Impact of Alternate b-Value Combinations and Metrics on the Predictive Performance and Repeatability of Diffusion-Weighted MRI in Breast Cancer Treatment: Results from the ECOG-ACRIN A6698 Trial
Abstract
:1. Introduction
2. Materials and Methods
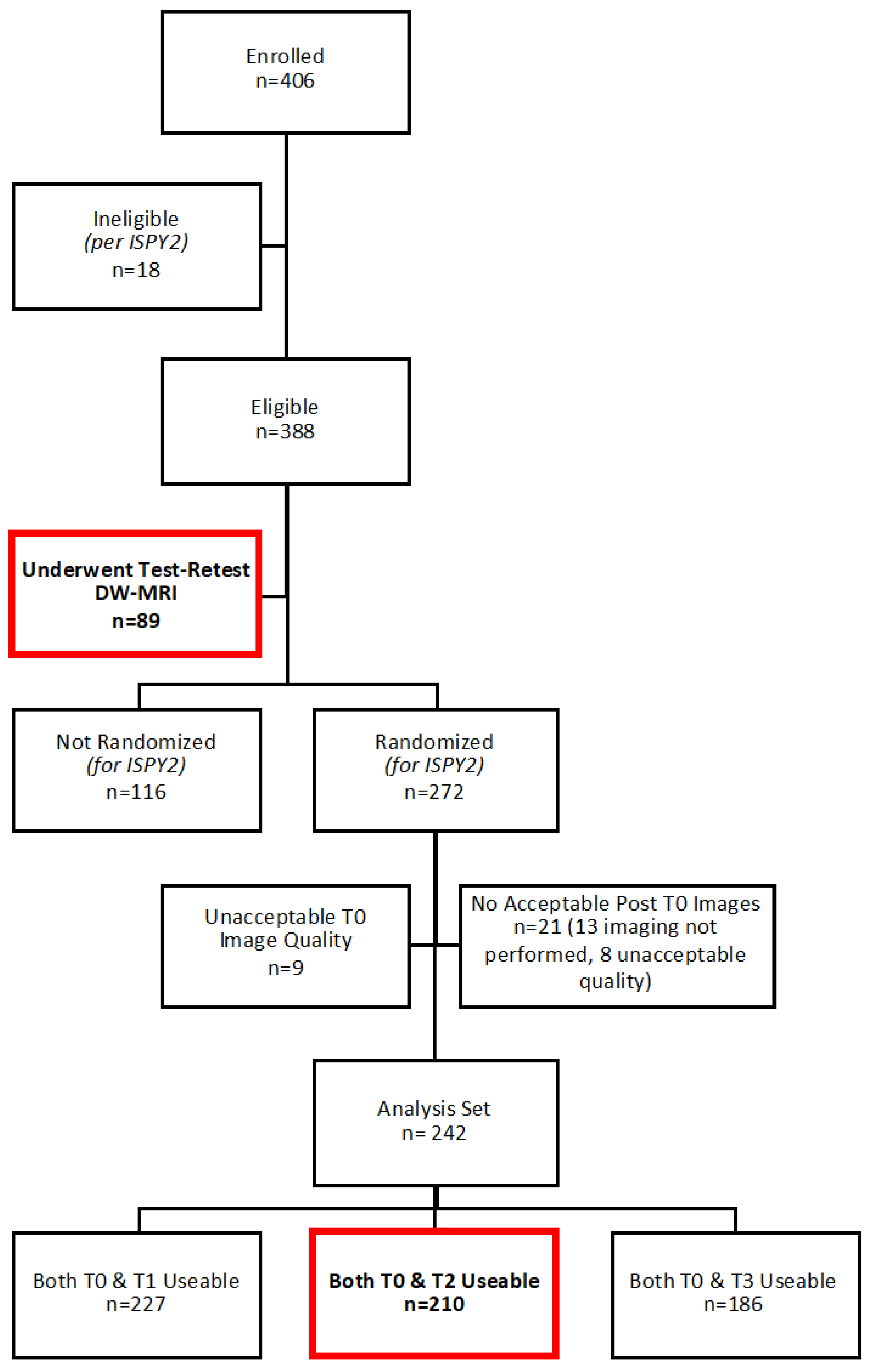
2.1. Study Participants
2.2. MRI Acquisition
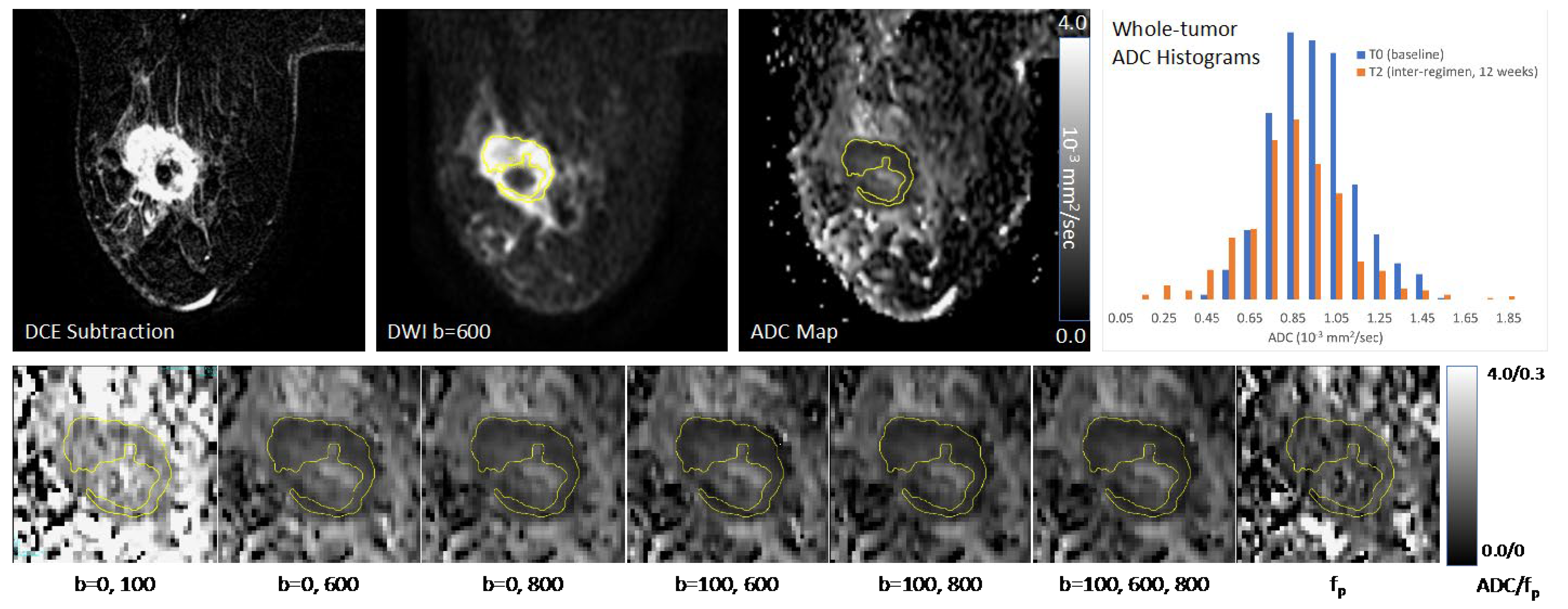
2.3. ADC Measurements
2.4. Reference Standard for Pathologic Response
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.1.1. Correlation between Metrics
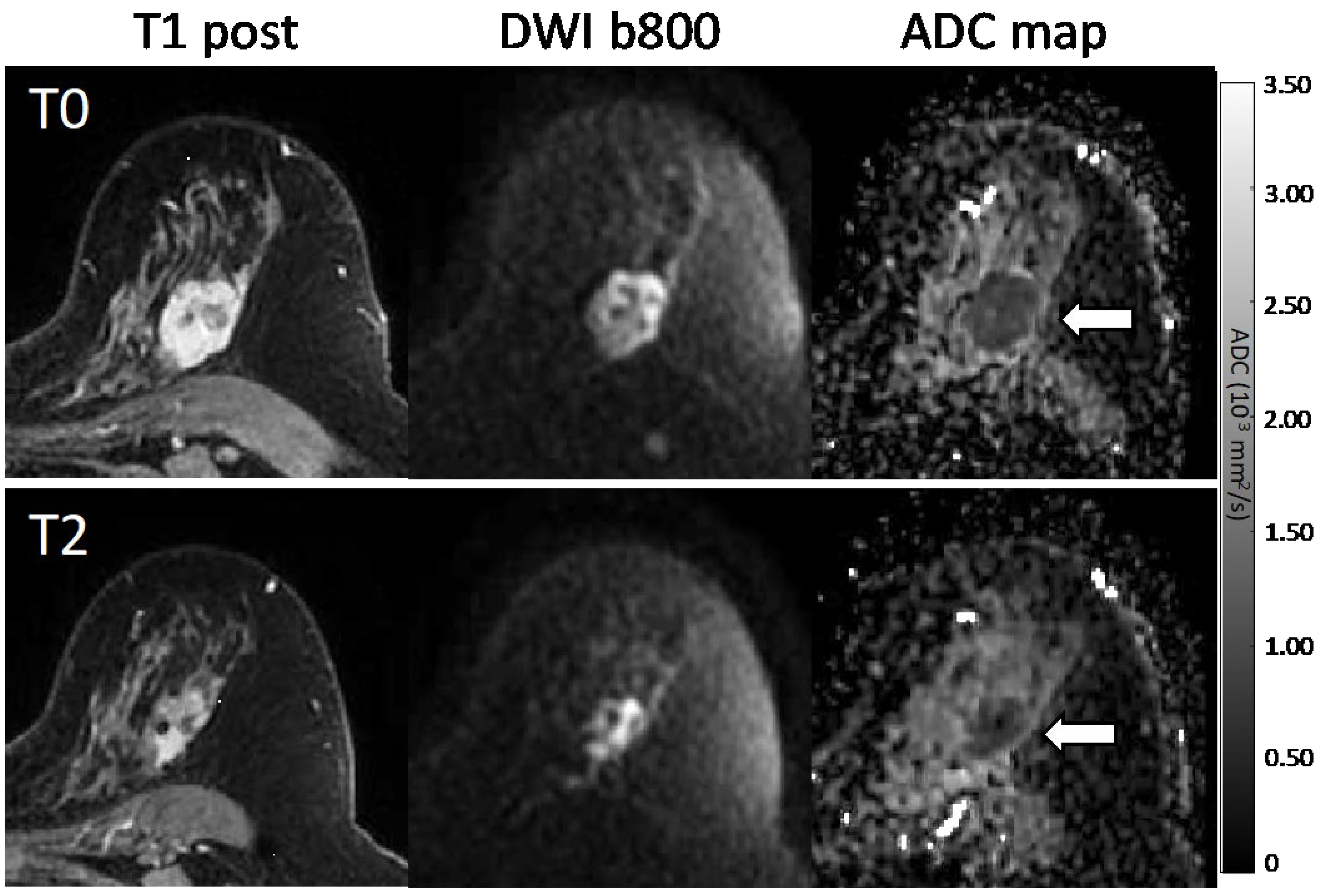
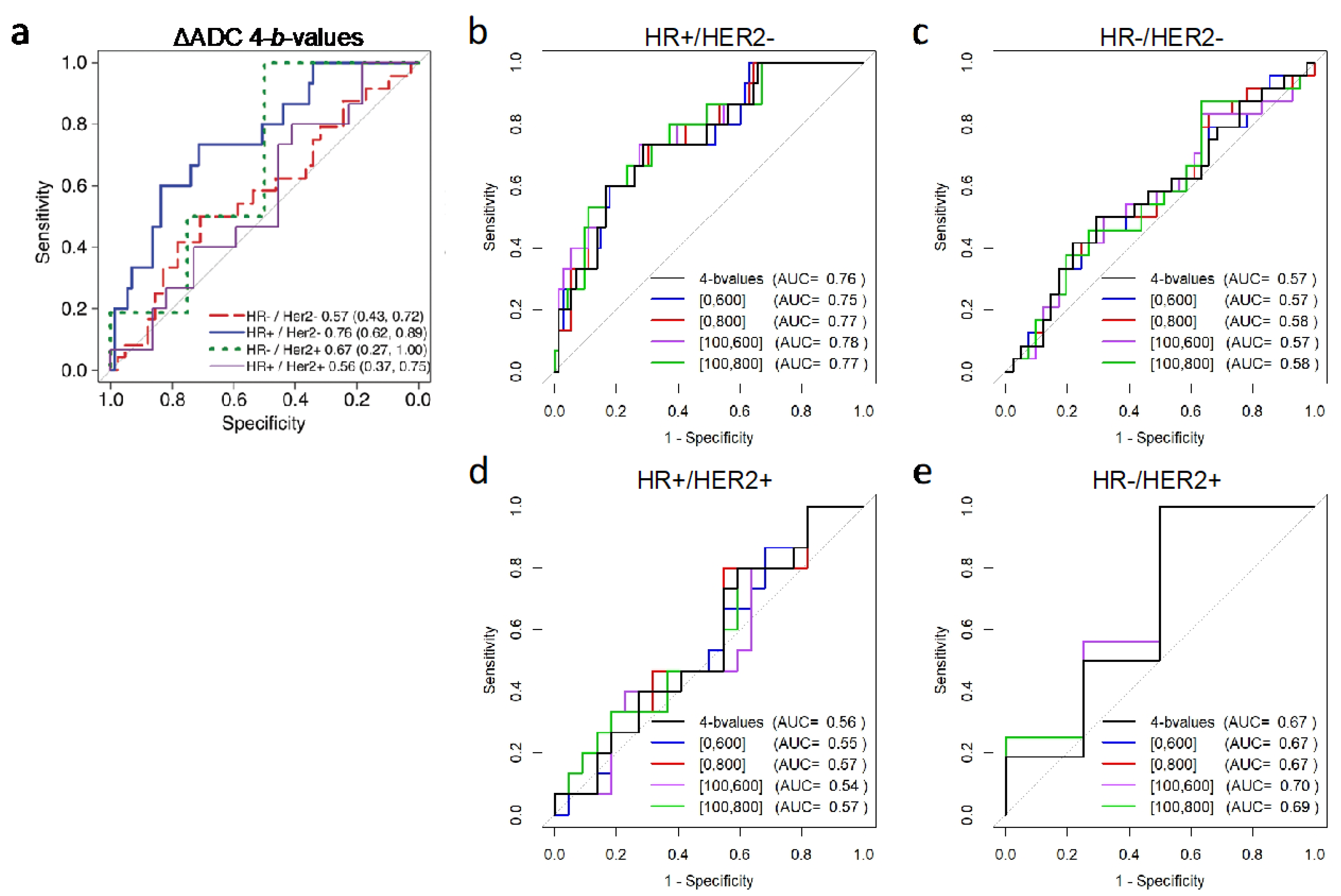
3.1.2. Association with Pathologic Response
3.1.3. Test–Retest Repeatability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chenevert, T.L.; Stegman, L.D.; Taylor, J.M.G.; Robertson, P.L.; Greenberg, H.S.; Rehemtulla, A.; Ross, B.D. Diffusion Magnetic Resonance Imaging: An Early Surrogate Marker of Therapeutic Efficacy in Brain Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Galbán, C.J.; Ma, B.; Malyarenko, D.; Pickles, M.D.; Heist, K.; Henry, N.L.; Schott, A.F.; Neal, C.H.; Hylton, N.M.; Rehemtulla, A.; et al. Multi-Site Clinical Evaluation of DW-MRI as a Treatment Response Metric for Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. PLoS ONE 2015, 10, e0122151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-R.; Cheng, L.-Q.; Liu, M.; Zhang, Y.-J.; Wang, J.-D.; Zhang, A.-L.; Song, X.; Li, J.; Zheng, Y.-Q.; Liu, L. DW-MRI ADC values can predict treatment response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. Med. Oncol. 2011, 29, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Danishad, K.K.A.; Seenu, V.; Jagannathan, N.R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009, 22, 104–113. [Google Scholar] [CrossRef]
- Richard, R.; Thomassin, I.; Chapellier, M.; Scemama, A.; de Cremoux, P.; Varna, M.; Giacchetti, S.; Espié, M.; de Kerviler, E.; de Bazelaire, C. Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur. Radiol. 2013, 23, 2420–2431. [Google Scholar] [CrossRef]
- Li, X.; Abramson, R.; Arlinghaus, L.; Kang, H.; Chakravarthy, A.B.; Abramson, V.G.; Farley, J.; Mayer, I.A.; Kelley, M.C.; Meszoely, I.M.; et al. Multiparametric Magnetic Resonance Imaging for Predicting Pathological Response After the First Cycle of Neoadjuvant Chemotherapy in Breast Cancer. Investig. Radiol. 2015, 50, 195–204. [Google Scholar] [CrossRef]
- Liu, S.; Ren, R.; Chen, Z.; Wang, Y.; Fan, T.; Li, C.; Zhang, P. Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. J. Magn. Reson. Imaging 2015, 42, 779–787. [Google Scholar] [CrossRef]
- Bufi, E.; Belli, P.; Costantini, M.; Cipriani, A.; Di Matteo, M.; Bonatesta, A.; Franceschini, G.; Terribile, D.; Mulé, A.; Nardone, L.; et al. Role of the Apparent Diffusion Coefficient in the Prediction of Response to Neoadjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer. Clin. Breast Cancer 2015, 15, 370–380. [Google Scholar] [CrossRef]
- Chu, W.; Jin, W.; Liu, D.; Wang, J.; Geng, C.; Chen, L.; Huang, X. Diffusion-weighted imaging in identifying breast cancer pathological response to neoadjuvant chemotherapy: A meta-analysis. Oncotarget 2017, 9, 7088–7100. [Google Scholar] [CrossRef] [Green Version]
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast—A consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450. [Google Scholar] [CrossRef] [Green Version]
- Quantitative Imaging Biomarkers Alliance (QIBA) Diffusion Weighted MRI Profile. Available online: https://qibawiki.rsna.org/index.php/Profiles (accessed on 1 March 2022).
- Newitt, D.C.; Zhang, Z.; Ba, J.E.G.; Partridge, S.C.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.; Ms, H.S.M.; Aliu, S.; Li, W.; et al. Test–retest repeatability and reproducibility of ADC measures by breast DWI: Results from the ACRIN 6698 trial. J. Magn. Reson. Imaging 2019, 49, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Wu, C.; Barnes, S.L.; Jarrett, A.M.; Avery, S.; Patt, D.; Goodgame, B.; Luci, J.J.; Kang, H.; Abramson, R.; et al. Repeatability, reproducibility, and accuracy of quantitative MRI of the breast in the community radiology setting. J. Magn. Reson. Imaging 2018, 48, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Zhang, Z.; Newitt, D.C.; Gibbs, J.E.; Chenevert, T.L.; Rosen, M.A.; Bolan, P.; Marques, H.S.; Romanoff, J.; Cimino, L.; et al. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology 2018, 289, 618–627. [Google Scholar] [CrossRef] [PubMed]
- DWI in Assessing Treatment Response in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy (ACRIN6698). Available online: https://clinicaltrials.gov/ct2/show/NCT01564368 (accessed on 1 March 2022).
- American College of Radiology Imaging Network (ACRIN) 6698 Protocol Documents. Available online: https://www.acr.org/Research/Clinical-Research/ACRIN-Legacy-Trials (accessed on 1 March 2022).
- Newitt, D.C.; Amouzandeh, G.; Partridge, S.C.; Marques, H.S.; Herman, B.A.; Ross, B.D.; Hylton, N.M.; Chenevert, T.L.; Malyarenko, D.I. Repeatability and Reproducibility of ADC Histogram Metrics from the ACRIN 6698 Breast Cancer Therapy Response Trial. Tomography 2020, 6, 177–185. [Google Scholar] [CrossRef]
- The Cancer Imaging Archive (TCIA). Available online: https://www.cancerimagingarchive.net (accessed on 1 March 2022).
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Lewin, M.; Fartoux, L.; Vignaud, A.; Arrivé, L.; Menu, Y.; Rosmorduc, O. The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: A pilot study. Eur. Radiol. 2010, 21, 281–290. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Jalnefjord, O.; Andersson, M.; Montelius, M.; Starck, G.; Elf, A.K.; Johanson, V.; Svensson, J.; Ljungberg, M. Comparison of methods for estimation of the intravoxel incoherent motion (IVIM) diffusion coefficient (D) and perfusion fraction (f). Magn. Reson. Mater. Phys. Biol. Med. 2018, 31, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Gurney-Champion, O.J.; Klaassen, R.; Froeling, M.; Barbieri, S.; Stoker, J.; Engelbrecht, M.R.W.; Wilmink, J.W.; Besselink, M.G.; Bel, A.; van Laarhoven, H.W.M.; et al. Comparison of six fit algorithms for the intra-voxel incoherent motion model of diffusion-weighted magnetic resonance imaging data of pancreatic cancer patients. PloS ONE 2018, 13, e0194590. [Google Scholar] [CrossRef]
- Park, J.W.; Liu, M.C.; Yee, D.; Yau, C.; van ’t Veer, L.J.; Symmans, W.F.; Paoloni, M.; Perlmutter, J.; Hylton, N.M.; Hogarth, M.; et al. Adaptive randomization of neratinib in early breast cancer. N. Engl. J. Med. 2016, 375, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of Residual Breast Cancer Burden to Predict Survival After Neoadjuvant Chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (FDA). Guidance for Industry: Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm305501.pdf (accessed on 1 March 2022).
- Dmitrienko, A.; Tamhane, A.C.; Bretz, F. (Eds.) Multiple Testing Problems in Pharmaceutical Statistics; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Shukla-Dave, A.; Obuchowski, N.A.; Chenevert, T.L.; Jambawalikar, S.; Schwartz, L.H.; Malyarenko, D.; Huang, W.; Noworolski, S.M.; Young, R.J.; Shiroishi, M.S.; et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reson. Imaging 2019, 49, e101–e121. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.C.; Graves, M.; Gilbert, F.J.; Patterson, A.J. A Meta-analysis of the Diagnostic Performance of Diffusion MRI for Breast Lesion Characterization. Radiology 2019, 291, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogner, W.; Gruber, S.; Pinker-Domenig, K.; Grabner, G.; Stadlbauer, A.; Weber, M.; Moser, E.; Helbich, T.H.; Trattnig, S. Diffusion-weighted MR for Differentiation of Breast Lesions at 3.0 T: How Does Selection of Diffusion Protocols Affect Diagnosis? Radiology 2009, 253, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Dorrius, M.D.; Dijkstra, H.; Oudkerk, M.; Sijens, P.E. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: A systematic review and meta-analysis. Eur. Radiol. 2014, 24, 2835–2847. [Google Scholar] [CrossRef]
- Pereira, F.P.A.; Martins, G.; Figueiredo, E.; Domingues, M.N.A.; Domingues, R.C.; Da Fonseca, L.M.B.; Gasparetto, E.L. Assessment of Breast Lesions with Diffusion-Weighted MRI: Comparing the Use of Different b Values. Am. J. Roentgenol. 2009, 193, 1030–1035. [Google Scholar] [CrossRef] [Green Version]
- McDonald, E.S.; Romanoff, J.; Rahbar, H.; Kitsch, A.E.; Harvey, S.M.; Whisenant, J.G.; Yankeelov, T.E.; Moy, L.; DeMartini, W.B.; Dogan, B.E.; et al. Mean Apparent Diffusion Coefficient Is a Sufficient Conventional Diffusion-weighted MRI Metric to Improve Breast MRI Diagnostic Performance: Results from the ECOG-ACRIN Cancer Research Group A6702 Diffusion Imaging Trial. Radiology 2021, 298, 60–70. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Bullen, J. Quantitative imaging biomarkers: Effect of sample size and bias on confidence interval coverage. Stat. Methods Med. Res. 2018, 27, 3139–3150. [Google Scholar] [CrossRef]
- Bedair, R.; Priest, A.N.; Patterson, A.J.; McLean, M.A.; Graves, M.J.; Manavaki, R.; Gill, A.B.; Abeyakoon, O.; Griffiths, J.R.; Gilbert, F.J. Assessment of early treatment response to neoadjuvant chemotherapy in breast cancer using non-mono-exponential diffusion models: A feasibility study comparing the baseline and mid-treatment MRI examinations. Eur. Radiol. 2016, 27, 2726–2736. [Google Scholar] [CrossRef] [Green Version]
- Iima, M.; Kataoka, M.; Kanao, S.; Onishi, N.; Kawai, M.; Ohashi, A.; Sakaguchi, R.; Toi, M.; Togashi, K. Intravoxel Incoherent Motion and Quantitative Non-Gaussian Diffusion MR Imaging: Evaluation of the Diagnostic and Prognostic Value of Several Markers of Malignant and Benign Breast Lesions. Radiology 2018, 287, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Yu, T.; Li, L.; Lu, L.; Zhuo, Y.; Lian, J.; Xiong, Y.; Kong, D.; Li, K. Use of diffusion kurtosis imaging and quantitative dynamic contrast-enhanced MRI for the differentiation of breast tumors. J. Magn. Reson. Imaging 2018, 48, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Panagiotaki, E.; Chan, R.W.; Dikaios, N.; Ahmed, H.U.; O’Callaghan, J.; Freeman, A.; Atkinson, D.; Punwani, S.; Hawkes, D.J.; Alexander, D.C. Microstructural Characterization of Normal and Malignant Human Prostate Tissue with Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours Magnetic Resonance Imaging. Investig. Radiol. 2015, 50, 218–227. [Google Scholar] [CrossRef] [PubMed]



| Analysis Set N = 210 | Test–Retest Set N = 71 | |||
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± Std Dev | 48.3 ± 10.7 | 46.3 ± 11.1 | ||
| Median (Min–Max) | 48.0 | (25.0–77.0) | 46.0 | (27.0–71.0) |
| Race, n (%) | ||||
| White | 153 | (72.9) | 53 | (74.6) |
| Black | 21 | (10.0) | 8 | (11.3) |
| Asian | 14 | (6.7) | 7 | (9.9) |
| Native Hawaiian/Pacific Islander | 1 | (0.5) | 0 | 0 |
| Not Reported/Unknown | 21 | (10.0) | 3 | (4.2) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 20 | (9.5) | 5 | (7.0) |
| Not Hispanic or Latino | 137 | (65.2) | 62 | (87.3) |
| Not Reported/Unknown | 53 | (25.2) | 4 | (5.6) |
| HR/HER2 Subtype, n (%) | ||||
| HR−/HER2− (TN) | 65 | (31.0) | 17 | (23.9) |
| HR+/HER2− | 88 | (41.9) | 31 | (43.7) |
| HR−/HER2+ | 20 | (9.5) | 9 | (12.7) |
| HR+/HER2+ | 37 | (17.6) | 8 | (11.3) |
| Missing | 0 | 6 | (8.5) | |
| MRI Longest Diameter at Baseline (cm) | ||||
| Mean (Std Dev) | 4.2 ± 2.3 | 4.5 ± 2.3 | ||
| Median (Min–Max) | 3.5 | (0.4–15.0) | 3.9 | (0.4–13.2) |
| Missing, n (%) | 0 | 8 | (11.3) | |
| Lesion Type, n (%) | ||||
| Single mass | 81 | (38.6) | 25 | (35.2) |
| Single NME | 9 | (4.3) | 7 | (9.9) |
| Multiple masses | 109 | (51.9) | 35 | (49.3) |
| Multiple NME | 11 | (5.2) | 4 | (5.6) |
| Tumor Grade, n (%) | ||||
| I (Low) | 5 | (2.4) | 2 | (2.8) |
| II (Intermediate) | 58 | (27.6) | 16 | (22.5) |
| III (High) | 146 | (69.5) | 48 | (67.6) |
| N/A | 1 | (0.5) | 4 | (5.6) |
| Missing | 0 | 1 | (1.4) | |
| Pathologic Response, n (%) | ||||
| Non-pCR | 140 | (66.7) | 42 | (59.2) |
| pCR | 70 | (33.3) | 17 | (23.9) |
| Missing | 0 | 12 | (16.9) | |
| Pearson’s Correlation Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| All b-Values | 0/600 | 0/800 | 100/600 | 100/800 | ADCfast | ADCslow | |
| 0/600 | 0.99 | ||||||
| 0/800 | 0.99 | 0.95 | |||||
| 100/600 | 0.98 | 0.98 | 0.94 | ||||
| 100/800 | 0.97 | 0.92 | 0.99 | 0.94 | |||
| ADCfast | 0.67 | 0.71 | 0.67 | 0.57 | 0.56 | ||
| ADCslow | 0.98 | 0.94 | 0.99 | 0.96 | 1.00 | 0.57 | |
| fp | 0.13 | 0.19 | 0.14 | 0.06 | 0.03 | 0.75 | 0.04 |
| Mid-Treatment Δ | pCR (N = 70) Mean ± SD (%) | Non-pCR (N = 140) Mean ± SD (%) | AUC [95% CI] | p-Value |
|---|---|---|---|---|
| ΔADC: All b-values (0, 100, 600, 800) | 50.3 ± 48.8 | 35.7 ± 43.7 | 0.60 [0.52, 0.68] | Reference |
| Alternative 2-b-value combinations (Non-inferiority test) | ||||
| ΔADC: b = 0, 600 | 47.1 ± 45.8 | 32.4 ± 40.9 | 0.60 [0.52, 0.68] | <0.001 a |
| ΔADC: b = 0, 800 | 48.2 ± 47.5 | 34.1 ± 42.2 | 0.60 [0.52, 0.68] | <0.001 a |
| ΔADC: b = 100, 600 | 55.9 ± 52.7 | 38.8 ± 45.8 | 0.61 [0.53, 0.69] | <0.001 a |
| ΔADC: b = 100, 800 | 55.1 ± 53.2 | 39.3 ± 46.6 | 0.60 [0.52, 0.68] | 0.006 a |
| Alternative diffusion metrics (Superiority test) | ||||
| ΔADCfast (0, 100) | 19.2 ± 31.4 | 14.4 ± 30.3 | 0.54 [0.46, 0.62] | 0.08 b |
| ΔADCslow (100, 600, 800) | 55.3 ± 53.0 | 39.1 ± 46.4 | 0.60 [0.52, 0.68] | 0.81 b |
| Δfp | –1.0 ± 44.1 | 4.7 ± 44.3 c | 0.56 [0.47, 0.64] | 0.46 b |
| Tumor ΔADC | pCR Mean ± SD (%) | Non-pCR Mean ± SD (%) | AUC (95% CI) | p-Value * |
|---|---|---|---|---|
| HR+/HER2– (N = 88) | N = 15 | N = 73 | ||
| All b-values (0, 100, 600, 800) | 75.1 ± 42.7 | 35.4 ± 39.6 | 0.76 [0.62, 0.89] | Reference |
| b = 0, 600 | 69.2 ± 41.2 | 32.2 ± 36.9 | 0.75 [0.62, 0.88] | 0.003 |
| b = 0, 800 | 72.2 ± 40.7 | 33.4 ± 37.6 | 0.77 [0.64, 0.89] | <0.001 |
| b = 100, 600 | 84.7 ± 46.2 | 38.3 ± 40.6 | 0.78 [0.65, 0.91] | <0.001 |
| b = 100, 800 | 84.9 ± 46.3 | 38.1 ± 40.7 | 0.77 [0.65, 0.90] | 0.003 |
| HR−/HER2− (N = 65) | N = 24 | N = 41 | ||
| All b-values (0, 100, 600, 800) | 32.7 ± 35.9 | 25.5 ± 39.6 | 0.57 [0.43, 0.72] | - |
| b = 0, 600 | 30.3 ± 33.9 | 24.0 ± 37.5 | 0.57 [0.42, 0.72] | - |
| b = 0, 800 | 31.3 ± 34.5 | 24.5 ± 38.5 | 0.58 [0.43, 0.72] | - |
| b = 100, 600 | 35.6 ± 39.9 | 28.6 ± 43.0 | 0.57 [0.43, 0.72] | - |
| b = 100, 800 | 35.6 ± 39.1 | 28.2 ± 43.2 | 0.58 [0.43, 0.72] | - |
| HR−/HER2+ (N = 20) | N = 16 | N = 4 | ||
| All b-values (0, 100, 600, 800) | 63.2 ± 64.7 | 35.0 ± 56.9 | 0.67 [0.27, 1.00] | - |
| b = 0, 600 | 60.2 ± 60.3 | 32.0 ± 52.8 | 0.67 [0.27, 1.00] | - |
| b = 0, 800 | 59.8 ± 63.5 | 34.1 ± 55.8 | 0.67 [0.27, 1.00] | - |
| b = 100, 600 | 69.3 ± 68.5 | 37.9 ± 60.2 | 0.70 [0.33, 1.00] | - |
| b = 100, 800 | 66.2 ± 69.1 | 38.9 ± 62.1 | 0.69 [0.31, 1.00] | - |
| HR+/HER2+ (N = 37) | N = 15 | N = 22 | ||
| All b-values (0, 100, 600, 800) | 39.8 ± 42.6 | 56.2 ± 56.3 | 0.56 [0.37, 0.75] | - |
| b = 0, 600 | 37.9 ± 39.4 | 48.9 ± 53.8 | 0.55 [0.36, 0.74] | - |
| b = 0, 800 | 38.8 ± 42.9 | 54.7 ± 55.2 | 0.57 [0.37, 0.76] | - |
| b = 100, 600 | 45.3 ± 44.5 | 59.3 ± 59.5 | 0.54 [0.35, 0.74] | - |
| b = 100, 800 | 44.8 ± 48.2 | 63.9 ± 60.9 | 0.57 [0.38, 0.76] | - |
| Metric | Mean ± SD a,b | Limits of Agreement | wCV (%) (95% CI) |
|---|---|---|---|
| Mean Difference b (95% CI) | |||
| ADC: All b-values (0, 100, 600, 800) | 1.17 ± 0.31 | 0.0097 [−0.1467, 0.1700] | 5.36 c [4.60, 6.41] |
| Alternative b-value Combinations, Metrics | |||
| ADC: b = 0, 600 | 1.22 ± 0.29 | 0.0085 [−0.1431, 0.1600] | 4.94 [4.25, 5.91] |
| ADC: b = 0, 800 | 1.14 ± 0.28 | 0.0084 [−0.1446, 0.1600] | 5.25 [4.51, 6.28] |
| ADC: b = 100, 600 | 1.13 ± 0.29 | 0.0085 [−0.1629, 0.1800] | 6.01 [5.16, 7.19] |
| ADC: b = 100, 800 | 1.07 ± 0.28 | 0.0069 [−0.1597, 0.1700] | 6.07 [5.21, 7.26] |
| ADCfast (b = 0, 100) | 1.76 ± 0.32 | 0.0094 [−0.3122, 0.3300] | 6.71 [5.76, 8.02] |
| ADCslow (b = 100, 600, 800) | 1.08 ± 0.29 | 0.0072 [−0.1589, 0.1700] | 6.01 [5.16, 7.19] |
| fp | 0.09 ± 0.02 | 0.0009 [−0.0308, 0.0300] | 12.37 [10.63, 14.80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partridge, S.C.; Steingrimsson, J.; Newitt, D.C.; Gibbs, J.E.; Marques, H.S.; Bolan, P.J.; Boss, M.A.; Chenevert, T.L.; Rosen, M.A.; Hylton, N.M. Impact of Alternate b-Value Combinations and Metrics on the Predictive Performance and Repeatability of Diffusion-Weighted MRI in Breast Cancer Treatment: Results from the ECOG-ACRIN A6698 Trial. Tomography 2022, 8, 701-717. https://doi.org/10.3390/tomography8020058
Partridge SC, Steingrimsson J, Newitt DC, Gibbs JE, Marques HS, Bolan PJ, Boss MA, Chenevert TL, Rosen MA, Hylton NM. Impact of Alternate b-Value Combinations and Metrics on the Predictive Performance and Repeatability of Diffusion-Weighted MRI in Breast Cancer Treatment: Results from the ECOG-ACRIN A6698 Trial. Tomography. 2022; 8(2):701-717. https://doi.org/10.3390/tomography8020058
Chicago/Turabian StylePartridge, Savannah C., Jon Steingrimsson, David C. Newitt, Jessica E. Gibbs, Helga S. Marques, Patrick J. Bolan, Michael A. Boss, Thomas L. Chenevert, Mark A. Rosen, and Nola M. Hylton. 2022. "Impact of Alternate b-Value Combinations and Metrics on the Predictive Performance and Repeatability of Diffusion-Weighted MRI in Breast Cancer Treatment: Results from the ECOG-ACRIN A6698 Trial" Tomography 8, no. 2: 701-717. https://doi.org/10.3390/tomography8020058
APA StylePartridge, S. C., Steingrimsson, J., Newitt, D. C., Gibbs, J. E., Marques, H. S., Bolan, P. J., Boss, M. A., Chenevert, T. L., Rosen, M. A., & Hylton, N. M. (2022). Impact of Alternate b-Value Combinations and Metrics on the Predictive Performance and Repeatability of Diffusion-Weighted MRI in Breast Cancer Treatment: Results from the ECOG-ACRIN A6698 Trial. Tomography, 8(2), 701-717. https://doi.org/10.3390/tomography8020058






