[18F] Sodium Fluoride PET Kinetic Parameters in Bone Imaging
Abstract
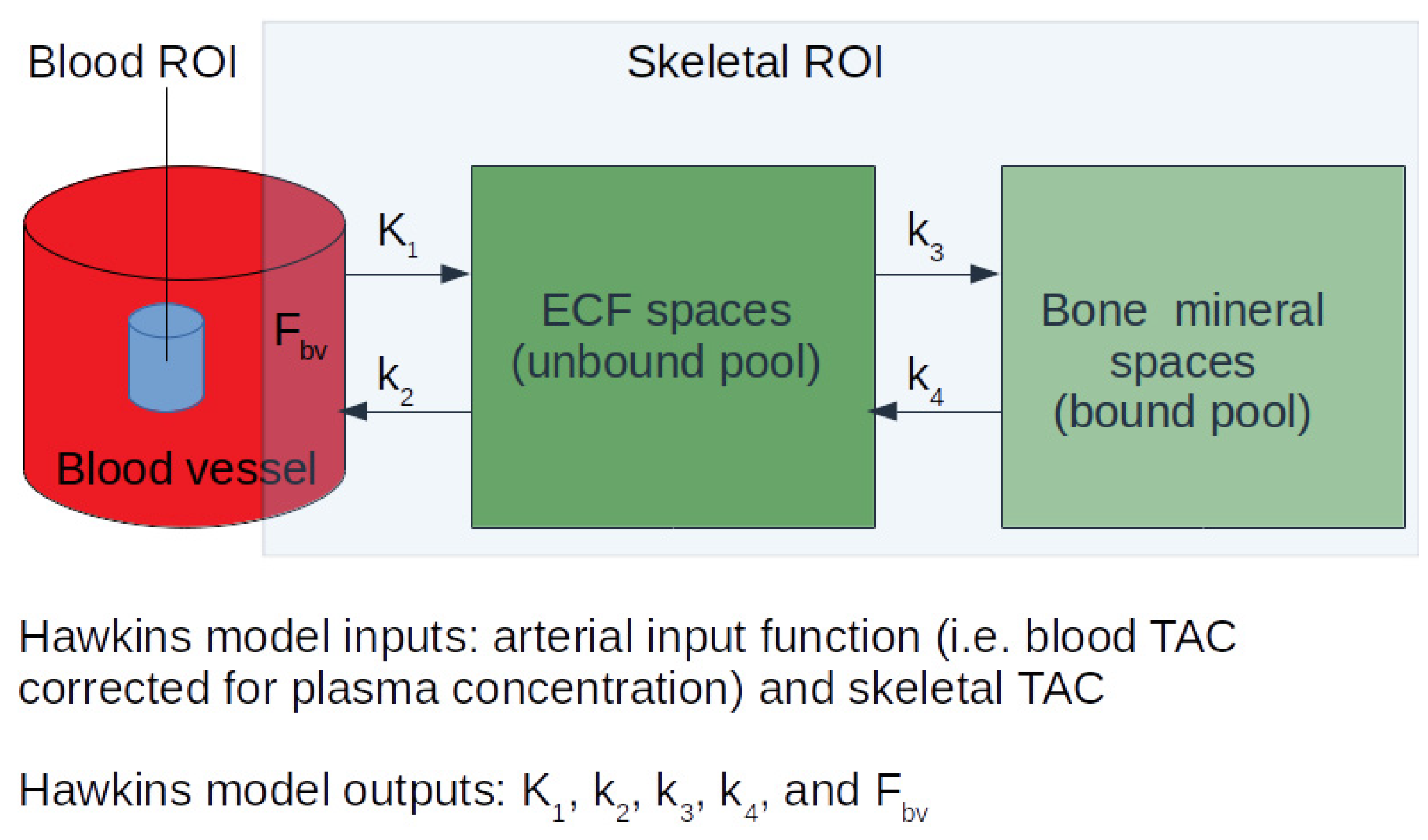
1. Introduction
2. Ki
3. K1
4. k2 and k3
5. k4
6. K1/k2
7. k3/(k2 + k3)
8. Changes in K-Parameters in Response to Treatment
9. Precision Errors in Measuring K-Parameters
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heaney, R.P. Is the paradigm shifting? Bone 2003, 33, 457–465. [Google Scholar] [CrossRef]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Tong, X.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Correlation between (18)F-Sodium Fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone 2020, 134, 115267. [Google Scholar] [CrossRef]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Burton, I.S.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Bone Histomorphometry and (18)F-Sodium Fluoride Positron Emission Tomography Imaging: Comparison Between only Bone Turnover-based and Unified TMV-based Classification of Renal Osteodystrophy. Calcif. Tissue Int. 2021, 109, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Arlot, M.; Meunier, P.J.; Boivin, G.; Haddock, L.; Tamayo, J.; Correa-Rotter, R.; Jasqui, S.; Donley, D.W.; Dalsky, G.P.; Martin, J.S.; et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J. Bone Miner. Res. 2005, 20, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Compston, E.J.; Croucher, P.I. Histomorphometric assessment of trabecular bone remodelling in osteoporosis. Bone Miner. 1991, 14, 91–102. [Google Scholar] [CrossRef]
- Dempster, D.W.; Cosman, F.; Kurland, E.S.; Zhou, H.; Nieves, J.; Woelfert, L.; Shane, E.; Plavetić, K.; Müller, R.; Bilezikian, J.; et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. J. Bone Miner. Res. 2001, 16, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Zhou, H.; Cosman, F.; Nieves, J.; Dempster, D.W.; Hodsman, A.B. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J. Bone Miner. Res. 2007, 22, 495–502. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.J.; Mitlak, B.H.; Wang, O.; Genant, H.K.; Eriksen, E.F. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J. Bone Miner. Res. 2003, 18, 1932–1941. [Google Scholar] [CrossRef]
- Glover, S.J.; Eastell, R.; McCloskey, E.V.; Rogers, A.; Garnero, P.; Lowery, J.; Belleli, R.; Wright, T.M.; John, M.R. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 2009, 45, 1053–1058. [Google Scholar] [CrossRef]
- Garnero, P.; Shih, W.J.; Gineyts, E.; Karpf, D.B.; Delmas, P.D. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J. Clin. Endocrinol. Metab. 1994, 79, 1693–1700. [Google Scholar]
- Garnero, P.; Hausherr, E.; Chapuy, M.C.; Marcelli, C.; Grandjean, H.; Muller, C.; Cormier, C.; Bréart, G.; Meunier, P.J.; Delmas, P.D. Markers of bone resorption predict hip fracture in elderly women: The EPIDOS Prospective Study. J. Bone Miner. Res. 1996, 11, 1531–1538. [Google Scholar] [CrossRef]
- Beck-Jensen, J.E.; Kollerup, G.; Sørensen, H.A.; Pors Nielsen, S.; Sørensen, O.H. A single measurement of biochemical markers of bone turnover has limited utility in the individual person. Scand J. Clin. Lab. Investig. 1997, 57, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.L.; Fogelman, I.; Blake, G.M.; Marsden, P.K.; Cook, G., Jr. Dissociation between global markers of bone formation and direct measurement of spinal bone formation in osteoporosis. J. Bone Miner. Res. 2004, 19, 1797–1804. [Google Scholar] [CrossRef]
- Lenora, J.; Norrgren, K.; Thorsson, O.; Wollmer, P.; Obrant, K.J.; Ivaska, K.K. Bone turnover markers are correlated with total skeletal uptake of 99mTc-methylene diphosphonate (99mTc-MDP). BMC Med. Phys. 2009, 9, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schiepers, C.; Nuyts, J.; Bormans, G.; Dequeker, J.; Bouillon, R.; Mortelmans, L.; Verbruggen, A.; De Roo, M. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J. Nucl. Med. 1997, 38, 1970–1976. [Google Scholar] [PubMed]
- Messa, C.; Goodman, W.G.; Hoh, C.K.; Choi, Y.; Nissenson, A.R.; Salusky, I.B.; Phelps, M.E.; Hawkins, R.A. Bone metabolic activity measured with positron emission tomography and [18F]fluoride ion in renal osteodystrophy: Correlation with bone histomorphometry. J. Clin. Endocrinol. Metab. 1993, 77, 949–955. [Google Scholar] [PubMed]
- O’Connor, J.P.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef]
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Saunders, W.B., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Vandyke, D.; Anger, H.O.; Yano, Y.; Bozzini, C. Bone blood flow shown with F18 and the positron camera. Am. J. Physiol. 1965, 209, 65–70. [Google Scholar]
- Blake, G.M.; Park-Holohan, S.J.; Cook, G.J.; Fogelman, I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin. Nucl. Med. 2001, 31, 28–49. [Google Scholar] [CrossRef]
- Park-Holohan, J.S.; Blake, G.M.; Fogelman, I. Quantitative studies of bone using (18)F-fluoride and (99m)Tc-methylene diphosphonate: Evaluation of renal and whole-blood kinetics. Nucl. Med. Commun. 2001, 22, 1037–1044. [Google Scholar] [CrossRef]
- Moore, E.A.; Blake, G.M.; Fogelman, I. Quantitative measurements of bone remodeling using 99mTc-methylene diphosphonate bone scans and blood sampling. J. Nucl. Med. 2008, 49, 375–382. [Google Scholar] [CrossRef]
- Guillemart, A.; Besnard, J.C.; Le Pape, A.; Galy, G.; Fetissoff, F. Skeletal uptake of pyrophosphate labeled with technetium-95m and technetium-96, as evaluated by autoradiography. J. Nucl. Med. 1978, 19, 895–899. [Google Scholar]
- Schümichen, C.; Rempfle, H.; Wagner, M.; Hoffmann, G. The short-term fixation of radiopharmaceuticals in bone. Eur. J. Nucl. Med. 1979, 4, 423–428. [Google Scholar] [CrossRef]
- Einhorn, A.T.; Vigorita, V.J.; Aaron, A. Localization of technetium-99m methylene diphosphonate in bone using microautoradiography. J. Orthop. Res. 1986, 4, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. Misconceptions (2): Turnover is always higher in cancellous than in cortical bone. Bone 2002, 30, 807–809. [Google Scholar] [CrossRef]
- Fazzalari, N.L. Bone remodeling: A review of the bone microenvironment perspective for fragility fracture (osteoporosis) of the hip. Semin. Cell Dev. Biol. 2008, 19, 467–472. [Google Scholar] [CrossRef]
- Crockett, J.C.; Rogers, M.J.; Coxon, F.P.; Hocking, L.J.; Helfrich, M.H. Bone remodelling at a glance. J. Cell Sci. 2011, 124 Pt 7, 991–998. [Google Scholar] [CrossRef]
- Garnero, P.; Cremers, S. Bone Turnover Markers. Principles of Bone Biolog; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1801–1832. [Google Scholar]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Parfitt, A.M. What is the normal rate of bone remodeling? Bone 2004, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Choi, Y.; Huang, S.C.; Hoh, C.K.; Dahlbom, M.; Schiepers, C.; Satyamurthy, N.; Barrio, J.R.; Phelps, M.E. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J. Nucl. Med. 1992, 33, 633–642. [Google Scholar]
- Azad, G.K.; Siddique, M.; Taylor, B.; Green, A.; O’Doherty, J.; Gariani, J.; Blake, G.M.; Mansi, J.; Goh, V.; Cook, G.J.R. Is Response Assessment of Breast Cancer Bone Metastases Better with Measurement of (18)F-Fluoride Metabolic Flux Than with Measurement of (18)F-Fluoride PET/CT SUV? J. Nucl. Med. 2019, 60, 322–327. [Google Scholar] [CrossRef]
- Frost, M.L.; Cook, G.J.; Blake, G.M.; Marsden, P.K.; Benatar, N.A.; Fogelman, I. A prospective study of risedronate on regional bone metabolism and blood flow at the lumbar spine measured by 18F-fluoride positron emission tomography. J. Bone Miner. Res. 2003, 18, 2215–2222. [Google Scholar] [CrossRef]
- Frost, M.L.; Blake, G.M.; Cook, G.J.; Marsden, P.K.; Fogelman, I. Differences in regional bone perfusion and turnover between lumbar spine and distal humerus: (18)F-fluoride PET study of treatment-naïve and treated postmenopausal women. Bone 2009, 45, 942–948. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Miyazaki, T.; Yayama, T.; Kawahara, H.; Kobayashi, S.; Tsuchida, T.; Okazawa, H.; Fujibayashi, Y.; Baba, H. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: A prospective study. J. Nucl. Med. 2009, 50, 1808–1814. [Google Scholar] [CrossRef]
- Frost, M.L.; Siddique, M.; Blake, G.M.; Moore, A.E.; Schleyer, P.J.; Dunn, J.T.; Somer, E.J.; Marsden, P.K.; Eastell, R.; Fogelman, I. Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J. Bone Miner. Res. 2011, 26, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.L.; Moore, A.E.; Siddique, M.; Blake, G.M.; Laurent, D.; Borah, B.; Schramm, U.; Valentin, M.A.; Pellas, T.C.; Marsden, P.K.; et al. ¹⁸F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: A prospective, randomized, controlled clinical study. J. Bone Miner. Res. 2013, 28, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Installé, J.; Nzeusseu, A.; Bol, A.; Depresseux, G.; Devogelaer, J.P.; Lonneux, M. (18)F-fluoride PET for monitoring therapeutic response in Paget’s disease of bone. J. Nucl. Med. 2005, 46, 1650–1658. [Google Scholar]
- Mathavan, N.; Koopman, J.; Raina, D.B.; Turkiewicz, A.; Tägil, M.; Isaksson, H. (18)F-fluoride as a prognostic indicator of bone regeneration. Acta Biomater. 2019, 90, 403–411. [Google Scholar] [CrossRef]
- Siddique, M.; Blake, G.M.; Frost, M.L.; Moore, A.E.; Puri, T.; Marsden, P.K.; Fogelman, I. Estimation of regional bone metabolism from whole-body 18F-fluoride PET static images. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Siddique, M.M.; Frost, M.L.; Moore, A.E.B.; Blake, G.M. A Short Dynamic Scan Method of Measuring Bone Metabolic Flux Using [18F]NaF PET. Tomography 2021, 7, 623–635. [Google Scholar] [CrossRef]
- Blake, G.M.; Puri, T.; Siddique, M.; Frost, M.L.; Moore, A.E.B.; Fogelman, I. Site specific measurements of bone formation using [(18)F] sodium fluoride PET/CT. Quant. Imaging Med. Surg. 2018, 8, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Kato, K.; Nakagaki, H.; Ohno, N.; Kameyama, Y.; Weatherell, J.A. Distribution of fluoride concentration in the rat’s bone. Calcif. Tissue Int. 1990, 46, 200–204. [Google Scholar] [CrossRef]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Farlay, D.; Khebbab, M.T.; Jaurand, X.; Delmas, P.D.; Meunier, P.J. In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos. Int. 2010, 21, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Puri, T.; Siddique, M.M.; Frost, M.L.; Moore, A.E.B.; Blake, G.M.; Fogelman, I. Comparison of ordered-subset expectation maximization and filtered back projection reconstruction based on quantitative outcome from dynamic [18F]NaF PET images. Nucl. Med. Commun. 2021, 42, 699–706. [Google Scholar] [CrossRef]
- Puri, T.; Blake, G.M.; Curran, K.M.; Carr, H.; Moore, A.E.; Colgan, N.; O’Connell, M.J.; Marsden, P.K.; Fogelman, I.; Frost, M.L. Semiautomatic region-of-interest validation at the femur in (18)F-fluoride PET/CT. J. Nucl. Med. Technol. 2012, 40, 168–174. [Google Scholar] [CrossRef]
- Cook, G.J.; Lodge, M.A.; Blake, G.M.; Marsden, P.K.; Fogelman, I. Differences in skeletal kinetics between vertebral and humeral bone measured by 18F-fluoride positron emission tomography in postmenopausal women. J. Bone Miner. Res. 2000, 15, 763–769. [Google Scholar] [CrossRef]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Greenhalgh, T.A.; Wilson, J.M.; Franklin, J.; Wang, L.M.; Strauss, V.; Cunningham, C.; Partridge, M.; Maughan, T. [(18)F]Fluoromisonidazole PET in rectal cancer. EJNMMI Res. 2017, 7, 78. [Google Scholar] [CrossRef]
- Puri, T.; Blake, G.M.; Frost, M.L.; Moore, A.E.; Siddique, M.; Cook, G.J.; Marsden, P.K.; Fogelman, I.; Curran, K.M. Validation of image-derived arterial input functions at the femoral artery using 18F-fluoride positron emission tomography. Nucl. Med. Commun. 2011, 32, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Blake, G.M.; Siddique, M.; Frost, M.L.; Cook, G.J.; Marsden, P.K.; Fogelman, I.; Curran, K.M. Validation of new image-derived arterial input functions at the aorta using 18F-fluoride positron emission tomography. Nucl. Med. Commun. 2011, 32, 486–495. [Google Scholar] [CrossRef]
- Puri, T.; Blake, G.M.; Frost, M.L.; Siddique, M.; Moore, A.E.; Marsden, P.K.; Cook, G.J.; Fogelman, I.; Curran, K.M. Comparison of six quantitative methods for the measurement of bone turnover at the hip and lumbar spine using 18F-fluoride PET-CT. Nucl. Med. Commun. 2012, 33, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Piert, M.; Zittel, T.T.; Becker, G.A.; Jahn, M.; Stahlschmidt, A.; Maier, G.; Machulla, H.J.; Bares, R. Assessment of Porcine Bone Metabolism by Dynamic [18F]Fluoride Ion PET: Correlation with Bone Histomorphometry. J. Nucl. Med. 2001, 42, 1091–1100. [Google Scholar]
- Blake, M.G.; Frost, M.L.; Fogelman, I. Quantitative radionuclide studies of bone. J. Nucl. Med. 2009, 50, 1747–1750. [Google Scholar] [CrossRef]
- Blake, G.M.; Siddique, M.; Frost, M.L.; Moore, A.E.; Fogelman, I. Radionuclide studies of bone metabolism: Do bone uptake and bone plasma clearance provide equivalent measurements of bone turnover? Bone 2011, 49, 537–542. [Google Scholar] [CrossRef]
- Eastell, R.; Delmas, P.D.; Hodgson, S.F.; Eriksen, E.F.; Mann, K.G.; Riggs, B.L. Bone formation rate in older normal women: Concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J. Clin. Endocrinol. Metab. 1988, 67, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Sornay-Rendu, E.; Chapuy, M.C.; Delmas, P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996, 11, 337–349. [Google Scholar] [CrossRef]
- Cook, G.J.; Blake, G.M.; Marsden, P.K.; Cronin, B.; Fogelman, I. Quantification of skeletal kinetic indices in Paget’s disease using dynamic 18F-fluoride positron emission tomography. J. Bone Miner. Res. 2002, 17, 854–859. [Google Scholar] [CrossRef]
- Mosekilde, L.; Hasling, C.; Tågehøj Jensen, P.C.; Tågehøj Jensen, F. Bisphosphonate whole body retention test: Relations to bone mineralization rate, renal function and bone mineral content in osteoporosis and metabolic bone disorders. Eur. J. Clin. Investig. 1987, 17, 530–537. [Google Scholar] [CrossRef]
- Szulc, P.; Montella, A.; Delmas, P.D. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: The prospective MINOS study. Ann. Rheum Dis. 2008, 67, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Arden, N.K.; Griffiths, G.; Delmas, P.D.; Spector, T.D. Genetic influence on bone turnover in postmenopausal twins. J. Clin. Endocrinol. Metab. 1996, 81, 140–146. [Google Scholar]
- Grados, F.; Brazier, M.; Kamel, S.; Mathieu, M.; Hurtebize, N.; Maamer, M.; Garabédian, M.; Sebert, J.L.; Fardellone, P. Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J. Clin. Endocrinol. Metab. 2003, 88, 5175–5179. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R.; San Martin, J.; Miller, P.D.; Civitelli, R.; Bandeira, F.; Omizo, M.; Donley, D.W.; Dalsky, G.P.; Eriksen, E.F. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch. Intern. Med. 2005, 165, 1762–1768. [Google Scholar] [CrossRef]
- Eastell, R.; Barton, I.; Hannon, R.A.; Chines, A.; Garnero, P.; Delmas, P.D. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J. Bone Miner. Res. 2003, 18, 1051–1056. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Greenspan, S.; Wasnich, R.D.; Miller, P.; Thompson, D.E.; Ross, P.D. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J. Clin. Endocrinol. Metab. 2002, 87, 1586–1592. [Google Scholar] [CrossRef]
- Hansen, M.A.; Overgaard, K.; Riis, B.J.; Christiansen, C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ 1991, 303, 961–964. [Google Scholar] [CrossRef]
- Riggs, L.B.; Melton, L.J., 3rd; O’Fallon, W.M. Drug therapy for vertebral fractures in osteoporosis: Evidence that decreases in bone turnover and increases in bone mass both determine antifracture efficacy. Bone 1996, 18 (Suppl. 3), 197s–201s. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Khosla, S.; Atkinson, E.J.; O’Fallon, W.M.; Riggs, B.L. Relationship of bone turnover to bone density and fractures. J. Bone Miner. Res. 1997, 12, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P. Markers of bone turnover for the prediction of fracture risk. Osteoporos. Int. 2000, 11 (Suppl. 6), S55–S65. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone Miner. Res. 2000, 15, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, N.H.; Sarkar, S.; Duong, T.; Mitlak, B.; Delmas, P.D.; Christiansen, C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos. Int. 2001, 12, 922–930. [Google Scholar] [CrossRef]
- Delmas, P.D.; Recker, R.R.; Chesnut, C.H., 3rd; Skag, A.; Stakkestad, J.A.; Emkey, R.; Gilbride, J.; Schimmer, R.C.; Christiansen, C. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: Results from the BONE study. Osteoporos. Int. 2004, 15, 792–798. [Google Scholar] [CrossRef]
- Sarkar, S.; Reginster, J.Y.; Crans, G.G.; Diez-Perez, A.; Pinette, K.V.; Delmas, P.D. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J. Bone Miner. Res. 2004, 19, 394–401. [Google Scholar] [CrossRef]
- Wootton, R.; Doré, C. The single-passage extraction of 18F in rabbit bone. Clin. Phys. Physiol Meas. 1986, 7, 333–343. [Google Scholar] [CrossRef]
- Piert, M.; Machulla, H.J.; Jahn, M.; Stahlschmidt, A.; Becker, G.A.; Zittel, T.T. Coupling of porcine bone blood flow and metabolism in high-turnover bone disease measured by [(15)O]H(2)O and [(18)F]fluoride ion positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 907–914. [Google Scholar] [CrossRef]
- Piert, M.; Zittel, T.T.; Machulla, H.J.; Becker, G.A.; Jahn, M.; Maier, G.; Bares, R.; Becker, H.D. Blood flow measurements with [(15)O]H2O and [18F]fluoride ion PET in porcine vertebrae. J. Bone Miner. Res. 1998, 13, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Prisby, R.D.; Ramsey, M.W.; Behnke, B.J.; Dominguez, J.M., 2nd; Donato, A.J.; Allen, M.R.; Delp, M.D. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J. Bone Miner. Res. 2007, 22, 1280–1288. [Google Scholar] [CrossRef]
- McCarthy, I. The physiology of bone blood flow: A review. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 3), 4–9. [Google Scholar] [CrossRef]
- Bloomfield, A.S.; Hogan, H.A.; Delp, M.D. Decreases in bone blood flow and bone material properties in aging Fischer-344 rats. Clin. Orthop. Relat. Res. 2002, 396, 248–257. [Google Scholar] [CrossRef]
- Griffith, J.F.; Yeung, D.K.; Antonio, G.E.; Wong, S.Y.; Kwok, T.C.; Woo, J.; Leung, P.C. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology 2006, 241, 831–838. [Google Scholar] [CrossRef]
- Griffith, J.F.; Yeung, D.K.; Tsang, P.H.; Choi, K.C.; Kwok, T.C.; Ahuja, A.T.; Leung, K.S.; Leung, P.C. Compromised bone marrow perfusion in osteoporosis. J. Bone Miner. Res. 2008, 23, 1068–1075. [Google Scholar] [CrossRef]
- Bridgeman, G.; Brookes, M. Blood supply to the human femoral diaphysis in youth and senescence. J. Anat. 1996, 188 Pt 3, 611–621. [Google Scholar]
- Eriksen, F.E.; Eghbali-Fatourechi, G.Z.; Khosla, S. Remodeling and vascular spaces in bone. J. Bone Miner. Res. 2007, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.T.; Cauley, J.A.; Kuller, L.H.; Nevitt, M.C. Bone mineral density and blood flow to the lower extremities: The study of osteoporotic fractures. J. Bone Miner. Res. 1997, 12, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H. The Michael Mason Prize Essay 1997. Nitric oxide and bone: What a gas! Br. J. Rheumatol. 1997, 36, 831–838. [Google Scholar] [CrossRef]
- Reeve, J.; Arlot, M.; Wootton, R.; Edouard, C.; Tellez, M.; Hesp, R.; Green, J.R.; Meunier, P.J. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: A relationship between blood flow and corrected apposition rate. J. Clin. Endocrinol. Metab. 1988, 66, 1124–1131. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Juby, A.; Hanley, D.; Tymchak, W.; Sclater, A. Role of Vascular Factors in Osteoporosis. J. Gerontol. Ser. A 2003, 58, M362–M366. [Google Scholar] [CrossRef]
- Parfitt, A.M. The mechanism of coupling: A role for the vasculature. Bone 2000, 26, 319–323. [Google Scholar] [CrossRef]
- Rawlinson, S.C.; El-Haj, A.J.; Minter, S.L.; Tavares, I.A.; Bennett, A.; Lanyon, L.E. Loading-related increases in prostaglandin production in cores of adult canine cancellous bone in vitro: A role for prostacyclin in adaptive bone remodeling? J. Bone Miner. Res. 1991, 6, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Ludot, I.; Thiechart, M.; Arlet, J.; Pieraggi, M.; Chiron, P.; Moulinier, L.; Cantagrel, A.; Puget, J.; Utheza, G.; et al. Study of the intraosseous vessels of the femoral head in patients with fractures of the femoral neck or osteoarthritis of the hip. Osteoporos. Int. 1995, 5, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Schiepers, C.; Broos, P.; Miserez, M.; Bormans, G.; De Roo, M. Measurement of skeletal flow with positron emission tomography and 18F-fluoride in femoral head osteonecrosis. Arch. Orthop. Trauma Surg. 1998, 118, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.; Frost, M.L.; Curran, K.M.; Siddique, M.; Moore, A.E.; Cook, G.J.; Marsden, P.K.; Fogelman, I.; Blake, G.M. Differences in regional bone metabolism at the spine and hip: A quantitative study using (18)F-fluoride positron emission tomography. Osteoporos. Int. 2013, 24, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Patlak, C.S.; Blasberg, R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cereb. Blood Flow Metab. 1985, 5, 584–590. [Google Scholar] [CrossRef]
- Hosking, D.J.; Chamberlain, M.J. Studies in man with 18 F. Clin. Sci. 1972, 42, 153–161. [Google Scholar] [CrossRef]
- Hooper, G.; McCarthy, I.; Wootton, R.; Hughes, S. Fluid Spaces in Cannie Tribia; Williams and Wilkins: Baltimore, MD, USA, 1984. [Google Scholar]
- Papapoulos, S. Bisphosphonates: Pharmacology and Use in the Treatment of Osteoporosis; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Fleisch, H. Bisphosphonates: Mechanism of Action and Clinical Use; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Gnanasegaran, G.; Moore, A.E.; Blake, G.M.; Vijayanathan, S.; Clarke, S.E.; Fogelman, I. Atypical Paget’s disease with quantitative assessment of tracer kinetics. Clin. Nucl. Med. 2007, 32, 765–769. [Google Scholar] [CrossRef]
| k1 | k2 | k3 | k4 | Fbv | k3/(k2 + k3) | ki | |
|---|---|---|---|---|---|---|---|
| mL min−1 mL−1 | min−1 | min−1 | min−1 | mL min−1 mL−1 | |||
| %CV (95% CI) | 36% (29–45%) | 52% (42–67%) | 28% (23–36%) | 33% (27–42%) | 55% (45–70%) | 19% (15–24%) | 15% (12–19%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, T.; Frost, M.L.; Cook, G.J.; Blake, G.M. [18F] Sodium Fluoride PET Kinetic Parameters in Bone Imaging. Tomography 2021, 7, 843-854. https://doi.org/10.3390/tomography7040071
Puri T, Frost ML, Cook GJ, Blake GM. [18F] Sodium Fluoride PET Kinetic Parameters in Bone Imaging. Tomography. 2021; 7(4):843-854. https://doi.org/10.3390/tomography7040071
Chicago/Turabian StylePuri, Tanuj, Michelle L. Frost, Gary J. Cook, and Glen M. Blake. 2021. "[18F] Sodium Fluoride PET Kinetic Parameters in Bone Imaging" Tomography 7, no. 4: 843-854. https://doi.org/10.3390/tomography7040071
APA StylePuri, T., Frost, M. L., Cook, G. J., & Blake, G. M. (2021). [18F] Sodium Fluoride PET Kinetic Parameters in Bone Imaging. Tomography, 7(4), 843-854. https://doi.org/10.3390/tomography7040071









