Diastolic Cardiac Function by MRI—Imaging Capabilities and Clinical Applications
Abstract
1. Introduction
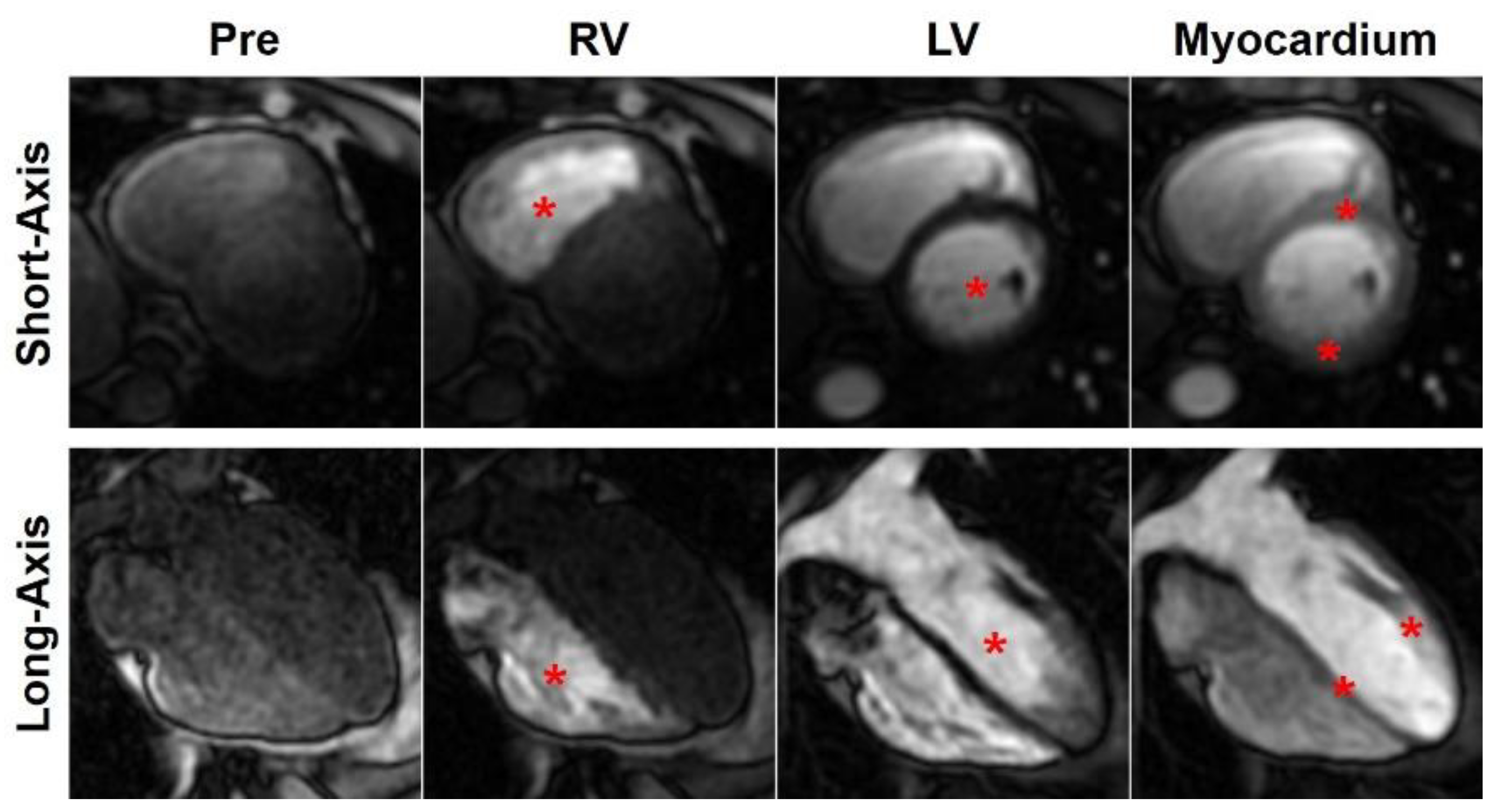
2. Cardiac MRI Techniques
2.1. MRI Tagging
2.2. Complementary Spatial Modulation of Magnetization
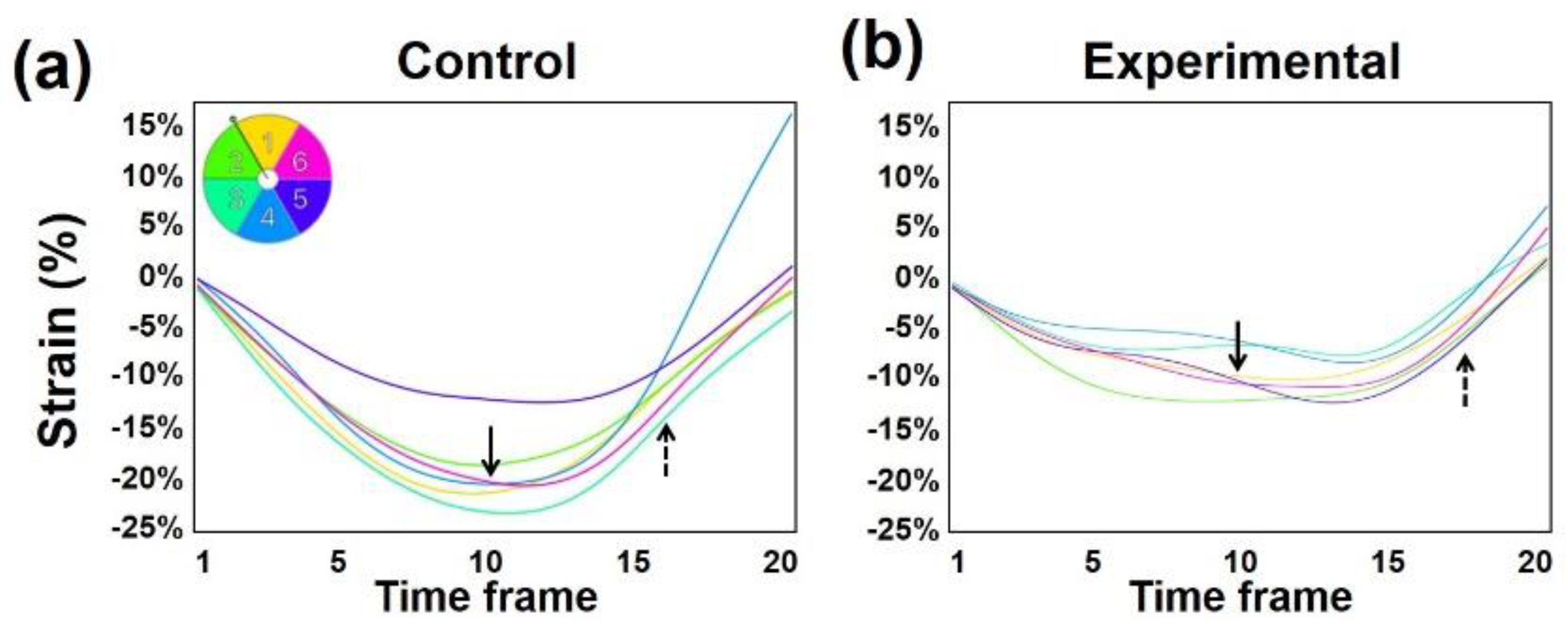
2.3. MRI Feature-Tracking
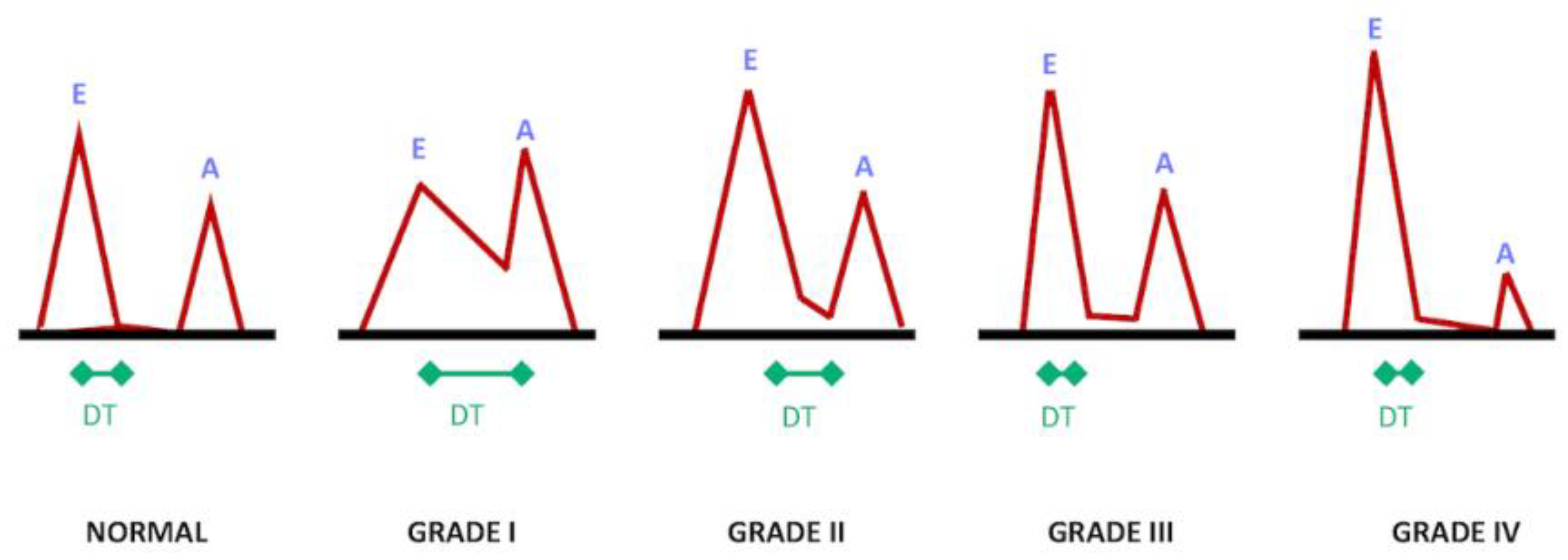
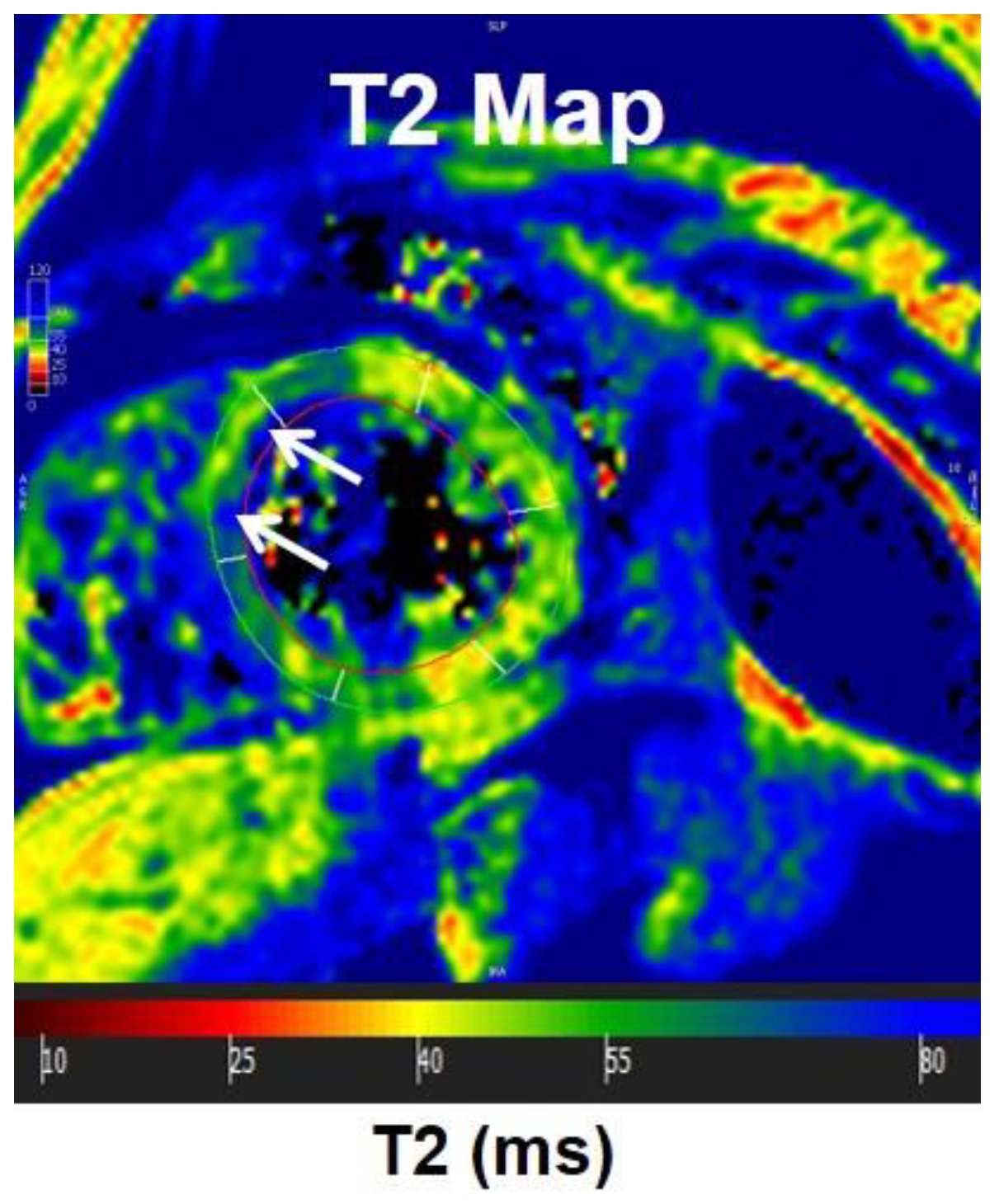
2.4. Phase-Contrast MRI
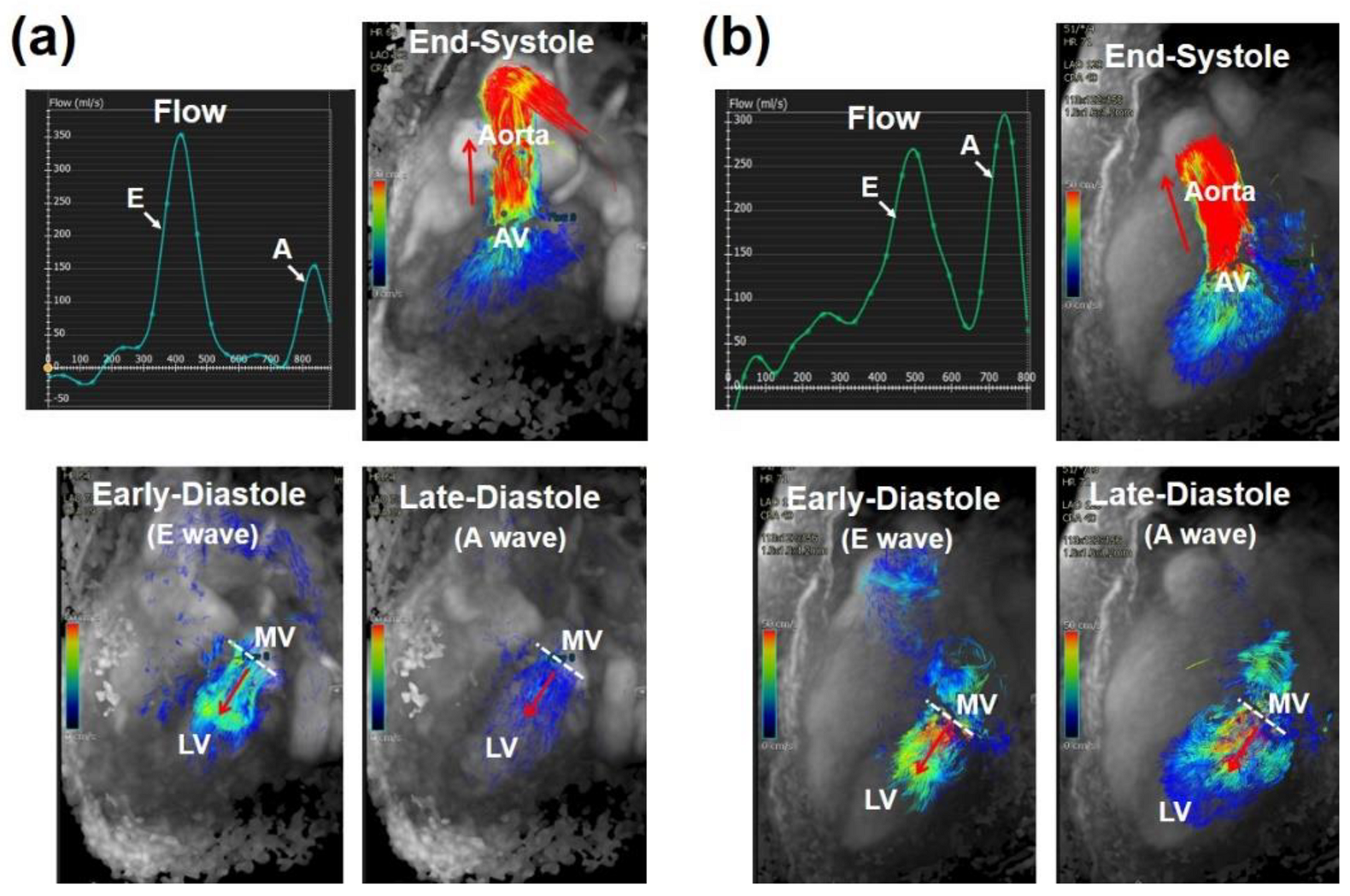
2.5. Four-Dimensional Flow Cardiac MRI (4D-Flow)
3. Cardiovascular Measures
3.1. Temporal Resolution
3.2. Late Diastole
3.3. Untwisting Motion
3.4. Vorticity
3.5. Left Atrium
3.6. Mitral Annulus
4. Heart Failure with Preserved Ejection Fraction
5. Effectors of Diastolic Function
5.1. Aging
5.2. Diabetes
5.3. Metabolic Syndrome
6. Coronary Artery Disease
6.1. Acute Coronary Syndrome
6.2. Myocardial Infarction
7. The Right Ventricle
7.1. Pulmonary Hypertension
7.2. Chronic Obstructive Pulmonary Disease
7.3. Tetralogy of Fallot
8. Other Related Heart Diseases
8.1. Aortic Stenosis
8.2. Hypertrophy
8.3. Thalassemia
8.4. Pericarditis
8.5. Cardiotoxicity
8.6. Cardiac Allograft
9. Summary
Funding
Conflicts of Interest
References
- Cheng-Baron, J.; Chow, K.; Khoo, N.S.; Esch, B.T.; Scott, J.M.; Haykowsky, M.J.; Tyberg, J.V.; Thompson, R.B. Measurements of changes in left ventricular volume, strain, and twist during isovolumic relaxation using MRI. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1908–H1918. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson, R.B.; Paterson, I.; Chow, K.; Cheng-Baron, J.; Scott, J.M.; Esch, B.T.; Ennis, D.B.; Haykowsky, M.J. Characterization of the relationship between systolic shear strain and early diastolic shear strain rates: Insights into torsional recoil. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H898–H907. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Zile, M.R. Left ventricular diastolic dysfunction and diastolic heart failure. Annu. Rev. Med. 2004, 55, 373–394. [Google Scholar] [CrossRef] [PubMed]
- From, A.M.; Scott, C.G.; Chen, H.H. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Dusch, M.N.; Thadani, S.R.; Dhillon, G.S.; Hope, M.D. Diastolic function assessed by cardiac MRI using longitudinal left ventricular fractional shortening. Clin. Imaging 2014, 38, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Steendijk, P. Heart failure with preserved ejection fraction. Diastolic dysfunction, subtle systolic dysfunction, systolic-ventricular and arterial stiffening, or misdiagnosis? Cardiovasc. Res. 2004, 64, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Kermer, J.; Traber, J.; Utz, W.; Hennig, P.; Menza, M.; Jung, B.; Greiser, A.; Barckow, P.; von Knobelsdorff-Brenkenhoff, F.; Topper, A.; et al. Assessment of diastolic dysfunction: Comparison of different cardiovascular magnetic resonance techniques. ESC Heart Fail. 2020, 7, 2637–2649. [Google Scholar] [CrossRef]
- Wu, V.; Chyou, J.Y.; Chung, S.; Bhagavatula, S.; Axel, L. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: Comparison with tissue Doppler imaging. J. Cardiovasc. Magn. Reson. 2014, 16, 71. [Google Scholar] [CrossRef]
- Paelinck, B.P.; Lamb, H.J.; Bax, J.J.; Van der Wall, E.E.; de Roos, A. Assessment of diastolic function by cardiovascular magnetic resonance. Am. Heart J. 2002, 144, 198–205. [Google Scholar] [CrossRef]
- Yamanari, H.; Kakishita, M.; Fujimoto, Y.; Hashimoto, K.; Kiyooka, T.; Katayama, Y.; Otsuka, F.; Emori, T.; Uchida, S.; Ohe, T. Effect of regional myocardial perfusion abnormalities on regional myocardial early diastolic function in patients with hypertrophic cardiomyopathy. Heart Vessel. 1997, 12, 192–198. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.; Doornbos, J.; Luyten, P.R.; Oosterwaal, L.J.; van der Wall, E.E.; den Hollander, J.A. Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: Assessment with proton-decoupled P-31 MR spectroscopy. J. Magn. Reson. Imaging 1992, 2, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.I.; Sieverding, L.; Breuer, J.; Hoess, T.; Widmaier, S.; Schmidt, O.; Bunse, M.; van Erckelens, F.; Apitz, J.; Lutz, O.; et al. 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 1998, 97, 2536–2542. [Google Scholar] [CrossRef]
- Petrank, Y.F.; Dong, S.J.; Tyberg, J.; Sideman, S.; Beyar, R. Regional differences in shape and load in normal and diseased hearts studied by three dimensional tagged magnetic resonance imaging. Int. J. Card. Imaging 1999, 15, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Stuber, M.; Scheidegger, M.B.; Fischer, S.E.; Nagel, E.; Steinemann, F.; Hess, O.M.; Boesiger, P. Alterations in the local myocardial motion pattern in patients suffering from pressure overload due to aortic stenosis. Circulation 1999, 100, 361–368. [Google Scholar] [CrossRef]
- Hartiala, J.J.; Foster, E.; Fujita, N.; Mostbeck, G.H.; Caputo, G.R.; Fazio, G.P.; Winslow, T.; Higgins, C.B. Evaluation of left atrial contribution to left ventricular filling in aortic stenosis by velocity-encoded cine MRI. Am. Heart J. 1994, 127, 593–600. [Google Scholar] [CrossRef]
- Kudelka, A.M.; Turner, D.A.; Liebson, P.R.; Macioch, J.E.; Wang, J.Z.; Barron, J.T. Comparison of cine magnetic resonance imaging and Doppler echocardiography for evaluation of left ventricular diastolic function. Am. J. Cardiol. 1997, 80, 384–386. [Google Scholar] [CrossRef]
- Nagel, E.; Stuber, M.; Burkhard, B.; Fischer, S.E.; Scheidegger, M.B.; Boesiger, P.; Hess, O.M. Cardiac rotation and relaxation in patients with aortic valve stenosis. Eur. Heart J. 2000, 21, 582–589. [Google Scholar] [CrossRef]
- Lamb, H.J.; Beyerbacht, H.P.; van der Laarse, A.; Stoel, B.C.; Doornbos, J.; van der Wall, E.E.; de Roos, A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 1999, 99, 2261–2267. [Google Scholar] [CrossRef]
- Karwatowski, S.P.; Brecker, S.J.; Yang, G.Z.; Firmin, D.N.; St John Sutton, M.; Underwood, S.R. A comparison of left ventricular myocardial velocity in diastole measured by magnetic resonance and left ventricular filling measured by Doppler echocardiography. Eur. Heart J. 1996, 17, 795–802. [Google Scholar] [CrossRef]
- Bogaert, J.; Bosmans, H.; Maes, A.; Suetens, P.; Marchal, G.; Rademakers, F.E. Remote myocardial dysfunction after acute anterior myocardial infarction: Impact of left ventricular shape on regional function: A magnetic resonance myocardial tagging study. J. Am. Coll. Cardiol. 2000, 35, 1525–1534. [Google Scholar] [CrossRef]
- Nagel, E.; Stuber, M.; Lakatos, M.; Scheidegger, M.B.; Boesiger, P.; Hess, O.M. Cardiac rotation and relaxation after anterolateral myocardial infarction. Coron. Artery Dis. 2000, 11, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Garot, J.; Bluemke, D.A.; Osman, N.F.; Rochitte, C.E.; McVeigh, E.R.; Zerhouni, E.A.; Prince, J.L.; Lima, J.A. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation 2000, 101, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Helbing, W.A.; Niezen, R.A.; Le Cessie, S.; van der Geest, R.J.; Ottenkamp, J.; de Roos, A. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: Volumetric evaluation by magnetic resonance velocity mapping. J. Am. Coll. Cardiol. 1996, 28, 1827–1835. [Google Scholar] [CrossRef]
- Rebergen, S.A.; Helbing, W.A.; van der Wall, E.E.; Maliepaard, C.; Chin, J.G.; de Roos, A. MR velocity mapping of tricuspid flow in healthy children and in patients who have undergone Mustard or Senning repair. Radiology 1995, 194, 505–512. [Google Scholar] [CrossRef]
- Singh, G.K.; Greenberg, S.B.; Yap, Y.S.; Delany, D.P.; Keeton, B.R.; Monro, J.L. Right ventricular function and exercise performance late after primary repair of tetralogy of Fallot with the transannular patch in infancy. Am. J. Cardiol. 1998, 81, 1378–1382. [Google Scholar] [CrossRef]
- Fogel, M.A.; Weinberg, P.M.; Gupta, K.B.; Rychik, J.; Hubbard, A.; Hoffman, E.A.; Haselgrove, J. Mechanics of the single left ventricle: A study in ventricular-ventricular interaction II. Circulation 1998, 98, 330–338. [Google Scholar] [CrossRef]
- Young, A.A.; Cowan, B.R.; Thrupp, S.F.; Hedley, W.J.; Dell’Italia, L.J. Left ventricular mass and volume: Fast calculation with guide-point modeling on MR images. Radiology 2000, 216, 597–602. [Google Scholar] [CrossRef]
- Nacif, M.S.; Almeida, A.L.C.; Young, A.A.; Cowan, B.R.; Armstrong, A.C.; Yang, E.; Sibley, C.T.; Hundley, W.G.; Liu, S.; Lima, J.A.; et al. Three-Dimensional Volumetric Assessment of Diastolic Function by Cardiac Magnetic Resonance Imaging: The Multi-Ethnic Study of Atherosclerosis (MESA). Arq. Bras. Cardiol. 2017, 108, 552–563. [Google Scholar] [CrossRef]
- Ibrahim, E.S.H. Heart Mechanics: Magnetic Resonance Imaging; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Edvardsen, T.; Rosen, B.D.; Pan, L.; Jerosch-Herold, M.; Lai, S.; Hundley, W.G.; Sinha, S.; Kronmal, R.A.; Bluemke, D.A.; Lima, J.A. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging—the Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2006, 151, 109–114. [Google Scholar] [CrossRef]
- Ambale-Venkatesh, B.; Armstrong, A.C.; Liu, C.Y.; Donekal, S.; Yoneyama, K.; Wu, C.O.; Gomes, A.S.; Hundley, G.W.; Bluemke, D.A.; Lima, J.A. Diastolic function assessed from tagged MRI predicts heart failure and atrial fibrillation over an 8-year follow-up period: The multi-ethnic study of atherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.E.; McKinnon, G.C.; Maier, S.E.; Boesiger, P. Improved myocardial tagging contrast. Magn. Reson. Med. 1993, 30, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, P.P.; Larghat, A.; Zaman, A.; Fairbairn, T.A.; Motwani, M.; Greenwood, J.P.; Plein, S. Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J. Magn. Reson. Imaging 2014, 39, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.S.H. Cardiovascular Magnetic Resonance Tagging for Assessment of Left Ventricular Diastolic Function. In Cardiovascular Magnetic Resonance: A Companion to Braunwald’s Heart Disease, 3rd ed.; Manning, W.J., Pennell, D.J., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 282–290. [Google Scholar]
- Feinstein, J.A.; Epstein, F.H.; Arai, A.E.; Foo, T.K.; Hartley, M.R.; Balaban, R.S.; Wolff, S.D. Using cardiac phase to order reconstruction (CAPTOR): A method to improve diastolic images. J. Magn. Reson. Imaging 1997, 7, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Ennis, D.B.; Epstein, F.H.; Kellman, P.; Fananapazir, L.; McVeigh, E.R.; Arai, A.E. Assessment of regional systolic and diastolic dysfunction in familial hypertrophic cardiomyopathy using MR tagging. Magn. Reson. Med. 2003, 50, 638–642. [Google Scholar] [CrossRef]
- Uddin, A.; Fairbairn, T.; Swoboda, P.; Kidambi, A.; Motwani, M.; Ripley, D.; Al Musa, T.; McDiarmid, A.; Plein, S.; Greenwood, J. 119 Cardiovascular Magnetic Resonance Evaluation of Change in Myocardial Strain following Transcatheter Aortic Valve Implantation (TAVI) and Surgical Aortic Valve Replacement (SAVR). Heart 2014, 100, A68. [Google Scholar] [CrossRef]
- Ng, M.Y.; Tong, X.; He, J.; Lin, Q.; Luo, L.; Chen, Y.; Shen, X.P.; Wan, E.Y.F.; Yan, A.T.; Yiu, K.H. Feature tracking for assessment of diastolic function by cardiovascular magnetic resonance imaging. Clin. Radiol. 2020, 75, 321.e1–321.e11. [Google Scholar] [CrossRef]
- Buss, S.J.; Krautz, B.; Schnackenburg, B.; Abdel-Aty, H.; Santos, M.F.; Andre, F.; Maertens, M.J.; Mereles, D.; Korosoglou, G.; Giannitsis, E.; et al. Classification of diastolic function with phase-contrast cardiac magnetic resonance imaging: Validation with echocardiography and age-related reference values. Clin. Res. Cardiol. 2014, 103, 441–450. [Google Scholar] [CrossRef]
- Paelinck, B.P.; de Roos, A.; Bax, J.J.; Bosmans, J.M.; van Der Geest, R.J.; Dhondt, D.; Parizel, P.M.; Vrints, C.J.; Lamb, H.J. Feasibility of tissue magnetic resonance imaging: A pilot study in comparison with tissue Doppler imaging and invasive measurement. J. Am. Coll. Cardiol. 2005, 45, 1109–1116. [Google Scholar] [CrossRef]
- Ashrafpoor, G.; Bollache, E.; Redheuil, A.; De Cesare, A.; Giron, A.; Defrance, C.; Azarine, A.; Perdrix, L.; Ladouceur, M.; Diebold, B.; et al. Age-specific changes in left ventricular diastolic function: A velocity-encoded magnetic resonance imaging study. Eur. Radiol. 2015, 25, 1077–1086. [Google Scholar] [CrossRef]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhall, C.J. Four-dimensional blood flow-specific markers of LV dysfunction in dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Svalbring, E.; Fredriksson, A.; Eriksson, J.; Dyverfeldt, P.; Ebbers, T.; Bolger, A.F.; Engvall, J.; Carlhall, C.J. Altered Diastolic Flow Patterns and Kinetic Energy in Subtle Left Ventricular Remodeling and Dysfunction Detected by 4D Flow MRI. PLoS ONE 2016, 11, e0161391. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Cheong, B.; Pednekar, A.; Muthupillai, R. High frame rate cardiac cine MRI for the evaluation of diastolic function and its direct correlation with echocardiography. J. Magn. Reson. Imaging 2019, 50, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H. Editorial for Cardiac MRI Reveals Late Diastolic Changes in Left Ventricular Relaxation Patterns During Healthy Aging. J. Magn. Reson. Imaging 2021, 53, 775–776. [Google Scholar] [CrossRef]
- Kuijer, J.P.; Marcus, J.T.; Gotte, M.J.; van Rossum, A.C.; Heethaar, R.M. Three-dimensional myocardial strains at end-systole and during diastole in the left ventricle of normal humans. J. Cardiovasc. Magn. Reson. 2002, 4, 341–351. [Google Scholar] [CrossRef]
- Wang, J.; Khoury, D.S.; Thohan, V.; Torre-Amione, G.; Nagueh, S.F. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 2007, 115, 1376–1383. [Google Scholar] [CrossRef]
- Kowallick, J.T.; Lamata, P.; Hussain, S.T.; Kutty, S.; Steinmetz, M.; Sohns, J.M.; Fasshauer, M.; Staab, W.; Unterberg-Buchwald, C.; Bigalke, B.; et al. Quantification of left ventricular torsion and diastolic recoil using cardiovascular magnetic resonance myocardial feature tracking. PLoS ONE 2014, 9, e109164. [Google Scholar] [CrossRef]
- Dorfman, T.A.; Rosen, B.D.; Perhonen, M.A.; Tillery, T.; McColl, R.; Peshock, R.M.; Levine, B.D. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. J. Appl. Physiol. 2008, 104, 1037–1044. [Google Scholar] [CrossRef]
- Matter, C.; Mandinov, L.; Kaufmann, P.; Nagel, E.; Boesiger, P.; Hess, O.M. Function of the residual myocardium after infarct and prognostic significance. Z. Kardiol. 1997, 86, 684–690. [Google Scholar] [CrossRef]
- Schafer, M.; Humphries, S.; Stenmark, K.R.; Kheyfets, V.O.; Buckner, J.K.; Hunter, K.S.; Fenster, B.E. 4D-flow cardiac magnetic resonance-derived vorticity is sensitive marker of left ventricular diastolic dysfunction in patients with mild-to-moderate chronic obstructive pulmonary disease. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 415–424. [Google Scholar] [CrossRef]
- Martinez-Legazpi, P.; Bermejo, J.; Benito, Y.; Yotti, R.; Perez Del Villar, C.; Gonzalez-Mansilla, A.; Barrio, A.; Villacorta, E.; Sanchez, P.L.; Fernandez-Aviles, F.; et al. Contribution of the diastolic vortex ring to left ventricular filling. J. Am. Coll. Cardiol. 2014, 64, 1711–1721. [Google Scholar] [CrossRef]
- Abe, H.; Caracciolo, G.; Kheradvar, A.; Pedrizzetti, G.; Khandheria, B.K.; Narula, J.; Sengupta, P.P. Contrast echocardiography for assessing left ventricular vortex strength in heart failure: A prospective cohort study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1049–1060. [Google Scholar] [CrossRef]
- Seemann, F.; Baldassarre, L.A.; Llanos-Chea, F.; Gonzales, R.A.; Grunseich, K.; Hu, C.; Sugeng, L.; Meadows, J.; Heiberg, E.; Peters, D.C. Assessment of diastolic function and atrial remodeling by MRI-validation and correlation with echocardiography and filling pressure. Physiol. Rep. 2018, 6, e13828. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Pizzino, F.; Terrizzi, A.; Carerj, S.; Khandheria, B.K.; Di Bella, G. Diastolic dysfunction evaluated by cardiac magnetic resonance: The value of the combined assessment of atrial and ventricular function. Eur. Radiol. 2019, 29, 1555–1564. [Google Scholar] [CrossRef]
- Morris, D.A.; Gailani, M.; Vaz Perez, A.; Blaschke, F.; Dietz, R.; Haverkamp, W.; Ozcelik, C. Left atrial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2011, 24, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Moreira, H.T.; Bettencourt, N.; Fontes-Carvalho, R.; Sampaio, F.; Ambale-Venkatesh, B.; Wu, C.; Liu, K.; Bertoni, A.G.; Ouyang, P.; et al. Metabolic Syndrome Is Associated With Impaired Diastolic Function Independently of MRI-Derived Myocardial Extracellular Volume: The MESA Study. Diabetes 2018, 67, 1007–1012. [Google Scholar] [CrossRef]
- Mordi, I.R.; Singh, S.; Rudd, A.; Srinivasan, J.; Frenneaux, M.; Tzemos, N.; Dawson, D.K. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC Cardiovasc. Imaging 2018, 11, 577–585. [Google Scholar] [CrossRef]
- Choi, E.Y.; Rosen, B.D.; Fernandes, V.R.; Yan, R.T.; Yoneyama, K.; Donekal, S.; Opdahl, A.; Almeida, A.L.; Wu, C.O.; Gomes, A.S.; et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 2013, 34, 2354–2361. [Google Scholar] [CrossRef]
- Ito, H.; Ishida, M.; Makino, W.; Goto, Y.; Ichikawa, Y.; Kitagawa, K.; Omori, T.; Dohi, K.; Ito, M.; Sakuma, H. Cardiovascular magnetic resonance feature tracking for characterization of patients with heart failure with preserved ejection fraction: Correlation of global longitudinal strain with invasive diastolic functional indices. J. Cardiovasc. Magn. Reson. 2020, 22, 42. [Google Scholar] [CrossRef]
- Rommel, K.P.; von Roeder, M.; Latuscynski, K.; Oberueck, C.; Blazek, S.; Fengler, K.; Besler, C.; Sandri, M.; Lucke, C.; Gutberlet, M.; et al. Extracellular Volume Fraction for Characterization of Patients With Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2016, 67, 1815–1825. [Google Scholar] [CrossRef]
- Eichhorn, E.J.; Willard, J.E.; Alvarez, L.; Kim, A.S.; Glamann, D.B.; Risser, R.C.; Grayburn, P.A. Are contraction and relaxation coupled in patients with and without congestive heart failure? Circulation 1992, 85, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Claus, P.; Omar, A.M.S.; Pedrizzetti, G.; Sengupta, P.P.; Nagel, E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc. Imaging 2015, 8, 1444–1460. [Google Scholar] [CrossRef]
- van Heerebeek, L.; Hamdani, N.; Handoko, M.L.; Falcao-Pires, I.; Musters, R.J.; Kupreishvili, K.; Ijsselmuiden, A.J.; Schalkwijk, C.G.; Bronzwaer, J.G.; Diamant, M.; et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008, 117, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ellims, A.H.; Iles, L.M.; Ling, L.H.; Hare, J.L.; Kaye, D.M.; Taylor, A.J. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J. Cardiovasc. Magn. Reson. 2012, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.S.H.; Miller, A.B.; White, R.D. The relationship between aortic stiffness and E/A filling ratio and myocardial strain in the context of left ventricular diastolic dysfunction in heart failure with normal ejection fraction: Insights from magnetic resonance imaging. Magn. Reson. Imaging 2011, 29, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ma, H.; Sarnari, R.; Li, D.; Lloyd-Jones, D.M.; Markl, M.; Carr, J.C. Cardiac MRI Reveals Late Diastolic Changes in Left Ventricular Relaxation Patterns During Healthy Aging. J. Magn. Reson. Imaging 2021, 53, 766–774. [Google Scholar] [CrossRef]
- Oxenham, H.C.; Young, A.A.; Cowan, B.R.; Gentles, T.L.; Occleshaw, C.J.; Fonseca, C.G.; Doughty, R.N.; Sharpe, N. Age-related changes in myocardial relaxation using three-dimensional tagged magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2003, 5, 421–430. [Google Scholar] [CrossRef]
- Foll, D.; Jung, B.; Schilli, E.; Staehle, F.; Geibel, A.; Hennig, J.; Bode, C.; Markl, M. Magnetic resonance tissue phase mapping of myocardial motion: New insight in age and gender. Circ. Cardiovasc. Imaging 2010, 3, 54–64. [Google Scholar] [CrossRef]
- Fonseca, C.G.; Oxenham, H.C.; Cowan, B.R.; Occleshaw, C.J.; Young, A.A. Aging alters patterns of regional nonuniformity in LV strain relaxation: A 3-D MR tissue tagging study. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H621–H630. [Google Scholar] [CrossRef]
- Maceira, A.M.; Prasad, S.K.; Khan, M.; Pennell, D.J. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur. Heart J. 2006, 27, 2879–2888. [Google Scholar] [CrossRef]
- Tandri, H.; Daya, S.K.; Nasir, K.; Bomma, C.; Lima, J.A.; Calkins, H.; Bluemke, D.A. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am. J. Cardiol. 2006, 98, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Graca, B.; Donato, P.; Ferreira, M.J.; Castelo-Branco, M.; Caseiro-Alves, F. Left ventricular diastolic function in type 2 diabetes mellitus and the association with coronary artery calcium score: A cardiac MRI study. Am. J. Roentgenol. 2014, 202, 1207–1214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Bonito, P.; Moio, N.; Cavuto, L.; Covino, G.; Murena, E.; Scilla, C.; Turco, S.; Capaldo, B.; Sibilio, G. Early detection of diabetic cardiomyopathy: Usefulness of tissue Doppler imaging. Diabet. Med. 2005, 22, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Henriksen, J.E.; Dahl, J.; Johansen, A.; Gerke, O.; Vach, W.; Haghfelt, T.; Hoilund-Carlsen, P.F.; Beck-Nielsen, H.; Moller, J.E. Left ventricular diastolic function in type 2 diabetes mellitus: Prevalence and association with myocardial and vascular disease. Circ. Cardiovasc. Imaging 2010, 3, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Little, W.C. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr. Opin. Cardiol. 2010, 25, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Oxlund, H.; Rasmussen, L.M.; Andreassen, T.T.; Heickendorff, L. Increased aortic stiffness in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1989, 32, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Jellis, C.; Martin, J.; Narula, J.; Marwick, T.H. Assessment of nonischemic myocardial fibrosis. J. Am. Coll. Cardiol. 2010, 56, 89–97. [Google Scholar] [CrossRef]
- Van Schinkel, L.D.; Auger, D.; van Elderen, S.G.; Ajmone Marsan, N.; Delgado, V.; Lamb, H.J.; Ng, A.C.; Smit, J.W.; Bax, J.J.; Westenberg, J.J.; et al. Aortic stiffness is related to left ventricular diastolic function in patients with diabetes mellitus type 1: Assessment with MRI and speckle tracking strain analysis. Int. J. Cardiovasc. Imaging 2013, 29, 633–641. [Google Scholar] [CrossRef]
- Leite-Moreira, A.F.; Correia-Pinto, J.; Gillebert, T.C. Afterload induced changes in myocardial relaxation: A mechanism for diastolic dysfunction. Cardiovasc. Res. 1999, 43, 344–353. [Google Scholar] [CrossRef]
- Aronson, D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J. Hypertens. 2003, 21, 3–12. [Google Scholar] [CrossRef]
- Witteles, R.M.; Fowler, M.B. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 2008, 51, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Colletti, P.M.; Dustin, L.D.; Wong, N.D.; Shriki, J.E.; Kawakubo, M.; Azen, S.P.; Detrano, R.C. Does coronary calcium score predict future cardiac function? Association of subclinical atherosclerosis with left ventricular systolic and diastolic dysfunction at MR imaging in an elderly cohort. Radiology 2010, 257, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Appleton, C.P.; Lopez, A.G.; Cha, S.; Hurst, R.T. Coronary artery plaque burden does not affect left ventricular diastolic function in asymptomatic adults with normal ejection fraction. J. Am. Soc. Echocardiogr. 2011, 24, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Azarisman, S.M.; Teo, K.S.; Worthley, M.I.; Worthley, S.G. Cardiac magnetic resonance assessment of diastolic dysfunction in acute coronary syndrome. J. Int. Med. Res. 2017, 45, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Antman, E.M.; Beasley, J.W.; Califf, R.M.; Cheitlin, M.D.; Hochman, J.S.; Jones, R.H.; Kereiakes, D.; Kupersmith, J.; Levin, T.N.; et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: Executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation 2000, 102, 1193–1209. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.H.; Aufderheide, T.P.; Ruthazer, R.; Woolard, R.H.; Feldman, J.A.; Beshansky, J.R.; Griffith, J.L.; Selker, H.P. Missed diagnoses of acute cardiac ischemia in the emergency department. N. Engl. J. Med. 2000, 342, 1163–1170. [Google Scholar] [CrossRef]
- Nijland, F.; Kamp, O.; Karreman, A.J.; van Eenige, M.J.; Visser, C.A. Prognostic implications of restrictive left ventricular filling in acute myocardial infarction: A serial Doppler ec.chocardiographic study. J. Am. Coll. Cardiol. 1997, 30, 1618–1624. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Schussheim, A.E.; Rekhraj, S.; Aletras, A.H.; Geller, N.; Davis, J.; Christian, T.F.; Balaban, R.S.; Arai, A.E. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 2003, 107, 531–537. [Google Scholar] [CrossRef]
- Azevedo, C.F.; Amado, L.C.; Kraitchman, D.L.; Gerber, B.L.; Osman, N.F.; Rochitte, C.E.; Edvardsen, T.; Lima, J.A. Persistent diastolic dysfunction despite complete systolic functional recovery after reperfused acute myocardial infarction demonstrated by tagged magnetic resonance imaging. Eur. Heart J. 2004, 25, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, F.E.; Rogers, W.J.; Guier, W.H.; Hutchins, G.M.; Siu, C.O.; Weisfeldt, M.L.; Weiss, J.L.; Shapiro, E.P. Relation of regional cross-fiber shortening to wall thickening in the intact heart. Three-dimensional strain analysis by NMR tagging. Circulation 1994, 89, 1174–1182. [Google Scholar] [CrossRef]
- Kroeker, C.A.; Tyberg, J.V.; Beyar, R. Effects of ischemia on left ventricular apex rotation. An experimental study in anesthetized dogs. Circulation 1995, 92, 3539–3548. [Google Scholar] [CrossRef]
- Tello, K.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Wilhelm, J.; Gall, H.; Richter, M.J. Cardiac Magnetic Resonance Imaging-Based Right Ventricular Strain Analysis for Assessment of Coupling and Diastolic Function in Pulmonary Hypertension. JACC Cardiovasc. Imaging 2019, 12, 2155–2164. [Google Scholar] [CrossRef]
- Watz, H.; Waschki, B.; Meyer, T.; Kretschmar, G.; Kirsten, A.; Claussen, M.; Magnussen, H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: Role of hyperinflation. Chest 2010, 138, 32–38. [Google Scholar] [CrossRef]
- Gatzoulis, M.A.; Clark, A.L.; Cullen, S.; Newman, C.G.; Redington, A.N. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation 1995, 91, 1775–1781. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef]
- Chacko, B.R.; Karur, G.R.; Connelly, K.A.; Yan, R.T.; Kirpalani, A.; Wald, R.; Jimenez-Juan, L.; Jacob, J.R.; Deva, D.P.; Yan, A.T. Left ventricular structure and diastolic function by cardiac magnetic resonance imaging in hypertrophic cardiomyopathy. Indian Heart J. 2018, 70, 75–81. [Google Scholar] [CrossRef]
- Li, Z.L.; He, S.; Xia, C.C.; Peng, W.L.; Li, L.; Liu, K.L.; Zhang, J.G.; Pu, J.; Guo, Y.K. Global longitudinal diastolic strain rate as a novel marker for predicting adverse outcomes in hypertrophic cardiomyopathy by cardiac magnetic resonance tissue tracking. Clin. Radiol. 2021, 76, 78.e19–78.e25. [Google Scholar] [CrossRef]
- Russel, I.K.; Brouwer, W.P.; Germans, T.; Knaapen, P.; Marcus, J.T.; van der Velden, J.; Gotte, M.J.; van Rossum, A.C. Increased left ventricular torsion in hypertrophic cardiomyopathy mutation carriers with normal wall thickness. J. Cardiovasc. Magn. Reson. 2011, 13, 3. [Google Scholar] [CrossRef]
- Chinprateep, B.; Ratanasit, N.; Kaolawanich, Y.; Karaketklang, K.; Saiviroonporn, P.; Viprakasit, V.; Krittayaphong, R. Prevalence of left ventricular diastolic dysfunction by cardiac magnetic resonance imaging in thalassemia major patients with normal left ventricular systolic function. BMC Cardiovasc. Disord. 2019, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, I.; Keren, A.; Yock, P.G.; Allen, M.D.; Modry, D.L.; Zusman, D.R.; Mitchell, R.S.; Miller, D.C.; Popp, R.L. Timing of abnormal interventricular septal motion after cardiopulmonary bypass operations. Lack of injury proved by preoperative, intraoperative, and postoperative echocardiography. J. Thorac. Cardiovasc. Surg. 1986, 91, 619–623. [Google Scholar] [CrossRef]
- Spottiswoode, B.; Russell, J.B.; Moosa, S.; Meintjes, E.M.; Epstein, F.H.; Mayosi, B.M. Abnormal diastolic and systolic septal motion following pericardiectomy demonstrated by cine DENSE MRI. Cardiovasc. J. Afr. 2008, 19, 208–209. [Google Scholar] [PubMed]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Brezden-Masley, C.; Ramanan, V.; Ghugre, N.; Barfett, J.J.; Chan, K.K.W.; Haq, R.; Petrella, T.; Dhir, V.; Jimenez-Juan, L.; et al. Serial Measurements of Left Ventricular Systolic and Diastolic Function by Cardiac Magnetic Resonance Imaging in Patients with Early Stage Breast Cancer on Trastuzumab. Am. J. Cardiol. 2019, 123, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Tjeerdsma, G.; Meinardi, M.T.; van Der Graaf, W.T.; van Den Berg, M.P.; Mulder, N.H.; Crijns, H.J.; de Vries, E.G.; van Veldhuisen, D.J. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: Autonomic versus echocardiographic variables. Heart 1999, 81, 419–423. [Google Scholar] [CrossRef]
- Gong, I.Y.; Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Graham, J.J.; Chow, C.M.; Thavendiranathan, P.; Dai, D.; et al. Early diastolic strain rate measurements by cardiac MRI in breast cancer patients treated with trastuzumab: A longitudinal study. Int. J. Cardiovasc. Imaging 2019, 35, 653–662. [Google Scholar] [CrossRef]
- Jordan, J.H.; Sukpraphrute, B.; Melendez, G.C.; Jolly, M.P.; D’Agostino, R.B., Jr.; Hundley, W.G. Early Myocardial Strain Changes During Potentially Cardiotoxic Chemotherapy May Occur as a Result of Reductions in Left Ventricular End-Diastolic Volume: The Need to Interpret Left Ventricular Strain With Volumes. Circulation 2017, 135, 2575–2577. [Google Scholar] [CrossRef]
- Machida, H.; Nunoda, S.; Okajima, K.; Shitakura, K.; Sekikawa, A.; Kubo, Y.; Otsuka, K.; Hirata, M.; Kojima, S.; Ueno, E. Magnetic resonance assessment of left ventricular diastolic dysfunction for detecting cardiac allograft vasculopathy in recipients of heart transplants. Int. J. Cardiovasc. Imaging 2012, 28, 555–562. [Google Scholar] [CrossRef]
- Derumeaux, G.; Ovize, M.; Loufoua, J.; Andre-Fouet, X.; Minaire, Y.; Cribier, A.; Letac, B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 1998, 97, 1970–1977. [Google Scholar] [CrossRef]
- Sheng, J.; Shi, Y.; Zhang, Q. Improved parallel magnetic resonance imaging reconstruction with multiple variable density sampling. Sci. Rep. 2021, 11, 9005. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Frank, L.; Baruah, D.; Arpinar, V.E.; Nencka, A.S.; Koch, K.M.; Muftuler, L.T.; Unal, O.; Stojanovska, J.; Rubenstein, J.C.; et al. Value CMR: Towards a Comprehensive, Rapid, Cost-Effective Cardiovascular Magnetic Resonance Imaging. Int. J. Biomed. Imaging 2021, 2021, 8851958. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Pennell, D.J. Cardiovascular Magnetic Resonance; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Leiner, T.; Rueckert, D.; Suinesiaputra, A.; Baessler, B.; Nezafat, R.; Isgum, I.; Young, A.A. Machine learning in cardiovascular magnetic resonance: Basic concepts and applications. J. Cardiovasc. Magn. Reson. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]

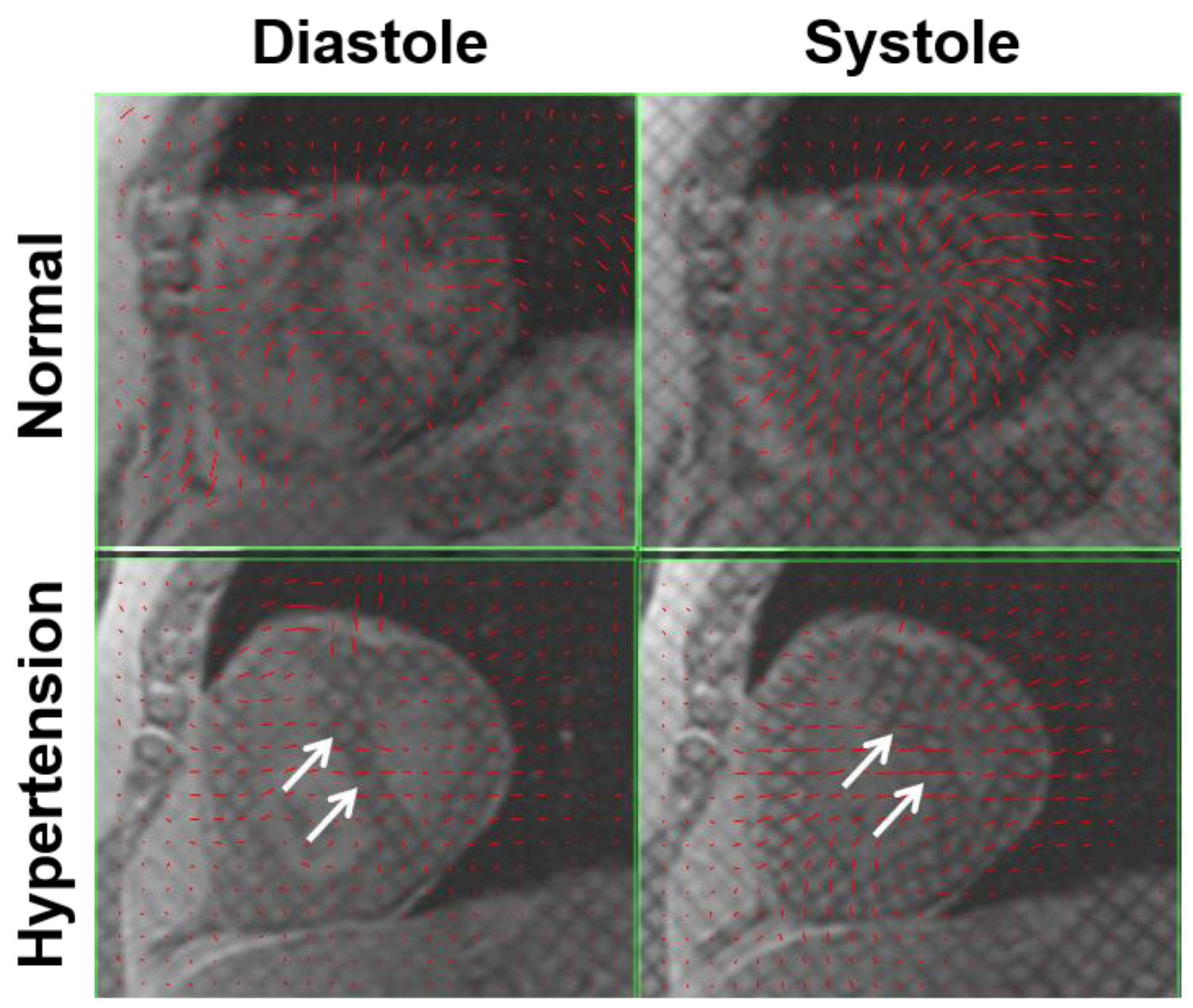
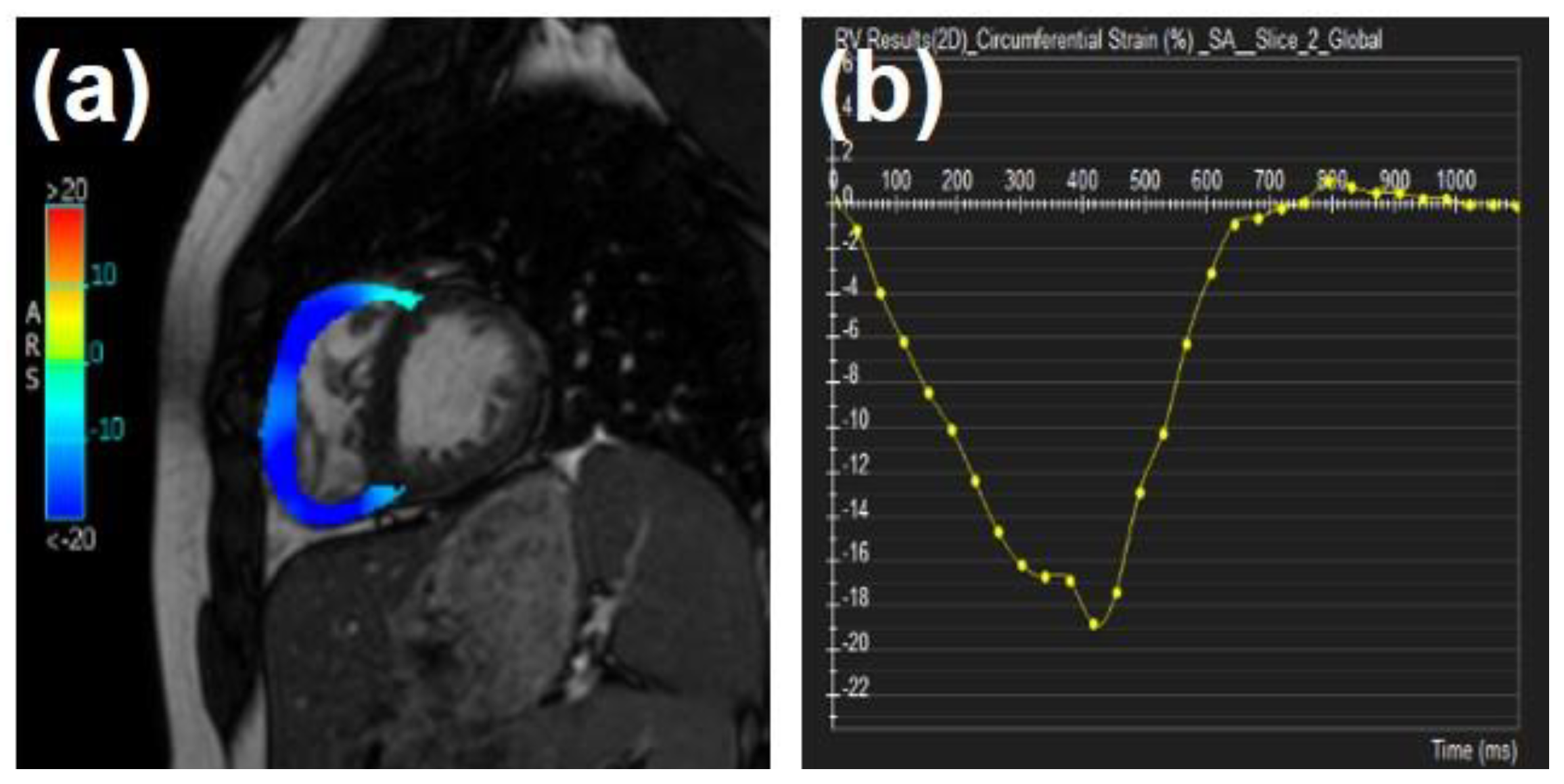
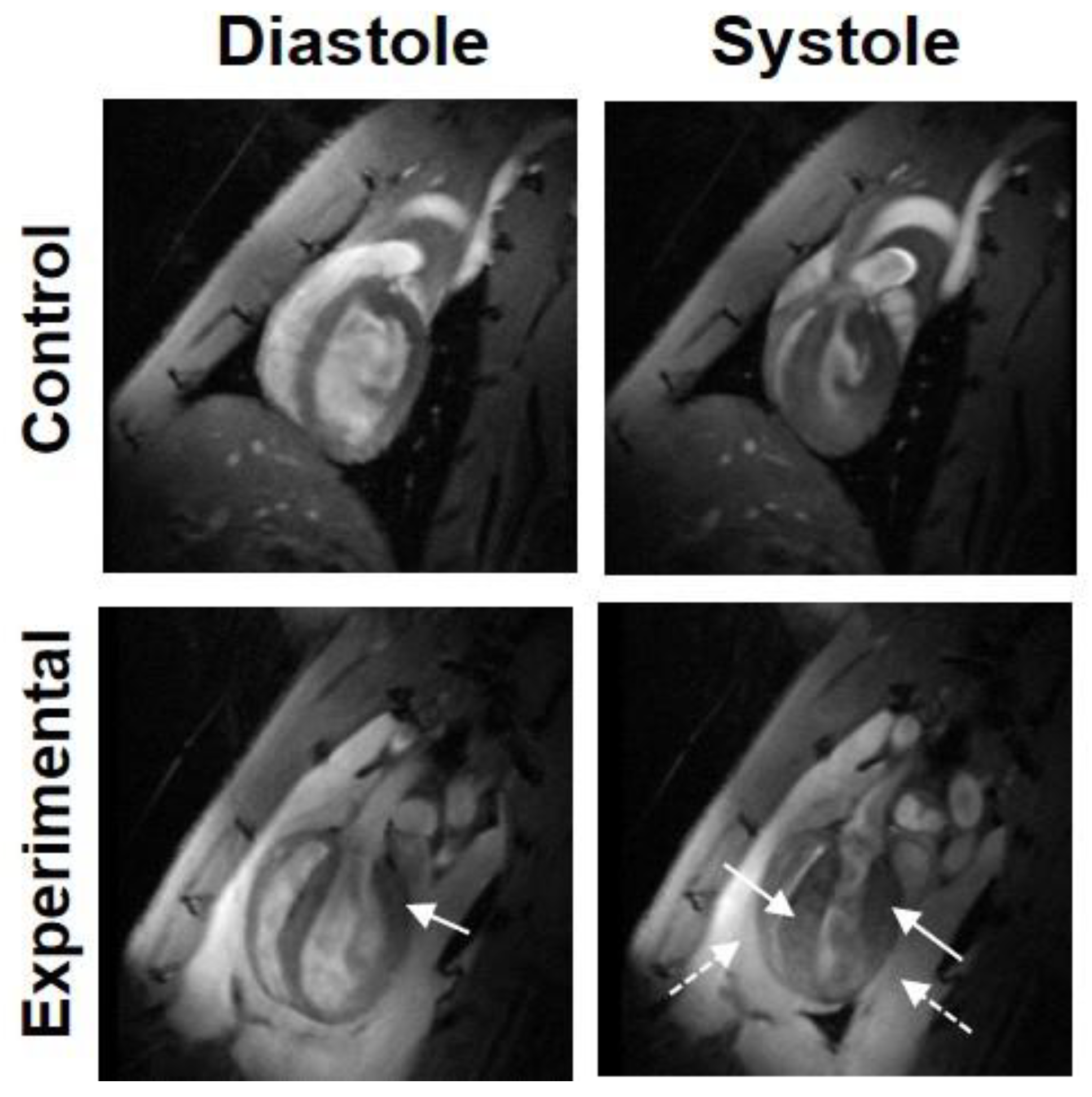
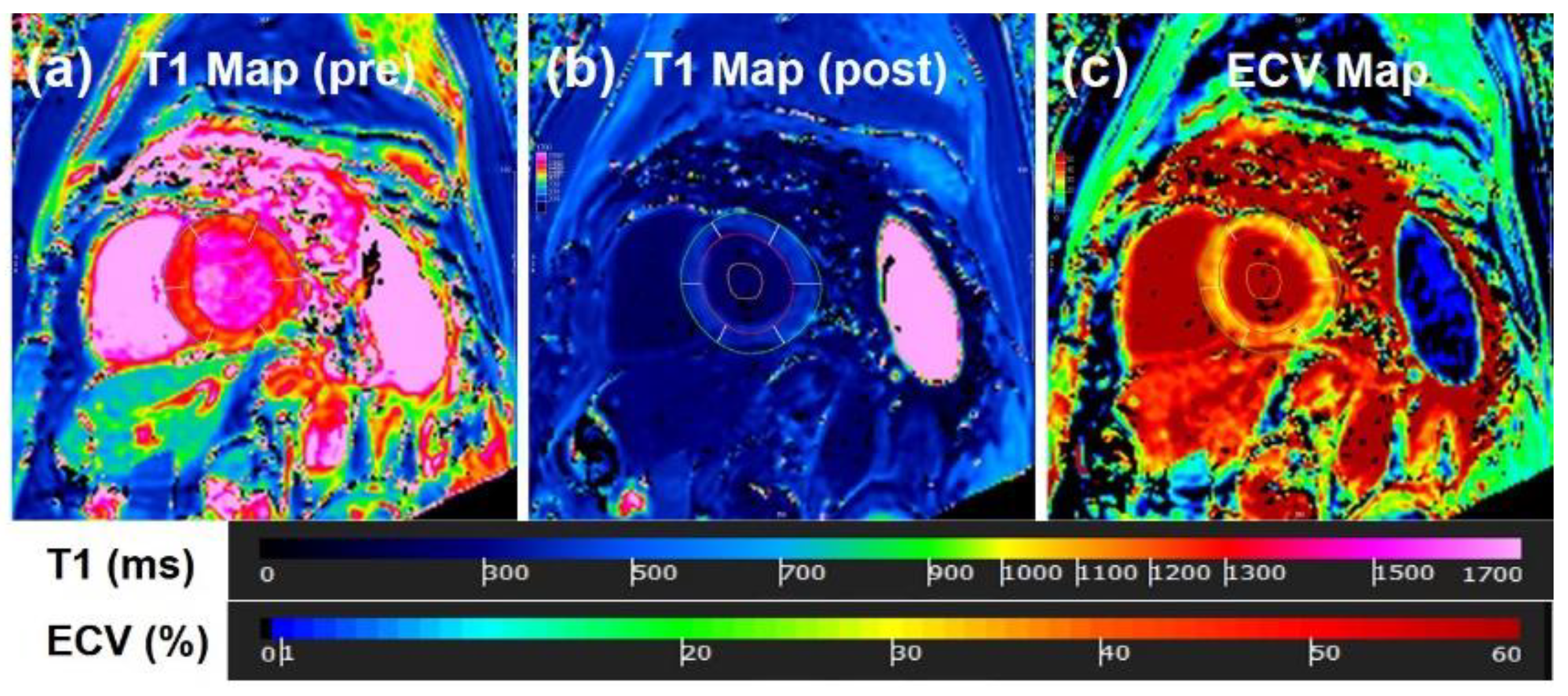
| Type of Disease | MRI Technique | n | Principal Findings |
|---|---|---|---|
| HCM [11] | GRE | 31 | Impairment of regional relaxation |
| HCM [12] | Spectroscopy | 8 | Decreased PCr/ATP in symptomatic patients |
| HCM [13] | Spectroscopy | 8 | Decreased PCr/ATP in asymptomatic patients |
| HCM [14] | Tagging | 17 | Smaller circumferential curvatures in hypertrophy |
| AS [15] | Tagging | 12 | Prolonged and delayed untwisting |
| AS [16] | Phase contrast | 9 | Volumetric mitral flow correlates with Doppler |
| LVH [17] | GRE | 9 | Early detection of filling abnormalities |
| AS [18] | Tagging | 13 | Prolonged and delayed untwisting |
| Hypertensive HD [19] | Spectroscopy | 11 | Decreased PCr/ATP correlates with impaired relaxation |
| Previous MI [20] | Phase contrast | 11 | Early diastolic filling velocities correlate with Doppler |
| Previous MI [21] | Tagging | 16 | Reduction of systolic strains in infarcted and remote area |
| Previous MI [22] | Tagging | 18 | Nonuniform, delayed, and prolonged untwisting |
| CAD/previous MI [20] | GRE | 10/15 | Reduced early diastolic long-axis velocity |
| Previous MI [23] | Tagging | 9 | Reduced systolic strains in asynergic segments |
| Fallot [24] | Phase contrast | 19 | Restrictive flow is associated with decreased exercise |
| Mustard/Senning [25] | Phase contrast | 12 | Restrictive tricuspid flow |
| Fallot [26] | GRE | 10 | Impaired ventricular filling correlates with exercise |
| RVPO [14] | Tagging | 9 | Heterogeneity in strain |
| Single ventricle [27] | Tagging | 10 | Regional decrease in systolic strains |
| Class of Diastolic Dysfunction | HC | I | II | III | p |
|---|---|---|---|---|---|
| n | 25 | 18 | 12 | 10 | |
| LV filling volume (mL) | 69 ± 16 | 66 ± 17 | 61 ± 23 | 69 ± 17 | 0.72 |
| PFR-E (mL/s) | 375 ± 63 I,II | 247 ± 47 H,II,III | 325 ± 47 H,I | 353 ± 92 I | 0.001 |
| PFR-E/LV filling volume (s) | 5.4 ± 1.3 I | 4 ± 0.8 H,III | 4.7 ± 2 | 5.4 ± 1.3 | 0.02 |
| PFR-A (mL/s) | 177 ± 56 I,III | 238 ± 59 H,III | 209 ± 83 III | 136 ± 37 H,I,II | 0.02 |
| PFR-A/LV filling volume (s) | 2.3 ± 1.1 I,II | 3.9 ± 1.2 H,II,III | 1.5 ± 0.8 H,I,III | 2.6 ± 1.6 H,I,II | 0.001 |
| PFR-E/PFR-A | 2.3 ± 1 I | 1.1 ± 0.4 H,II,III | 2.1 ± 1 I | 2.8 ± 1.3 I | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, E.-S.H.; Dennison, J.; Frank, L.; Stojanovska, J. Diastolic Cardiac Function by MRI—Imaging Capabilities and Clinical Applications. Tomography 2021, 7, 893-914. https://doi.org/10.3390/tomography7040075
Ibrahim E-SH, Dennison J, Frank L, Stojanovska J. Diastolic Cardiac Function by MRI—Imaging Capabilities and Clinical Applications. Tomography. 2021; 7(4):893-914. https://doi.org/10.3390/tomography7040075
Chicago/Turabian StyleIbrahim, El-Sayed H., Jennifer Dennison, Luba Frank, and Jadranka Stojanovska. 2021. "Diastolic Cardiac Function by MRI—Imaging Capabilities and Clinical Applications" Tomography 7, no. 4: 893-914. https://doi.org/10.3390/tomography7040075
APA StyleIbrahim, E.-S. H., Dennison, J., Frank, L., & Stojanovska, J. (2021). Diastolic Cardiac Function by MRI—Imaging Capabilities and Clinical Applications. Tomography, 7(4), 893-914. https://doi.org/10.3390/tomography7040075






