Branch Pulmonary Artery Regurgitation in Repaired Tetralogy of Fallot: Correlation with Pulmonary Artery Morphology, Distensibility, and Right Ventricular Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. MRI Protocol
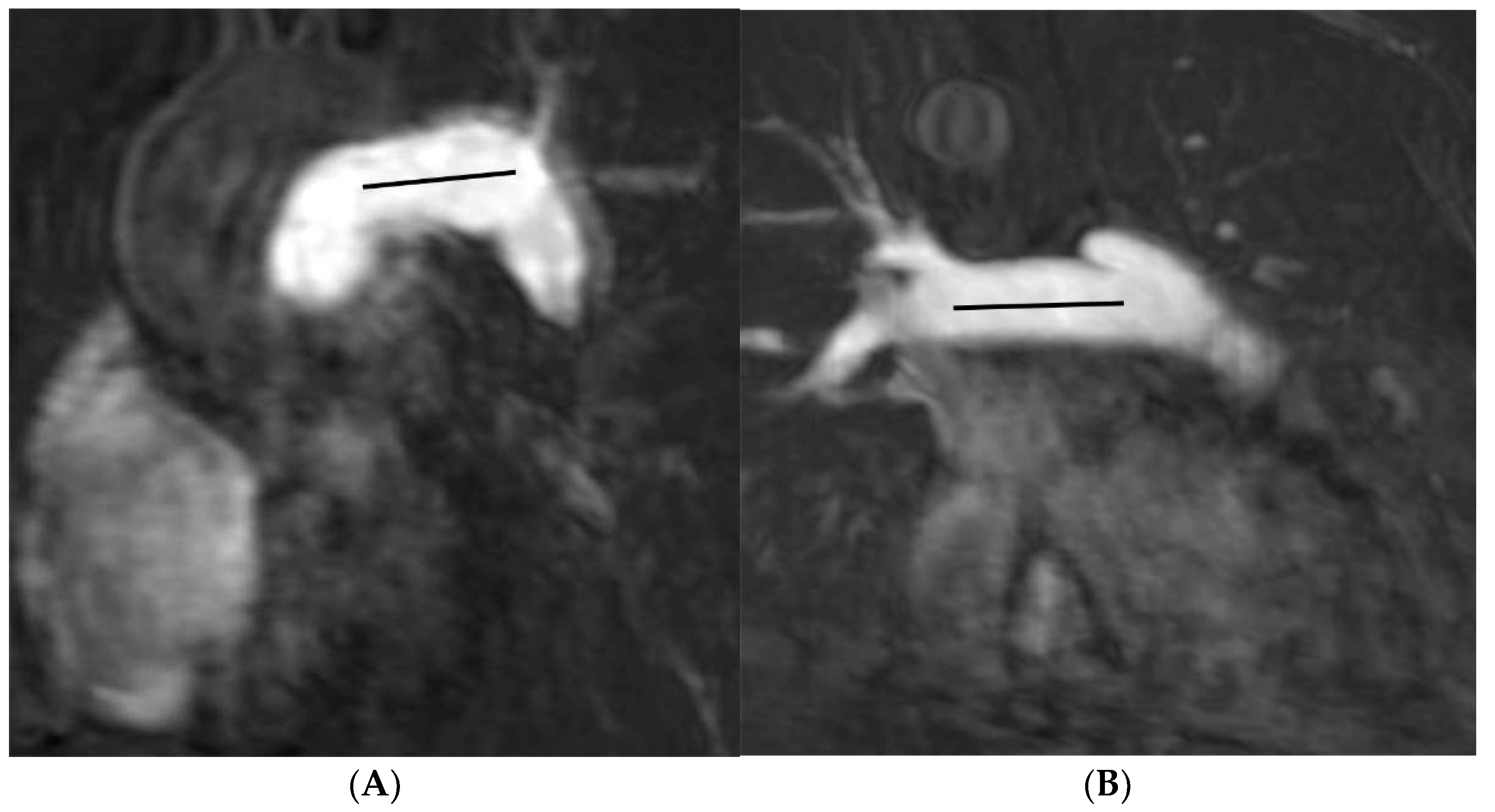
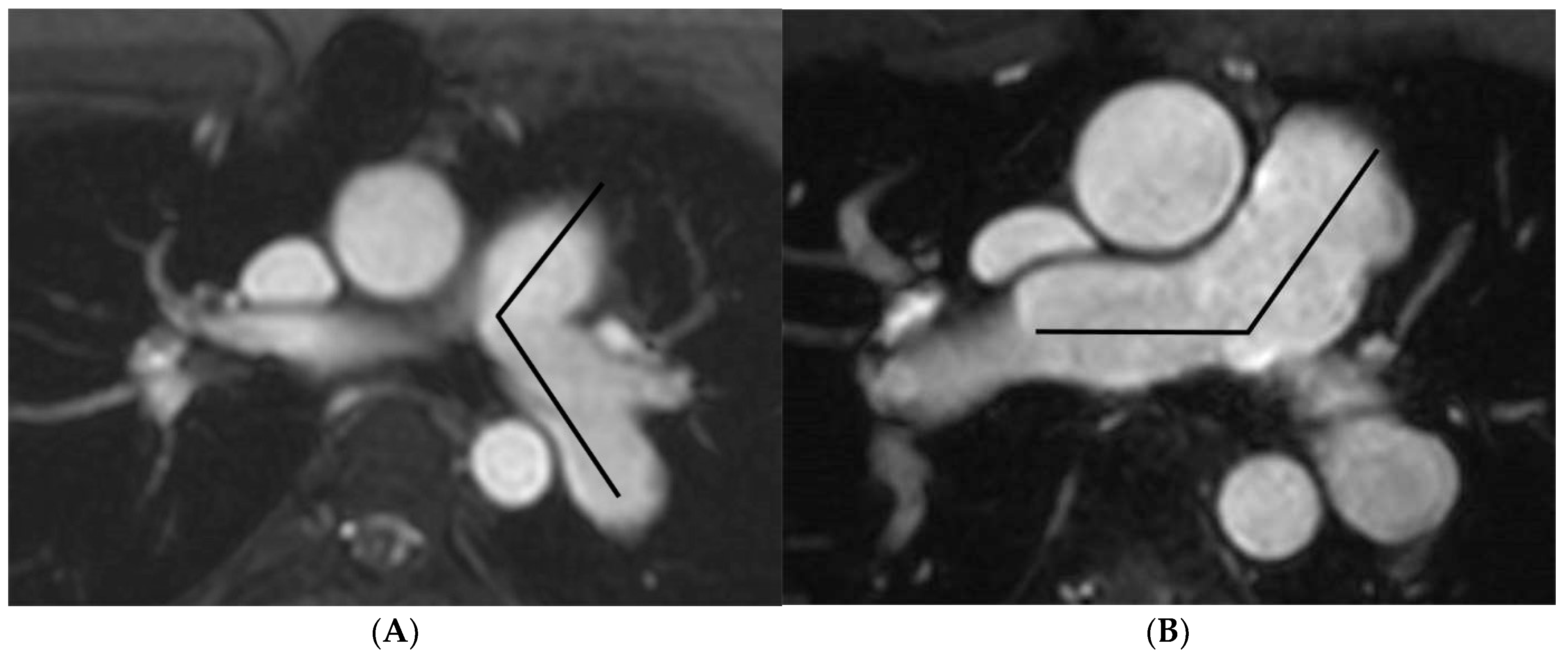
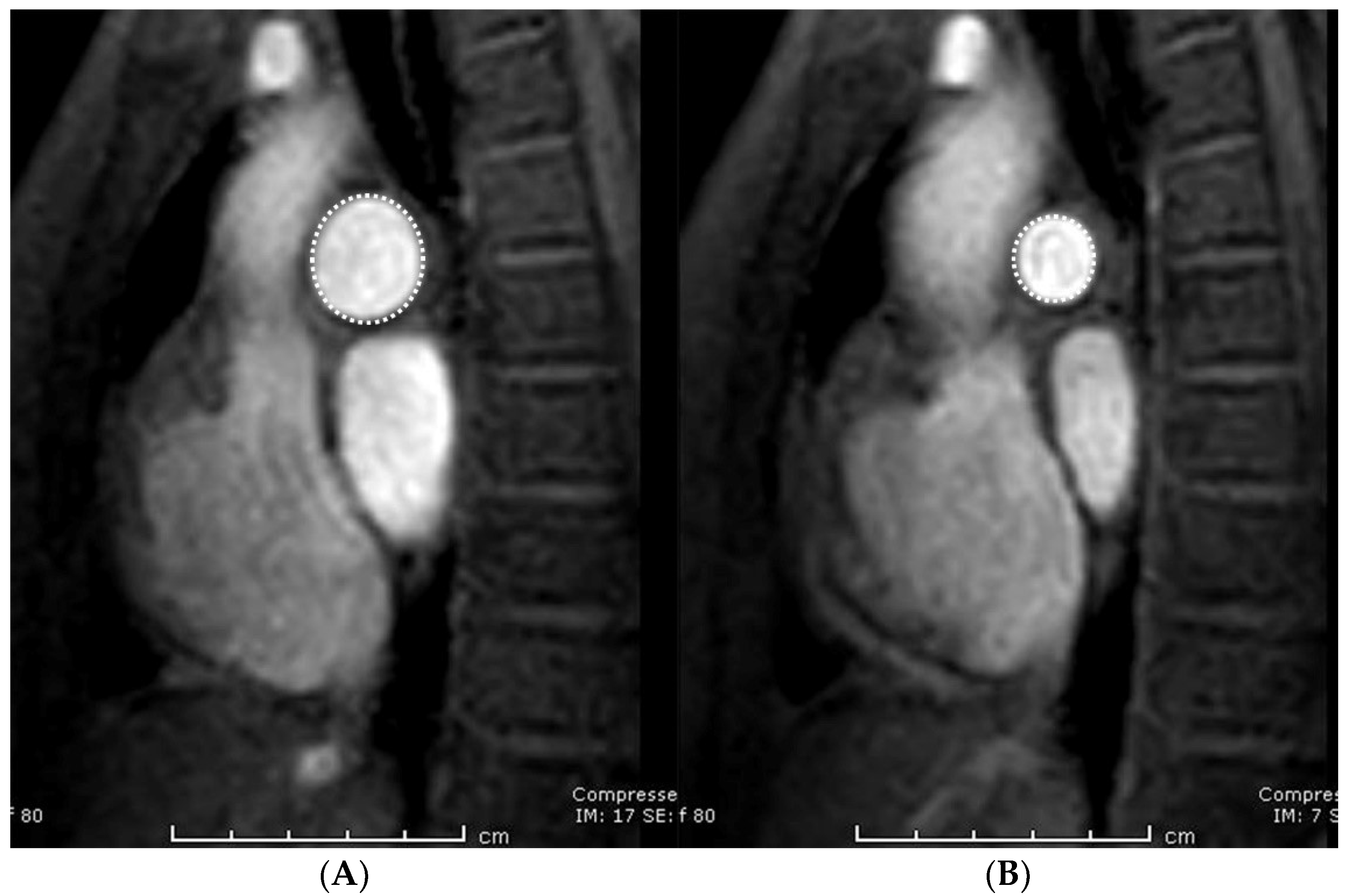
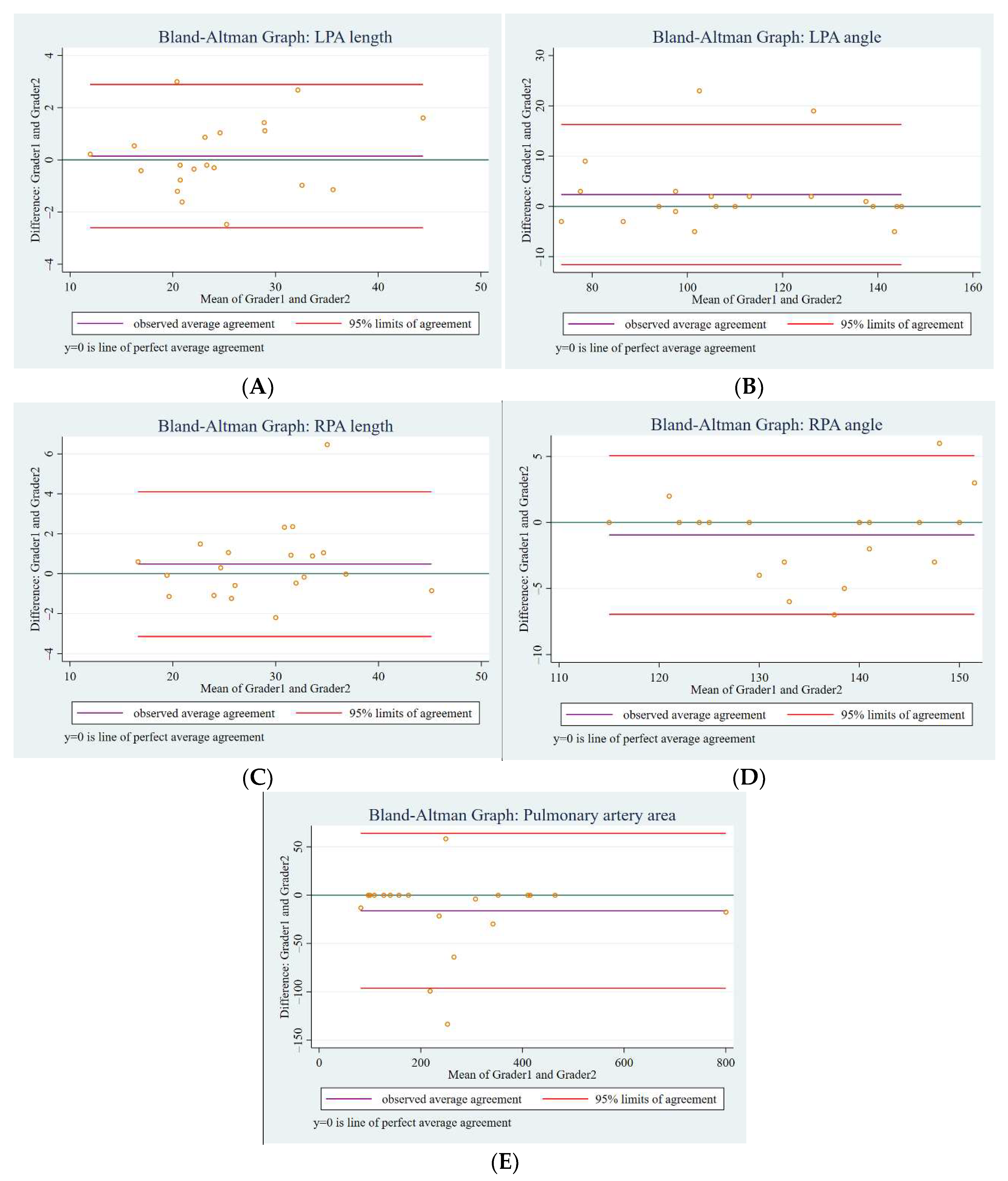
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Pulmonary Artery Morphology, Distensibility, and Differential Branch Regurgitation Fraction
4.2. Relationship of Pulmonary Insufficiency to Branch Pulmonary Artery Regurgitation Fraction and Distensibility, and Their Association with Right Ventricular Function
4.3. Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPA-RF | Branch pulmonary artery regurgitation fraction |
| LPA | Left pulmonary artery |
| MRI | Magnetic resonance imaging |
| PA | Pulmonary artery |
| PI | Pulmonary insufficiency |
| RF | Regurgitation fraction |
| rTOF | Repaired tetralogy of Fallot |
| RPA | Right pulmonary artery |
| RVF | Right ventricular function |
| RVEF | Right ventricular ejection fraction |
References
- Mitchell, S.C.; Korones, S.B.; Berendes, H.W. Congenital heart disease in 56,109 births incidence and natural history. Circulation 1971, 43, 323–332. [Google Scholar] [CrossRef]
- Pigula, F.A.; Khalil, P.N.; Mayer, J.E.; del Nido, P.J.; Jonas, R.A. Repair of Tetralogy of Fallot in Neonates and Young Infants. Circulation 1999, 100, 157–161. [Google Scholar] [CrossRef]
- Bacha, E.A.; Scheule, A.M.; Zurakowski, D.; Erickson, L.C.; Hung, J.; Lang, P.; Mayer, J.E.; del Nido, P.J.; Jonas, R.A. Long-term results after early primary repair of tetralogy of Fallot. J. Thorac. Cardiovasc. Surg. 2001, 122, 154–161. [Google Scholar] [CrossRef]
- Puranik, R.; Tsang, V.; Lurz, P.; Muthurangu, V.; Offen, S.; Frigiola, A.; Norman, W.; Walker, F.; Bonhoeffer, P.; Taylor, A.M. Long-term importance of right ventricular outflow tract patch function in patients with pulmonary regurgitation. J. Thorac. Cardiovasc. Surg. 2012, 143, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Burman, E.D.; Keegan, J.; Kilner, P.J. Pulmonary artery diameters, cross sectional areas and area changes measured by cine cardiovascular magnetic resonance in healthy volunteers. J. Cardiovasc. Magn. Reson. 2016, 18, 12. [Google Scholar] [CrossRef]
- Bouzas, B.; Kilner, P.J.; Gatzoulis, M.A. Pulmonary regurgitation: Not a benign lesion. Eur. Heart J. 2005, 26, 433–439. [Google Scholar] [CrossRef]
- Harris, M.A.; Whitehead, K.K.; Gillespie, M.J.; Liu, T.Y.; Cosulich, M.T.; Shin, D.C.; Goldmuntz, E.; Weinberg, P.M.; Fogel, M.A. Differential Branch Pulmonary Artery Regurgitant Fraction Is a Function of Differential Pulmonary Arterial Anatomy and Pulmonary Vascular Resistance. JACC Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Weinberg, P.M.; Whitehead, K.K.; Fogel, M.A. Usefulness of Branch Pulmonary Artery Regurgitant Fraction to Estimate the Relative Right and Left Pulmonary Vascular Resistances in Congenital Heart Disease. Am. J. Cardiol. 2005, 95, 1514–1517. [Google Scholar] [CrossRef]
- Kang, I.S.; Redington, A.N.; Benson, L.N.; Macgowan, C.; Valsangiacomo, E.R.; Roman, K.; Kellenberger, C.J.; Yoo, S.J. Differential regurgitation in branch pulmonary arteries after repair of tetralogy of Fallot: A phase-contrast cine magnetic resonance study. Circulation 2003, 107, 2938–2943. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Huang, Y.-L.; Hsieh, K.-S.; Huang, J.-T.; Peng, N.-J.; Pan, J.-Y.; Huang, J.-S.; Yang, T.-L. Influence of Pulmonary Regurgitation Inequality on Differential Perfusion of the Lungs in Tetralogy of Fallot after Repair: A Phase-Contrast Magnetic Resonance Imaging and Perfusion Scintigraphy Study. J. Am. Coll. Cardiol. 2007, 49, 1880–1886. [Google Scholar] [CrossRef]
- Voser, E.M.; Kellenberger, C.J.; Buechel, E.R.V. Effects of Pulmonary Regurgitation on Distensibility and Flow of the Branch Pulmonary Arteries in Tetralogy of Fallot. Pediatr. Cardiol. 2013, 34, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.-J.; Wu, M.-T.; Her, S.-W. Numerical Study for Blood Flow in Pulmonary Arteries after Repair of Tetralogy of Fallot. Comput. Math. Methods Med. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Yan, Q.; Hong, H.; Mao, L. Computational haemodynamic analysis of left pulmonary artery angulation effects on pulmonary blood flow. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Kim, W.-H.; Cho, S. Effects of Angle Correction Angioplasty for Pulmonary Artery Stenosis with Tetralogy of Fallot. Ann. Thorac. Surg. 2017, 103, 862–868. [Google Scholar] [CrossRef][Green Version]
- Rahkonen, O.; Chaturvedi, R.R.; Benson, L.; Honjo, O.; Caldarone, C.A.; Lee, K.-J. Pulmonary artery stenosis in hybrid single-ventricle palliation: High incidence of left pulmonary artery intervention. J. Thorac. Cardiovasc. Surg. 2015, 149, 1102–1110.e2. [Google Scholar] [CrossRef] [PubMed]
- Chaothawee, L. Diagnostic approach to assessment of valvular heart disease using magnetic resonance imaging, part II: A practical approach for native and prosthetic heart valve stenosis. Heart Asia 2012, 4, 171–175. [Google Scholar] [CrossRef][Green Version]
- Knobel, Z.; Kellenberger, C.J.; Kaiser, T.; Albisetti, M.; Bergsträsser, E.; Buechel, E.R. Geometry and dimensions of the pulmonary artery bifurcation in children and adolescents: Assessment in vivo by contrast-enhanced MR-angiography. Int. J. Cardiovasc. Imaging 2011, 27, 385–396. [Google Scholar] [CrossRef]
- Mercer-Rosa, L.; Yang, W.; Kutty, S.; Rychik, J.; Fogel, M.; Goldmuntz, E. Quantifying pulmonary regurgitation and right ventricular function in surgically repaired tetralogy of Fallot: A comparative analysis of echocardiography and magnetic resonance imaging. Circ. Cardiovasc. Imaging 2012, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.R.; Redington, A.N. Pulmonary regurgitation in congenital heart disease. Heart 2007, 93, 880–889. [Google Scholar] [CrossRef]
- Patel, D.J.; Schilder, D.P.; Mallos, A.J. Mechanical properties and dimensions of the major pulmonary arteries. J. Appl. Physiol. 1960, 15, 92–96. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Bishnoi, A.K.; Ray, R.; Kalaivani, M.; Kapoor, P.M.; Reddy, S.M.; Mishra, A.K.; Gonvindappa, R.M. Central Pulmonary Artery Histopathology in Patients with Cyanotic Congenital Heart Diseases. Ann. Thorac. Surg. 2009, 87, 589–596.e3. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.S.; Lammers, S.R.; Shandas, R. Pulmonary Vascular Stiffness: Measurement, Modeling, and Implications in Normal and Hypertensive Pulmonary. Compr. Physiol. 2011, 1, 1413–1435. [Google Scholar] [CrossRef]
| Characteristics | Values |
|---|---|
| Children (<18 years) | 104 (57.1%) |
| Male sex | 117 (64.3%) |
| Height (cm) | 154.2 ± 17.0 |
| Body weight (kg) | 48.3 ± 17.1 |
| Body surface area (m2) | 1.73 ± 0.78 |
| Age at MRI imaging (years) | 17.1 (12.5–21.0) |
| Operative age (years) * | 5 (4–7) |
| Surgical repair ** | 133 (73.1%) |
| Transannular patch | 156 (85.7%) |
| Augmentation outflow | 19 (10.4%) |
| Valve repair | 5 (2.8%) |
| Unknown | 2 (1.1%) |
| Hemodynamic Data | Values |
|---|---|
| Right ventricular ejection fraction (RVEF, %) | 46.6 ± 8.4 |
| RVEF ≥ 55% | 24 (13.2%) |
| RVEF ≥ 45% and <55% | 86 (47.2%) |
| RVEF ≥ 35% and <45% | 56 (30.8%) |
| RVEF < 35% | 16 (8.8%) |
| Pulmonary insufficiency (regurgitation fraction %) | 45.3 (35.5–52.2) |
| Mild to moderate degree (<40%) | 65 (35.7%) |
| Severe degree (≥40%) | 117 (64.3%) |
| Left pulmonary regurgitation fraction (%) | 43.1 (32.6–51.1) |
| Mild to moderate degree (<40%) | 75 (41.2%) |
| Severe degree (≥40%) | 107 (58.8%) |
| Right pulmonary regurgitation fraction (%) | 35.2 (24.7–44.7) |
| Mild to moderate degree (<40%) | 84 (64.8%) |
| Severe degree (≥40%) | 64 (35.2%) |
| Variables | Left Pulmonary Artery | Right Pulmonary Artery | Coefficient (95% CI) | p-Value |
|---|---|---|---|---|
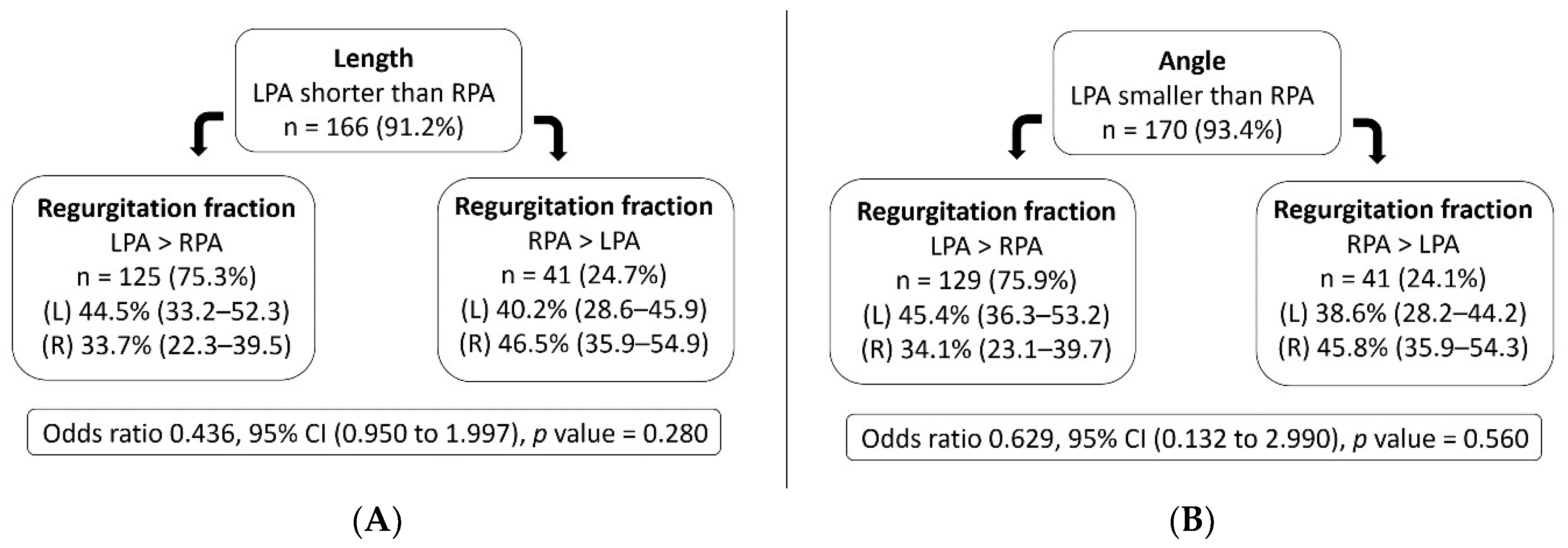
| Length (mm) | 23.9 (20.7–27.5) | 30.3 (26.0–34.9) | −6.4 a (−5.0, −7.8) | <0.001 |
| Angle (degrees) | 101.8 ± 18.3 | 141.0 ± 13.7 | −39.2 b (−43.1, −6.0) | <0.001 |
| Area-maximum (mm2) | 238.1 (166.8–329.1) | 247.8 (163.6–331.5) | - | 0.661 * |
| Area-minimum (mm2) | 119.4 (91.6–165.7) | 110.7 (85.0–160.9) | - | 0.024 * |
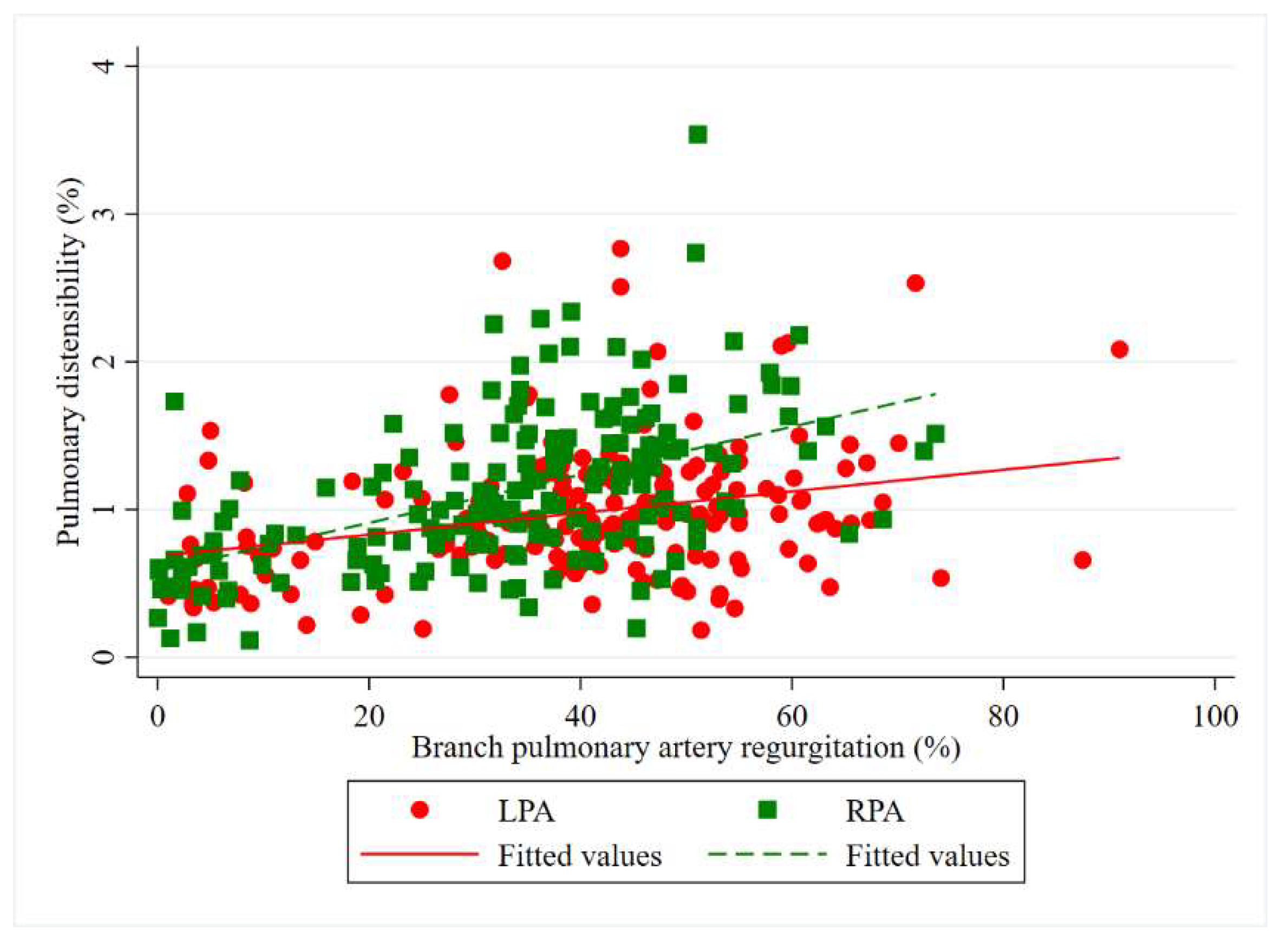
| Pulmonary distensibility (%) | 92.9 (68.0–121.3) | 105.5 (76.2–144.8) | - | 0.001 * |
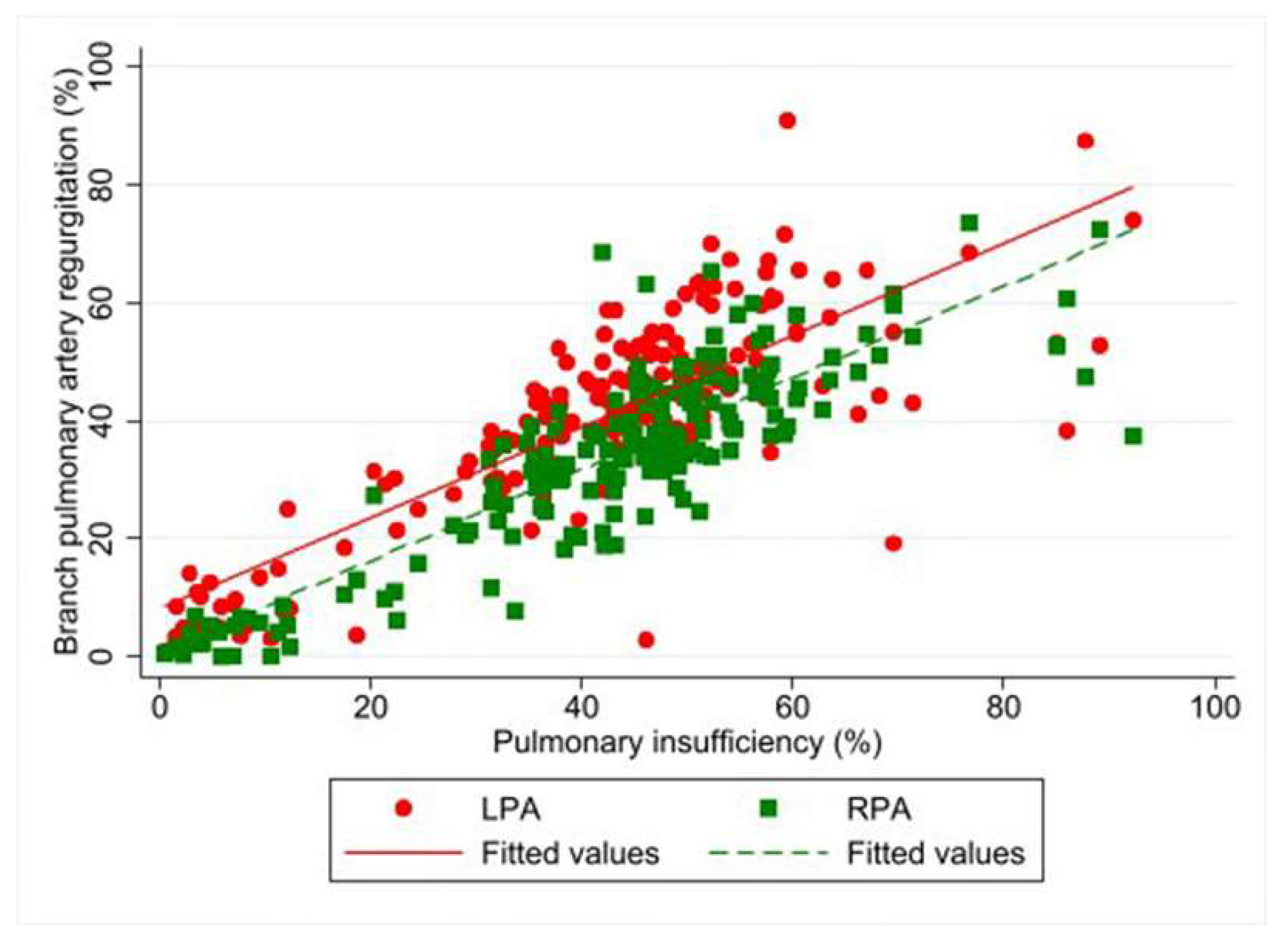
| Regurgitation fraction (%) | 43.1 (32.6–51.5) | 35.2 (24.7–44.7) | 7.9 a (4.8, 11.0) | <0.001 |
| Net pulmonary blood flow (mL) | 21.0 (15.7–27.1) | 31.3 (24.7–39.6) | −10.3 a (−7.7, 12.9) | <0.001 |
| Variables | Regurgitation Fraction (%) | Grading Branch Pulmonary Regurgitation Fraction (%) | Pulmonary Distensibility (%) Coefficients (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Total | Mild to Moderate | Severe | |||
| Left pulmonary artery | n = 182 (100%) | n = 75 (41.2%) | n = 107 (58.8%) | 0.73 a (0.37, 1.09) | <0.001 |
| 43.1 (32.6–51.1) | 29.7 (10.2–37.1) | 50.1 (44.5–57.6) | 19.02 b (6.08, 31.95) | 0.004 | |
| Right pulmonary artery | n = 182 (100%) | n = 118 (64.8%) | n = 64 (35.2%) | 1.63 a (1.22, 2.03) | <0.001 |
| 35.2 (24.7–44.7) | 30.3 (13.1–34.9) | 47.2 (43.9–53.2) | 40.33 b (23.47, 57.20) | <0.001 | |
| Factors (%) | Total | Grading Right Ventricular Ejection Fraction (RVEF, %) | p-Value | |||
|---|---|---|---|---|---|---|
| ≥55% | ≥45% and <55% | ≥35% and <45% | <35% | |||
| n = 182 (100%) | n = 24 (13.2%) | n = 86 (47.2%) | n = 56 (30.8%) | n = 16 (8.8%) | ||
| RVEF (mean ± SD) | 46.6 ± 8.4 | 59.1 ± 2.9 | 50.1 ± 3.1 | 40.4 ± 2.9 | 30.6 ± 3.6 | <0.001 |
| Pulm-insuff (mean ± SD) | 45.3 (35.5–52.2) | 37.6 (10.9–48.0) | 43.1 (31.7–50.6) | 47.1 (39.6–56.4) | 53.2 (46.6–67.6) | 0.042 |
| LPA distensibility median (IQR) | 92.9 (68.0–121.3) | 88.2 (70.8–129.2) | 97.4 (71.3–123.6) | 82.8 (64.6–108.6) | 99.8 (83.4–125.6) | 0.383 |
| RPA distensibility median (IQR) | 105.5 (76.2–144.8) | 88.4 (57.7–120.6) | 103.5 (76.4–144.9) | 115.4 (76.3–144.2) | 129.0 (95.4–157.4) | 0.368 |
| LPA-RF * (mean ± SE) | 40.8 ± 0.68 | 40.5 ± 2.14 | 41.6 ± 1.11 | 41.1 ± 1.39 | 35.9 ± 2.64 | 0.268 |
| RPA-RF * (mean ± SE) | 33.5 ± 0.68 | 32.1 ± 1.71 | 32.9 ± 0.89 | 34.3 ± 1.11 | 35.9 ± 2.11 | 0.434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siripornpitak, S.; Lueangwattanapong, D.; Sriprachyakul, A.; Wanitkun, S.; Limsuwan, A. Branch Pulmonary Artery Regurgitation in Repaired Tetralogy of Fallot: Correlation with Pulmonary Artery Morphology, Distensibility, and Right Ventricular Function. Tomography 2021, 7, 412-423. https://doi.org/10.3390/tomography7030036
Siripornpitak S, Lueangwattanapong D, Sriprachyakul A, Wanitkun S, Limsuwan A. Branch Pulmonary Artery Regurgitation in Repaired Tetralogy of Fallot: Correlation with Pulmonary Artery Morphology, Distensibility, and Right Ventricular Function. Tomography. 2021; 7(3):412-423. https://doi.org/10.3390/tomography7030036
Chicago/Turabian StyleSiripornpitak, Suvipaporn, Duangkanok Lueangwattanapong, Apichaya Sriprachyakul, Suthep Wanitkun, and Alisa Limsuwan. 2021. "Branch Pulmonary Artery Regurgitation in Repaired Tetralogy of Fallot: Correlation with Pulmonary Artery Morphology, Distensibility, and Right Ventricular Function" Tomography 7, no. 3: 412-423. https://doi.org/10.3390/tomography7030036
APA StyleSiripornpitak, S., Lueangwattanapong, D., Sriprachyakul, A., Wanitkun, S., & Limsuwan, A. (2021). Branch Pulmonary Artery Regurgitation in Repaired Tetralogy of Fallot: Correlation with Pulmonary Artery Morphology, Distensibility, and Right Ventricular Function. Tomography, 7(3), 412-423. https://doi.org/10.3390/tomography7030036





