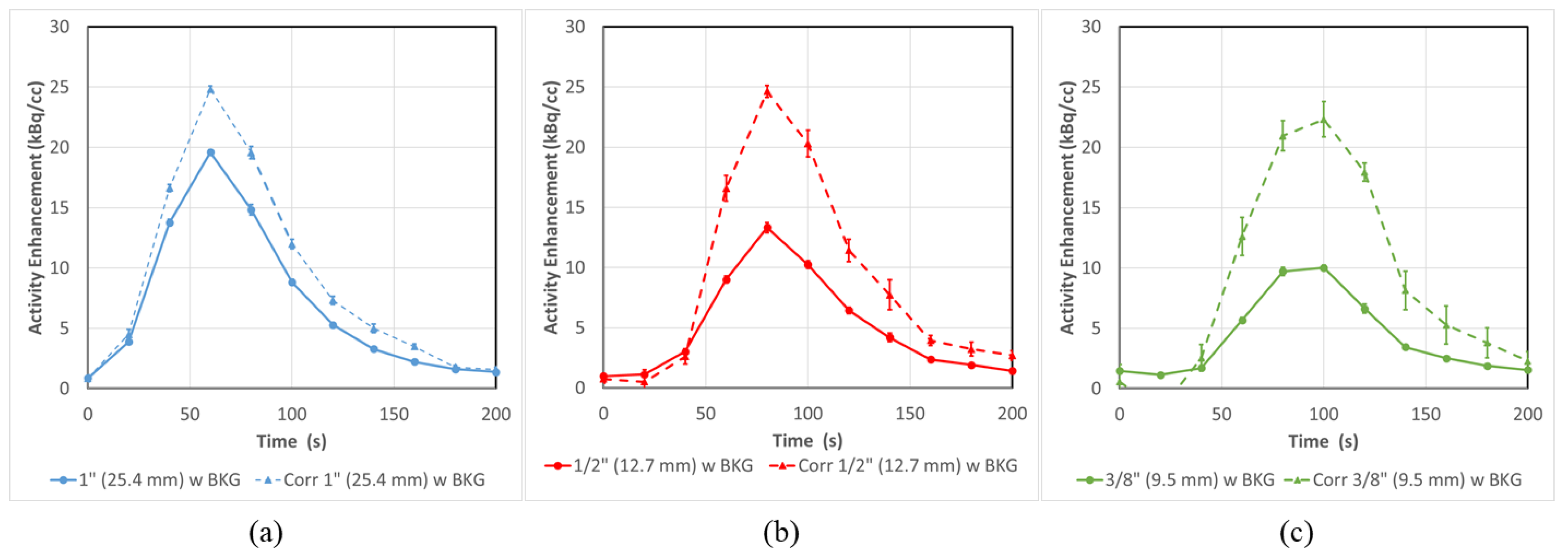

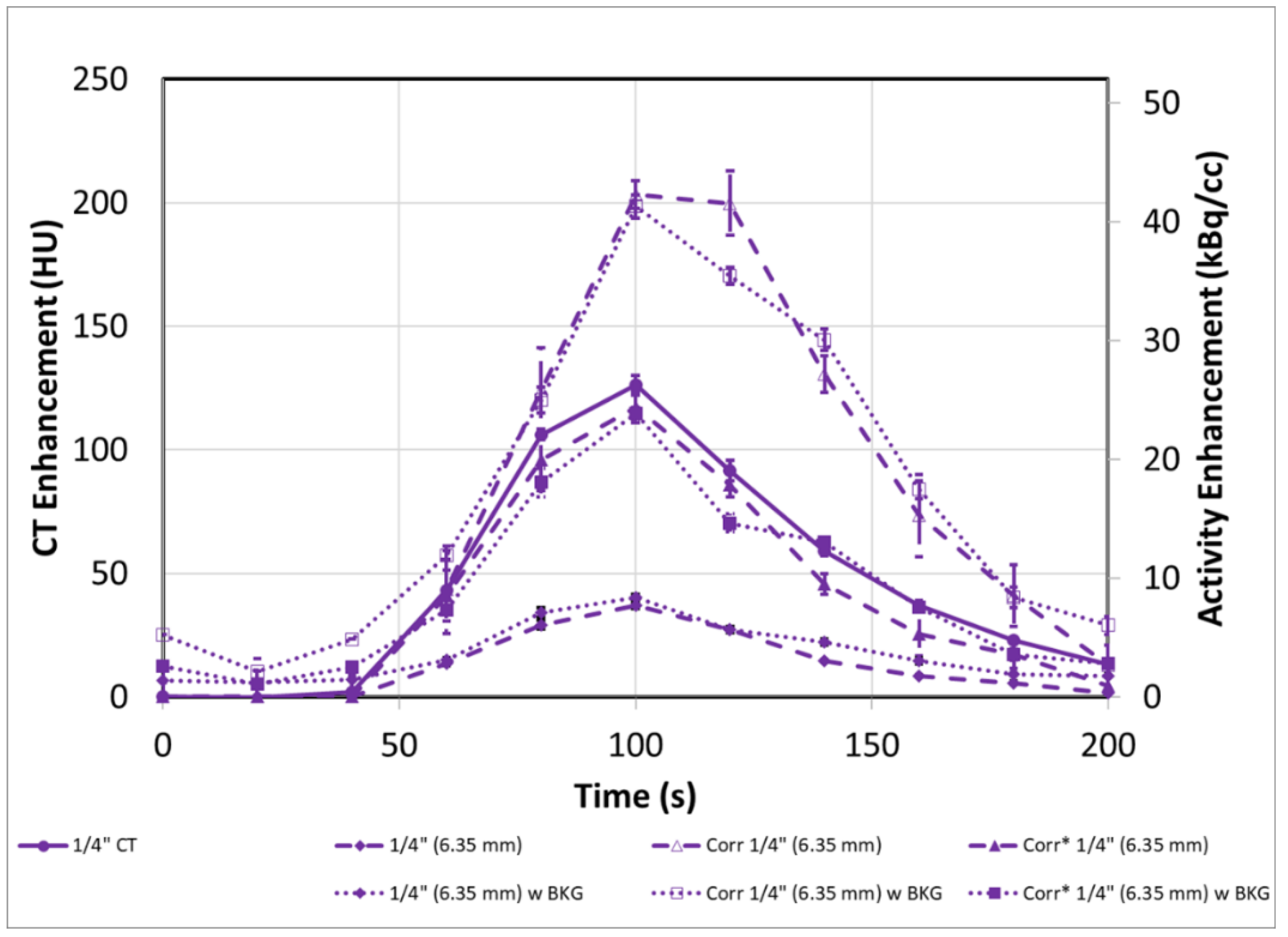
| 1” (25.4 mm) | Cor. 1” (25.4 mm) | 1/2” (12.7 mm) | Cor. 1/2” (12.7 mm) | 3/8” (9.5 mm) | Cor. 3/8” (9.5 mm) | 1/4” (6.35 mm) | Cor. 1/4” (6.35 mm) | |
|---|---|---|---|---|---|---|---|---|
| No Background | ||||||||
| AUC (kBq/cc × s) | 1399 ± 34 | 1827 ± 46 | 977 ± 39 | 1783 ± 68 | 747 ± 37 | 1610 ± 68 | 573 ± 31 | 1807 ± 87 |
| % Error vs. Model | −33% ± 2% | −12% ± 2% | −53% ± 2% | −14% ± 3% | −64% ± 2% | −22% ± 3% | −72% ± 1% | −13% ± 4% |
| % Improvement vs. uncorrected | 21% ± 3% | 39% ± 4% | 42% ± 4% | 60% ± 8% | ||||
| With Background | ||||||||
| AUC (kBq/cc × s) | 1535 ± 39 | 1975 ± 72 | 1104 ± 48 | 1902 ± 183 | 941 ± 44 | 1950 ± 300 | 828 ± 42 | 2056 ± 351 |
| % Error vs. Model | −26% ± 2% | −5% ± 3% | −47% ± 2% | −8% ± 9% | −55% ± 2% | −6% ± 14% | −60% ± 2% | −1% ± 17% |
| % Improvement vs. uncorrected | 21% ± 4% | 38% ± 9% | 49% ± 15% | 59% ± 35% | ||||
| K1 (mL/cc/min) | K2 (1/min) | K3 (1/min) | |
|---|---|---|---|
| Original AIF | 0.1352 | 0.0965 | 0.0071 |
| RC Corrected AIF | 0.0363 | 0.055 | 0 |
| CoA Corrected AIF | 0.0398 | 0.0616 | 0.0328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driscoll, B.; Shek, T.; Vines, D.; Sun, A.; Jaffray, D.; Yeung, I. Phantom Validation of a Conservation of Activity-Based Partial Volume Correction Method for Arterial Input Function in Dynamic PET Imaging. Tomography 2022, 8, 842-857. https://doi.org/10.3390/tomography8020069
Driscoll B, Shek T, Vines D, Sun A, Jaffray D, Yeung I. Phantom Validation of a Conservation of Activity-Based Partial Volume Correction Method for Arterial Input Function in Dynamic PET Imaging. Tomography. 2022; 8(2):842-857. https://doi.org/10.3390/tomography8020069
Chicago/Turabian StyleDriscoll, Brandon, Tina Shek, Douglass Vines, Alex Sun, David Jaffray, and Ivan Yeung. 2022. "Phantom Validation of a Conservation of Activity-Based Partial Volume Correction Method for Arterial Input Function in Dynamic PET Imaging" Tomography 8, no. 2: 842-857. https://doi.org/10.3390/tomography8020069
APA StyleDriscoll, B., Shek, T., Vines, D., Sun, A., Jaffray, D., & Yeung, I. (2022). Phantom Validation of a Conservation of Activity-Based Partial Volume Correction Method for Arterial Input Function in Dynamic PET Imaging. Tomography, 8(2), 842-857. https://doi.org/10.3390/tomography8020069





