Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma
Abstract
:1. Introduction
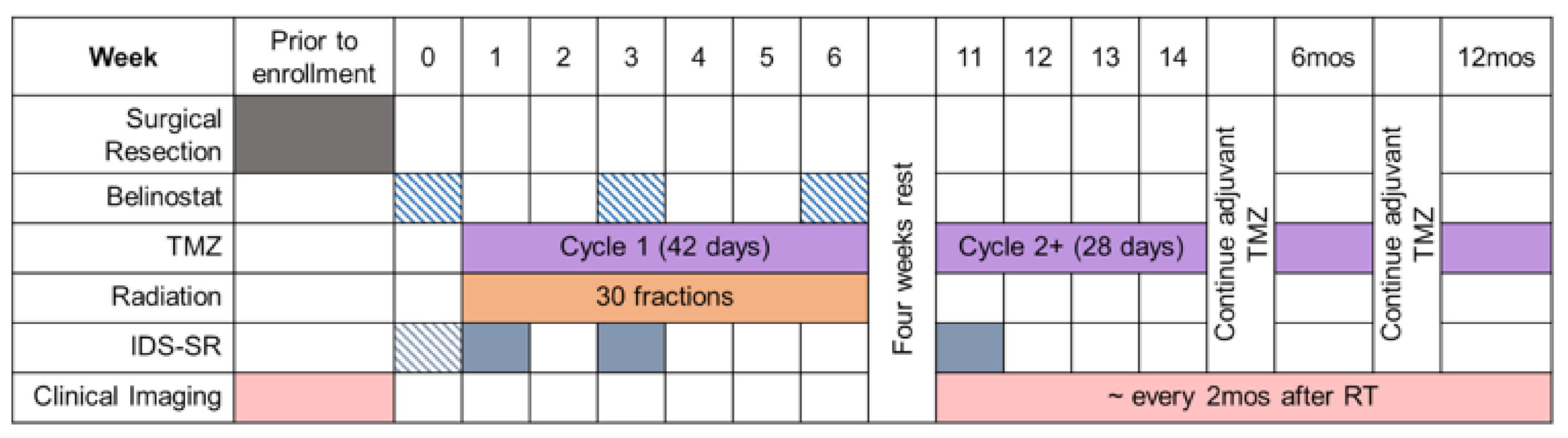
2. Materials and Methods
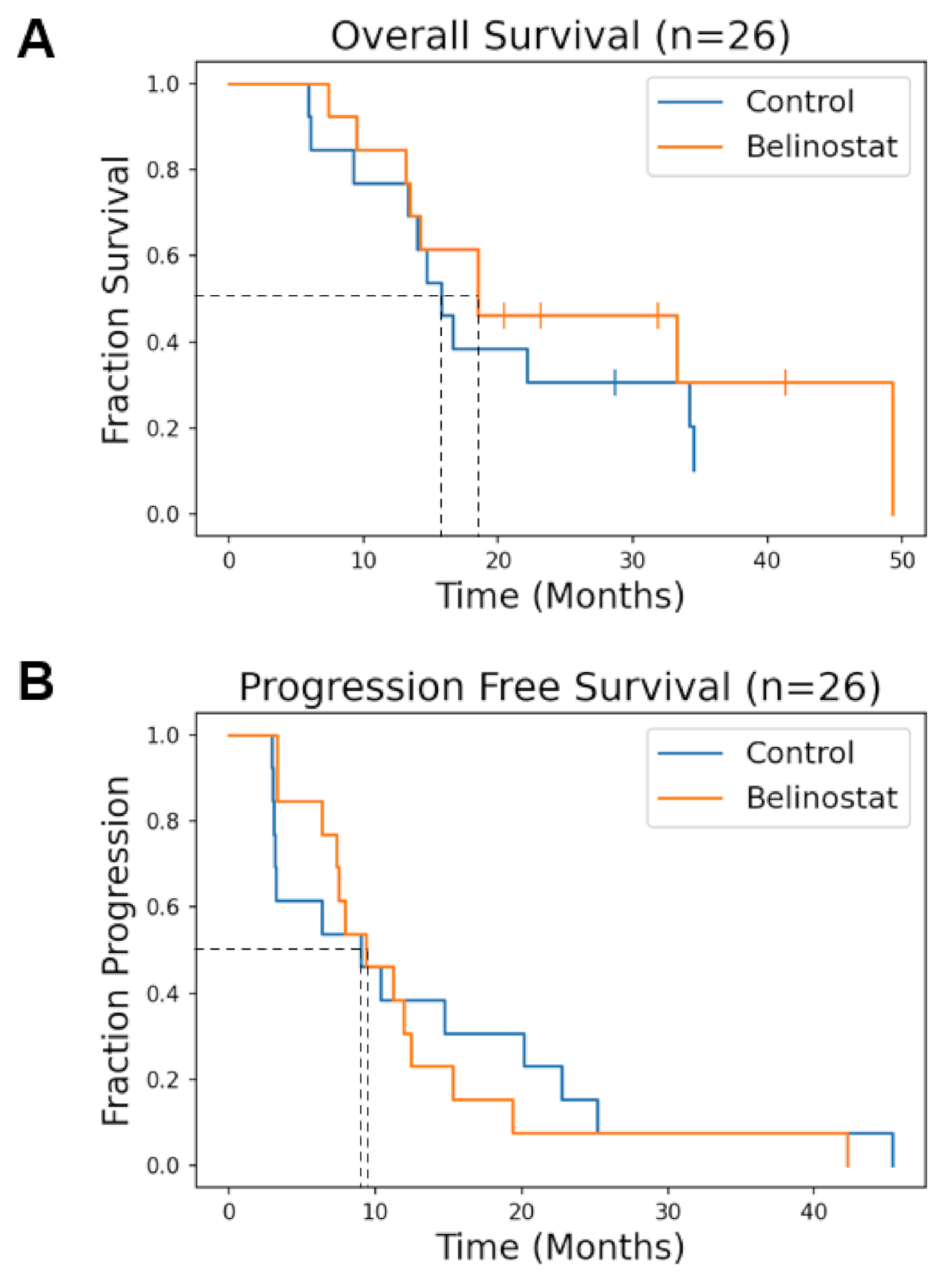
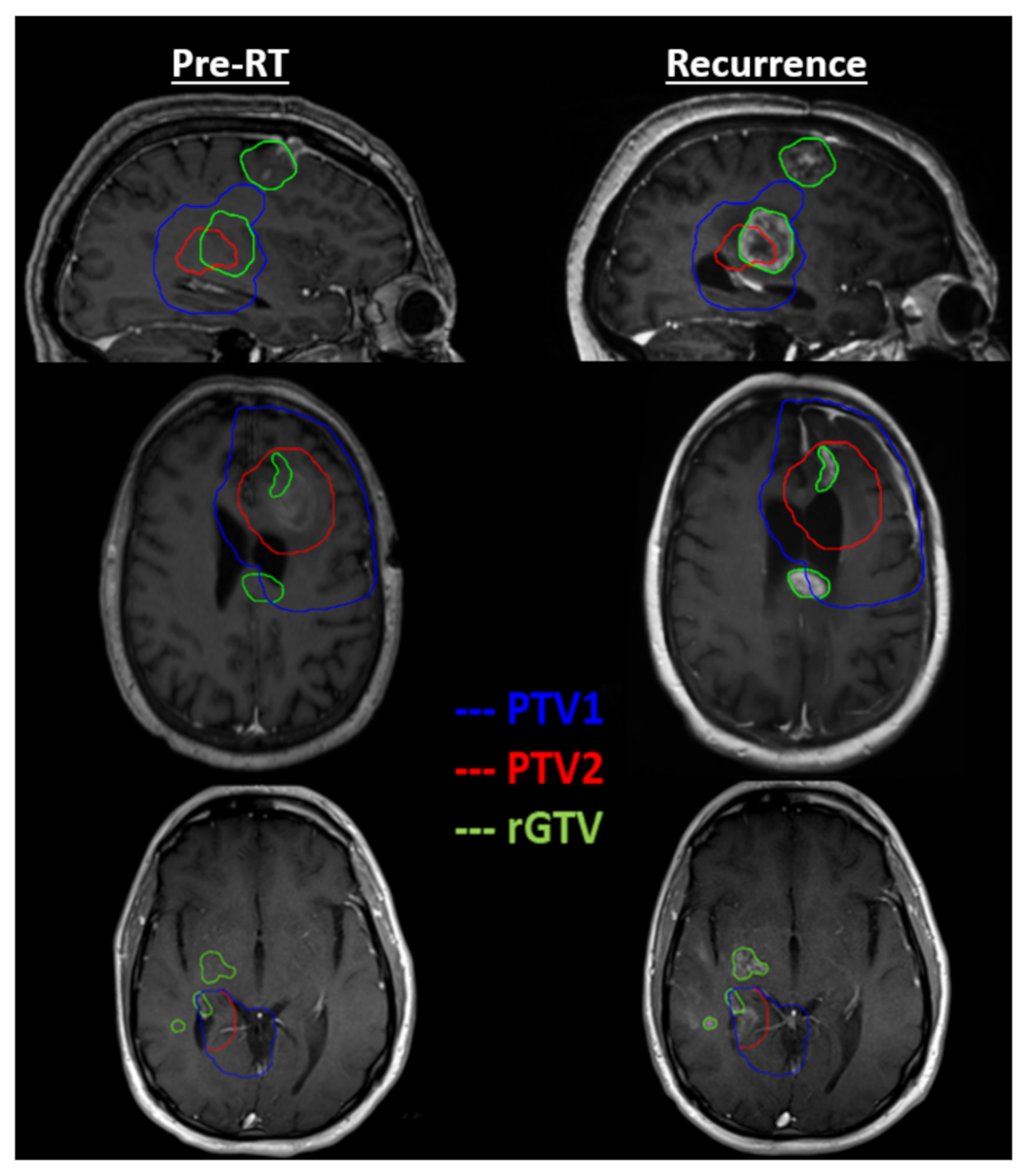
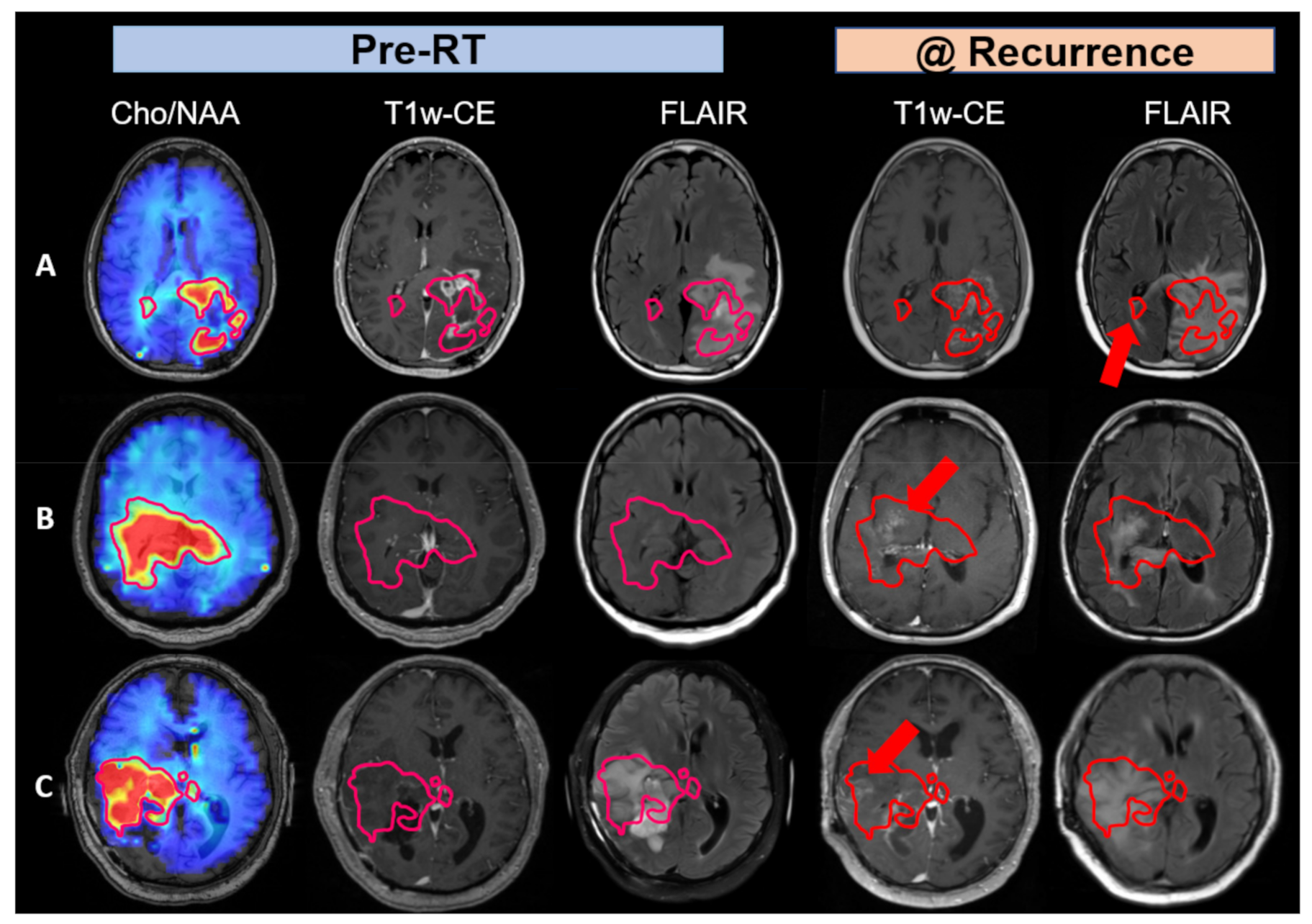
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study ID | Age (at RT) | IDH Mutation (Yes = 1, No = 0) | MGMT Methylation (Yes = 1, No = 0) | OS (Months since Surgery) | Event = 1, Censored = 0 | PFS (Months since Surgery) |
|---|---|---|---|---|---|---|
| COHORT 1 | ||||||
| QINU01EM001 | 61 | 0 | - | 16.6 | 1 | 6.4 |
| QINU01EM002 | 45 | 1 | 1 | 34.2 | 1 | 20.2 |
| QINU01EM003 | 55 | 0 | 0 | 28.7 | 0 | 25.2 |
| QINU01EM004 | 60 | 0 | 1 | 62.2 | 0 | 45.4 |
| QINU01EM005 | 71 | 0 | 1 | 14.7 | 1 | 9.0 |
| QINU01EM006 | 82 | 0 | 0 | 34.5 | 1 | 22.8 |
| QINU01EM007 | 45 | 0 | 0 | 22.1 | 1 | 14.7 |
| QINU01EM008 | 40 | 0 | 0 | 13.3 | 1 | 3.1 |
| QINU01EM010 | 61 | 0 | 0 | 15.8 | 1 | 3.2 |
| QINU01EM011 | 60 | 0 | 1 | 6.1 | 1 | 3.1 |
| QINU01EM012 | 63 | 0 | 0 | 9.2 | 1 | 2.9 |
| QINU01EM013 | 51 | 0 | 0 | 5.9 | 1 | 3.0 |
| QINU01JH001 | 67 | 0 | 0 | 14.0 | 1 | 10.4 |
| COHORT 2 | ||||||
| QINU01EM014 | 58 | 0 | 1 | 49.3 | 1 | 42.3 |
| QINU01EM015 | 52 | 0 | 0 | 18.5 | 1 | 7.5 |
| QINU01EM016 | 50 | 0 | - | 18.5 | 1 | 9.3 |
| QINU01EM017 | 44 | 0 | - | 7.4 | 1 | 3.3 |
| QINU01EM019 | 27 | 1 | 0 | 33.3 | 1 | 19.3 |
| QINU01EM021 | 68 | 0 | 0 | 13.4 | 1 | 3.3 |
| QINU01EM022 | 66 | 0 | 1 | 23.1 | 0 | 12.5 |
| QINU01EM023 | 61 | 0 | 1 | 20.5 | 0 | 11.2 |
| QINU01EM024 | 30 | 0 | 0 | 41.4 | 0 | 7.4 |
| QINU01JH002 | 55 | 0 | 0 | 13.1 | 1 | 7.9 |
| QINU01JH003 | 52 | 0 | 1 | 14.2 | 1 | 11.9 |
| QINU01EM025 | 50 | 0 | 0 | 9.4 | 1 | 6.4 |
| QINU01EM026 | 53 | 0 | 0 | 31.9 | 0 | 15.3 |
References
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Gondi, V.; Pugh, S.; Tsien, C.; Chenevert, T.; Gilbert, M.; Omuro, A.; Mcdonough, J.; Aldape, K.; Srinivasan, A.; Rogers, C.; et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S22–S23. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Lim, D.; Kattan, M.W.; Chakravarti, A.; Gilbert, M.R.; Lassman, A.B.; Lo, S.S.; Machtay, M.; Sloan, A.E.; Sulman, E.P.; et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro-Oncology 2016, 19, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Was, H.; Krol, S.K.; Rotili, D.; Mai, A.; Wojtas, B.; Kaminska, B.; Maleszewska, M. Histone deacetylase inhibitors exert anti-tumor effects on human adherent and stem-like glioma cells. Clin. Epigenet. 2019, 11, 11. [Google Scholar] [CrossRef]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef]
- Hanson, J.E.; La, H.; Plise, E.; Chen, Y.H.; Ding, X.; Hanania, T.; Sabath, E.V.; Alexandrov, V.; Brunner, D.; Leahy, E.; et al. SAHA enhances synaptic function and plasticity in vitro but has limited brain availability in vivo and does not impact cognition. PLoS ONE 2013, 8, e69964. [Google Scholar] [CrossRef]
- Wang, C.; Eessalu, T.E.; Barth, V.N.; Mitch, C.H.; Wagner, F.F.; Hong, Y.; Neelamegam, R.; Schroeder, F.A.; Holson, E.B.; Haggarty, S.J.; et al. Design, synthesis, and evaluation of hydroxamic acid-based molecular probes for in vivo imaging of histone deacetylase (HDAC) in brain. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 29–38. [Google Scholar]
- Lee, H.Z.; Kwitkowski, V.E.; Del Valle, P.L.; Ricci, M.S.; Saber, H.; Habtemariam, B.A.; Bullock, J.; Bloomquist, E.; Li Shen, Y.; Chen, X.H.; et al. FDA Approval: Belinostat for the Treatment of Patients with Relapsed or Refractory Peripheral T-cell Lymphoma. Clin. Cancer Res. 2015, 21, 2666–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurbani, S.S.; Yoon, Y.; Weinberg, B.D.; Salgado, E.; Press, R.H.; Cordova, J.S.; Ramesh, K.K.; Liang, Z.; Velazquez Vega, J.; Voloschin, A.; et al. Assessing Treatment Response of Glioblastoma to an HDAC Inhibitor Using Whole-Brain Spectroscopic MRI. Tomography 2019, 5, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.S.; Weinberg, B.D.; Salgado, E.; Voloschin, A.; Velazquez Vega, J.E.; Olson, J.J.; Shu, H.-K.G.; Shim, H. Remarkable response of a patient with secondary glioblastoma to a histone deacetylase inhibitor. Oxf. Med. Case Rep. 2020, 2020, omaa006. [Google Scholar] [CrossRef] [PubMed]
- Sulman, E.P.; Ismaila, N.; Armstrong, T.S.; Tsien, C.; Batchelor, T.T.; Cloughesy, T.; Galanis, E.; Gilbert, M.; Gondi, V.; Lovely, M.; et al. Radiation Therapy for Glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. J. Clin. Oncol. 2017, 35, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazda, T.; Dziacky, A.; Burkon, P.; Pospisil, P.; Slavik, M.; Rehak, Z.; Jancalek, R.; Slampa, P.; Slaby, O.; Lakomy, R. Radiotherapy of Glioblastoma 15 Years after the Landmark Stupp’s Trial: More Controversies than Standards? Radiol. Oncol. 2018, 52, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, K.; Mellon, E.A.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; Sheriff, S.A.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A Multi-Institutional Pilot Clinical Trial of Spectroscopic MRI-guided Radiation Dose Escalation for Newly-Diagnosed Glioblastoma. Neuro-Oncol. Adv. 2022. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Smith, A.W.; Taube, S.; Mehta, M.P. Glioblastoma: Radiation treatment margins, how small is large enough? Pract. Radiat. Oncol. 2016, 6, 298–305. [Google Scholar] [CrossRef]
- McDonald, M.W.; Shu, H.-K.G.; Curran Jr, W.J.; Crocker, I.R. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 130–136. [Google Scholar] [CrossRef]
- Sabati, M.; Sheriff, S.; Gu, M.; Wei, J.; Zhu, H.; Barker, P.B.; Spielman, D.M.; Alger, J.R.; Maudsley, A.A. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn. Reson. Med. 2015, 74, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Maudsley, A.A.; Darkazanli, A.; Alger, J.R.; Hall, L.O.; Schuff, N.; Studholme, C.; Yu, Y.; Ebel, A.; Frew, A.; Goldgof, D.; et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006, 19, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of Brain Metabolite Distributions by Volumetric Proton MR Spectroscopic Imaging (MRSI). Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordova, J.S.; Shu, H.-K.G.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.D.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro-Oncology 2016, 18, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.; Weinberg, B.; Cooper, L.; Mellon, E.; Schreibmann, E.; Sheriff, S.; Maudsley, A.; Goryawala, M.; Shu, H.K.; Shim, H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.D.; Gore, A.; Shu, H.-K.G.; Olson, J.J.; Duszak, R.; Voloschin, A.D.; Hoch, M.J. Management-based structured reporting of posttreatment glioma response with the brain tumor reporting and data system. J. Am. Coll. Radiol. 2018, 15, 767–771. [Google Scholar] [CrossRef]
- Gore, A.; Hoch, M.J.; Shu, H.-K.G.; Olson, J.J.; Voloschin, A.D.; Weinberg, B.D. Institutional implementation of a structured reporting system: Our experience with the brain tumor reporting and data system. Acad. Radiol. 2019, 26, 974–980. [Google Scholar] [CrossRef]
- Weinberg, B.; Ramesh, K.; Gurbani, S.; Schreibmann, E.; Kleinberg, L.; Shu, H.-K.; Shim, H.J.N.-O. NIMG-23. BRAIN TUMOR REPORTING AND DATA SYSTEM (BT-RADS) AND QUANTITATIVE TOOLS TO GUIDE ITS IMPLEMENTATION. Neuro-Oncology 2019, 21, vi166. [Google Scholar] [CrossRef]
- Kim, S.; Hoch, M.J.; Cooper, M.E.; Gore, A.; Weinberg, B.D. Using a website to teach a structured reporting system, the brain tumor reporting and data system. Curr. Probl. Diagn. Radiol. 2021, 50, 356–361. [Google Scholar] [CrossRef]
- Prensner, J.R.; Chinnaiyan, A.M. Metabolism unhinged: IDH mutations in cancer. Nat. Med. 2011, 17, 291–293. [Google Scholar] [CrossRef]
| Control | Belinostat | |
|---|---|---|
| Number of Patients | 13 | 13 |
| Age | 58.5 ± 11.1 | 51.2 ± 11.6 |
| IDH1 Mutation | 1 (7.7%) | 1 (7.7%) |
| MGMT Methylated | 4 (30.8%) | 4 (30.8%) |
| Toxicities (Grade) | ||
| Thrombocytopenia (4) | 0/13 | 2/13 * |
| Neutropenia (4) | 0/13 | 1/13 * |
| Lymphopenia (3) | 3/13 | 1/13 * |
| Constipation (3) | 1/13 | 1/13 |
| Fatigue (3) | 1/13 | 1/13 |
| Confusion (3) | 1/13 | 0/13 |
| Study ID | Minimum Dose (Gy) | Maximum Dose (Gy) | Mean Dose (Gy) | rGTV Overlap with PTV1 | rGTV Overlap with PTV2 |
|---|---|---|---|---|---|
| Cohort 1 | |||||
| QINU01EM001 | 59.0 | 65.2 | 62.3 | 100.0% | 100.0% |
| QINU01EM002 | 61.1 | 63.4 | 62.4 | 100.0% | 100.0% |
| QINU01EM003 | 59.4 | 63.8 | 61.8 | 100.0% | 100.0% |
| QINU01EM004 | 61.9 | 64.5 | 63.1 | 100.0% | 100.0% |
| QINU01EM005 | 60.3 | 63.5 | 62.5 | 100.0% | 100.0% |
| QINU01EM006 | 61.1 | 64.2 | 62.5 | 100.0% | 100.0% |
| QINU01EM007 | 60.2 | 63.6 | 62.0 | 100.0% | 100.0% |
| QINU01EM008 | 11.8 | 64.7 | 58.7 | 90.9% | 69.9% |
| QINU01EM010 | 52.2 | 64.7 | 62.3 | 100.0% | 99.1% |
| QINU01EM011 | 44.1 | 64.7 | 62.2 | 99.8% | 99.5% |
| QINU01EM012 | 58.7 | 63.6 | 61.9 | 100.0% | 94.5% |
| QINU01EM013 | 59.0 | 64.1 | 61.8 | 100.0% | 100.0% |
| QINU01JH001 | 60.1 | 63.2 | 61.4 | 100.0% | 99.8% |
| Cohort 2 | |||||
| QINU01EM014 | 52.2 | 63.2 | 58.2 | 100.0% | 50.1% |
| QINU01EM015 | 58.7 | 62.8 | 61.2 | 100.0% | 96.2% |
| QINU01EM016 | 56.2 | 64.9 | 62.8 | 99.2% | 99.2% |
| QINU01EM017 | 10.9 | 50.3 | 25.2 | 0.0% | 0.0% |
| QINU01EM019 | 60.2 | 63.2 | 61.6 | 100.0% | 100.0% |
| QINU01EM021 | * | * | * | * | * |
| QINU01EM022 | 59.6 | 62.8 | 61.0 | 100.0% | 100.0% |
| QINU01EM023 | 45.6 | 53.4 | 50.3 | 0.0% | 0.0% |
| QINU01EM024 | 59.5 | 63.8 | 61.6 | 100.0% | 100.0% |
| QINU01JH002 | 60.0 | 62.1 | 61.0 | 100.0% | 100.0% |
| QINU01JH003 | 1.9 | 63.8 | 32.3 | 13.4% | 13.4% |
| QINU01EM025 | 19.8 | 21.7 | 20.9 | 100.0% | 100.0% |
| QINU01EM026 | * | * | * | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Ramesh, K.; Huang, V.; Gurbani, S.S.; Cordova, J.S.; Schreibmann, E.; Weinberg, B.D.; Sengupta, S.; Voloschin, A.D.; Holdhoff, M.; et al. Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography 2022, 8, 688-700. https://doi.org/10.3390/tomography8020057
Xu K, Ramesh K, Huang V, Gurbani SS, Cordova JS, Schreibmann E, Weinberg BD, Sengupta S, Voloschin AD, Holdhoff M, et al. Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography. 2022; 8(2):688-700. https://doi.org/10.3390/tomography8020057
Chicago/Turabian StyleXu, Karen, Karthik Ramesh, Vicki Huang, Saumya S. Gurbani, James Scott Cordova, Eduard Schreibmann, Brent D. Weinberg, Soma Sengupta, Alfredo D. Voloschin, Matthias Holdhoff, and et al. 2022. "Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma" Tomography 8, no. 2: 688-700. https://doi.org/10.3390/tomography8020057
APA StyleXu, K., Ramesh, K., Huang, V., Gurbani, S. S., Cordova, J. S., Schreibmann, E., Weinberg, B. D., Sengupta, S., Voloschin, A. D., Holdhoff, M., Barker, P. B., Kleinberg, L. R., Olson, J. J., Shu, H.-K. G., & Shim, H. (2022). Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography, 8(2), 688-700. https://doi.org/10.3390/tomography8020057









