Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer
Abstract
:1. Introduction
2. Methods
2.1. Patient Cohort with Treatment Characteristics
2.2. Magnetic Resonance Imaging Technique
2.3. Conventional Image Analysis
2.4. Image Segmentation and Feature Extraction
2.5. Radiomic Feature Selection
2.6. Model Building
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and Disease Outcome
3.2. Application of Machine Learning Classifiers Algorithms to Predict Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging; |
| 18-FDG PET CT | 18F–fluorodeoxyglucose (FDG) positron emission tomography/computed tomography; |
| FIGO | International Federation of Gynecology and Obstetrics; |
| ADC | Apparent diffusion coefficient; |
| ICRT | Intracavitary brachytherapy; |
| FNAC | Fine needle aspiration cytology; |
| RFS | Recurrence-free survival; |
| VOI | Volumes of interest; |
| ROI | Region of interest; |
| GLCM | Gray level co-occurrence matrix; |
| GLRLM | Gray level run length matrix; |
| GLSZM | Gray level size zone matrix; |
| GLDM | Gray-level dependence matrix. |
References
- All Cancers Source: Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 9 September 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.D.; Neupane, D.; Vedsted, P.; Kallestrup, P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pac. J. Cancer Prev. 2018, 19, 319–324. [Google Scholar] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, N.M.; Soutter, W.P.; Rustin, G.; Mahon, M.M.; Jones, B.; Dina, R.; McIndoe, G.A. Use of neoadjuvant chemotherapy prior to radical hysterectomy in cervical cancer: Monitoring tumour shrinkage and molecular profile on magnetic resonance and assessment of 3-year outcome. Br. J. Cancer 2004, 90, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, D.; Zhang, J.; Yao, D.; Gao, K.; Wang, H.; Liu, C.; Yu, J.; Li, L. The efficacy and safety of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer: A randomized multicenter study. Gynecol. Oncol. 2016, 141, 231–239. [Google Scholar] [CrossRef]
- Angioli, R.; Plotti, F.; Montera, R.; Aloisi, A.; Luvero, D.; Capriglione, S.; Terranova, C.; Nardone, C.D.C.; Muzii, L.; Benedetti-Panici, P. Neoadjuvant chemotherapy plus radical surgery followed by chemotherapy in locally advanced cervical cancer. Gynecol. Oncol. 2012, 127, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Mayr, N.A.; Zhang, D.; Li, K.; Grecula, J.C.; Montebello, J.F.; Lo, S.S.; Yuh, W.T. Sequential magnetic resonance imaging of cervical cancer: The predictive value of absolute tumor volume and regression ratio measured before, during, and after radiation therapy. Cancer 2010, 116, 5093–5101. [Google Scholar] [CrossRef]
- Rose, P.G.; Java, J.; Whitney, C.W.; Stehman, F.B.; Lanciano, R.; Thomas, G.M.; DiSilvestro, P.A. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG oncology/Gynecologic oncology group randomized trials of chemoradiotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2136–2142. [Google Scholar]
- Herrera, F.G.; Prior, J.O. The role of PET/CT in cervical cancer. Front Oncol. 2013, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, H.J.; Jeong, Y.H.; Lee, J.H.; Cho, A.; Yun, M.; Lee, J.D.; Kim, Y.B.; Kim, Y.T.; Kang, W.J. The role of (18) F-FDG PET/CT in assessing therapy response in cervix cancer after concurrent Chemoradiation therapy. Nucl. Med. Mol. Imaging 2014, 48, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Joja, I.; Kodama, J.; Hongo, A.; Hiramatsu, Y. Measurement of SUVmax plus ADCmin of the primary tumour is a predictor of prognosis in patients with cervical cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 283–290. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Liang, B. The utility of diffusion-weighted MR imaging in cervical cancer. Eur. J. Radiol. 2010, 74, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Allen, P.K.; Bhosale, P.R.; Rauch, G.M.; Fuller, C.D.; Mohamed, A.S.; Frumovitz, M.; Jhingran, A.; Klopp, A.H. Diffusion-weighted magnetic resonance imaging as a predictor of outcome in cervical cancer after chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 546–553. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, Y.C.; Yin, L.L.; Pu, H. Can diffusion-weighted magnetic resonance imaging predict survival in patients with cervical cancer? A meta-analysis. Eur. J. Radiol. 2016, 85, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Z.; Sun, H.; Bai, R. Clinical application of diffusion-weighted magnetic resonance imaging in uterine cervical cancer. Int. J. Gynecol. Cancer 2015, 25, 1073–1078. [Google Scholar] [CrossRef]
- Parmar, C.; Leijenaar, R.T.; Grossmann, P.; Velazquez, E.R.; Bussink, J.; Rietveld, D.; Rietbergen, M.M.; Haibe-Kains, B.; Lambin, P.; Aerts, H.J. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci. Rep. 2015, 5, 11044. [Google Scholar] [PubMed]
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and radiogenomics in lung cancer: A review for the clinician. Lung Cancer 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Desseroit, M.C.; Miranda, O.; Malhaire, J.P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of outcome using pretreatment 18 F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef] [Green Version]
- Altazi, B.A.; Fernandez, D.C.; Zhang, G.G.; Hawkins, S.; Naqvi, S.M.; Kim, Y.; Hunt, D.; Latifi, K.; Biagioli, M.; Venkat, P.; et al. Investigating multi-radiomic models for enhancing prediction power of cervical cancer treatment outcomes. Phys. Med. 2018, 46, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Cheng, R.; Liu, S.; Qu, F.; Yin, X.; Wang, Q.; Xiao, B.; Ye, Z. Radiomics analysis of apparent diffusion coefficient in cervical cancer: A preliminary study on histological grade evaluation. J. Magn. Reson. Imaging 2019, 49, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, H.; Wang, S.; Dong, D.; Yin, F.; Chen, A.; Wang, S.; Zhao, G.; Fang, M.; Tian, J.; et al. MR-Based Radiomics Nomogram of Cervical Cancer in Prediction of the Lymph-Vascular Space Invasion preoperatively. J. Magn. Reson. Imaging 2019, 49, 1420–1426. [Google Scholar] [CrossRef]
- Sun, C.; Tian, X.; Liu, Z.; Li, W.; Li, P.; Chen, J.; Zhang, W.; Fang, Z.; Du, P.; Duan, H.; et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: A multicentre study. EBioMedicine 2019, 46, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Gao, T.; Yang, J.; Yan, X.; Wang, Y.; Zhou, X.; Tian, J.; Huang, L.; Zhang, M. Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur. J. Radiol. 2019, 114, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Chen, X.; Wang, Y.; Dong, L.; Liu, Z.; Tian, J.; Wang, M. Radiomics analysis of magnetic resonance imaging improves diagnostic performance of lymph node metastasis in patients with cervical cancer. Radiother. Oncol. 2019, 138, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Zhang, R.; Dong, R.T.; Hu, Q.Y.; Yu, T.; Liu, F.; Luo, Y.H.; Dong, Y. Feasibility of an ADC-based radiomics model for predicting pelvic lymph node metastases in patients with stage IB–IIA cervical squamous cell carcinoma. Br. J. Radiol. 2019, 92, 20180986. [Google Scholar] [CrossRef]
- Ciolina, M.; Vinci, V.; Villani, L.; Gigli, S.; Saldari, M.; Panici, P.B.; Perniola, G.; Laghi, A.; Catalano, C.; Manganaro, L. Texture analysis versus conventional MRI prognostic factors in predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced cancer of the uterine cervix. La Radiol. Med. 2019, 124, 955–964. [Google Scholar] [CrossRef]
- Horvat, J.V.; Bernard-Davila, B.; Helbich, T.H.; Zhang, M.; Morris, E.A.; Thakur, S.B.; Ochoa-Albiztegui, R.E.; Leithner, D.; Marino, M.A.; Baltzer, P.A.; et al. Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping as a quantitative imaging biomarker for prediction of immunohistochemical receptor status, proliferation rate, and molecular subtypes of breast cancer. J. Magn. Reson. Imaging 2019, 50, 836–846. [Google Scholar] [CrossRef]
- Park, S.H.; Hahm, M.H.; Bae, B.K.; Chong, G.O.; Jeong, S.Y.; Na, S.; Jeong, S.; Kim, J.C. Magnetic resonance imaging features of the tumor and lymph node to predict clinical outcome in node-positive cervical cancer: A retrospective analysis. Radiat. Oncol. 2020, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Schick, U.; Lucia, F.; Dissaux, G.; Visvikis, D.; Badic, B.; Masson, I.; Pradier, O.; Bourbonne, V.; Hatt, M. MRI-derived radiomics: Methodology and clinical applications in the field of pelvic oncology. Br. J. Radiol. 2019, 92, 20190105. [Google Scholar] [CrossRef]
- Egger, J.; Kapur, T.; Fedorov, A.; Pieper, S.; Miller, J.V.; Veeraraghavan, H.; Freisleben, B.; Golby, A.J.; Nimsky, C.; Kikinis, R. GBM volumetry using the 3D Slicer medical image computing platform. Sci. Rep. 2013, 3, 1364. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative—Feature definitions. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Galloway, M.M. Texture analysis using gray level run lengths. Comput. Graph. Image Process. 1975, 4, 172–179. [Google Scholar] [CrossRef]
- Rose, P.G.; Ali, S.; Watkins, E.; Thigpen, J.T.; Deppe, G.; Clarke-Pearson, D.L.; Insalaco, S. Long-term follow-up of a randomized trial comparing concurrent single agent cisplatin, cisplatin-based combination chemotherapy, or hydroxyurea during pelvic irradiation for locally advanced cervical cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Java, J.J.; Slaughter, K.N.; Rose, P.G.; Lanciano, R.; DiSilvestro, P.A.; Thigpen, J.T.; Lee, Y.C.; Tewari, K.S.; Chino, J.; et al. Is age a prognostic biomarker for survival among women with locally advanced cervical cancer treated with chemoradiation? An NRG Oncology/Gynecologic Oncology Group ancillary data analysis. Gynecoloncology 2016, 143, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.P.; Franckena, M.; Kirchheiner, K.; Sturdza, A.; Georg, P.; Dörr, W.; Pötter, R. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecoloncology 2014, 133, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chen, Z.; Liang, Y.; Shen, W.; Yang, F.; Dai, R.; Wu, N.; Tian, J. Staging of cervical cancer based on tumor heterogeneity characterized by texture features on 18F-FDG PET images. Phys. Med. Biol. 2015, 60, 5123. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wen, H.; Ju, X.; Chen, X.; Ke, G.; Zhou, Y.; Li, J.; Xia, L.; Tang, J.; Liang, S.; et al. Predictive factors of para-aortic lymph nodes metastasis in cervical cancer patients: A retrospective analysis based on 723 paraaortic lymphadenectomy cases. Oncotarget 2017, 8, 51840–51847. [Google Scholar] [CrossRef]
- Diab, Y. Sentinel lymph nodes mapping in cervical cancer a comprehensive review. Int. J. Gynecol. Cancer 2017, 27, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pol, F.J.; Zusterzeel, P.L.; Van Ham, M.A.; Kuijpers, D.A.; Bulten, J.; Massuger, L.F. Satellite lymphovascular space invasion: An independent risk factor in early stage cervical cancer. Gynecol. Oncol. 2015, 138, 579–584. [Google Scholar] [CrossRef]
- Sun, J.R.; Zhang, Y.N.; Sun, X.M.; Feng, S.Y.; Yan, M. Prediction model of pelvic lymph node metastasis in early stage cervical cancer and its clinical value. Minerva Chir. 2011, 66, 537. [Google Scholar]
- Ai, Y.; Zhu, H.; Xie, C.; Jin, X. Radiomics in cervical cancer: Current applications and future potential. Crit. Rev. Oncol. Hematol. 2020, 152, 102985. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shi, D.; Dou, S.; Shi, L.; Liu, M.; Dong, L.; Chang, X.; Wang, M. Radiomics analysis of multiparametric MRI evaluates the pathological features of cervical squamous cell carcinoma. J. Magn. Reson. Imaging 2019, 49, 1141–1148. [Google Scholar] [CrossRef]
- Kan, Y.; Dong, D.; Zhang, Y.; Jiang, W.; Zhao, N.; Han, L.; Fang, M.; Zang, Y.; Hu, C.; Tian, J.; et al. Radiomic signature as a predictive factor for lymph node metastasis in early-stage cervical cancer. J. Magn. Reson. Imaging 2019, 49, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Zhang, B.; Wang, S.; Jin, Y.; Wang, F.; Ding, Y.; Chen, Q.; Chen, L.; Li, Y.; Li, M.; et al. Association of MRI-derived radiomic biomarker with disease-free survival in patients with early-stage cervical cancer. Theranostics 2020, 10, 2284. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Liu, S.; Zhu, L.; Zhu, L.; Wang, H.; Xie, L.; Guan, Y.; He, J.; Yang, X.; Zhou, Z. Texture analysis as imaging biomarker for recurrence in advanced cervical cancer treated with CCRT. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.C.; Fang, Y.H.D.; Chung, H.W.; Yen, T.C.; Ho, T.Y.; Chou, H.H.; Hong, J.H.; Huang, Y.T.; Wang, C.C.; Lai, C.H. A preliminary investigation into textural features of intratumoral metabolic heterogeneity in 18F-FDG PET for overall survival prognosis in patients with bulky cervical cancer treated with definitive concurrent chemoradiotherapy. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 166. [Google Scholar]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Y.; Mao, X.; Qie, M. Prognostic value of fluorine-18-fluorodeoxyglucose positron emission tomography or PET-computed tomography in cervical cancer: A meta-analysis. Int. J. Gynecol. Cancer 2013, 23, 1184–1190. [Google Scholar] [CrossRef]
- Mu, W.; Chen, Z.; Shen, W.; Yang, F.; Liang, Y.; Dai, R.; Wu, N.; Tian, J. A segmentation algorithm for quantitative analysis of heterogeneous tumors of the cervix with 18F-FDG PET/CT. Biomed. Eng. IEEE Trans 2015, 62, 2465–2479. [Google Scholar] [CrossRef]
- Lee, S.L.; Lee, J.; Craig, T.; Berlin, A.; Chung, P.; Ménard, C.; Foltz, W.D. Changes in apparent diffusion coefficient radiomics features during dose-painted radiotherapy and high dose rate brachytherapy for prostate cancer. Phys. Imaging Radiat. Oncol. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladwish, A.; Milosevic, M.; Fyles, A.; Xie, J.; Halankar, J.; Metser, U.; Jiang, H.; Becker, N.; Levin, W.; Manchul, L.; et al. Association of apparent diffusion coefficient with disease recurrence in patients with locally advanced cervical cancer treated with radical chemotherapy and radiation therapy. Radiology 2015, 279, 158–166. [Google Scholar] [CrossRef]
- Gu, K.W.; Kim, C.K.; Choi, C.H.; Yoon, Y.C.; Park, W. Prognostic value of ADC quantification for clinical outcome in uterine cervical cancer treated with concurrent chemoradiotherapy. Eur. Radiol. 2019, 29, 6236–6244. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Joja, I.; Nagasaka, T.; Fukushima, C.; Kusumoto, T.; Seki, N.; Hongo, A.; Kodama, J.; Hiramatsu, Y. The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol. Oncol. 2012, 127, 7. [Google Scholar] [CrossRef]
- Traverso, A.; Kazmierski, M.; Welch, M.L.; Weiss, J.; Fiset, S.; Foltz, W.D.; Gladwish, A.; Dekker, A.; Jaffray, D.; Wee, L.; et al. Sensitivity of radiomic features to inter-observer variability and image pre-processing in Apparent Diffusion Coefficient (ADC) maps of cervix cancer patients. Radiother. Oncol. 2019, 143, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, F.; Kozarski, R.; Ganeshan, B.; Goh, V. Assessment of tumor heterogeneity by CT texture analysis: Can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur. J. Radiol. 2013, 82, 342–348. [Google Scholar] [CrossRef]
- Donati, O.F.; Chong, D.; Nanz, D.; Boss, A.; Froehlich, J.M.; Andres, E.; Seifert, B.; Thoeny, H.C. Diffusion-weighted MR imaging of upper abdominal organs: Field strength and intervendor variability of apparent diffusion coefficients. Radiology 2014, 270, 454–463. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Szomolanyi, P.; Jirak, D.; Materka, A.; Trattnig, S. Effects of MRI acquisition parameter variations and protocol heterogeneity on the results of texture analysis and pattern discrimination: An application-oriented study. Med. Phys. 2009, 36, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.; Aerts, H.J. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
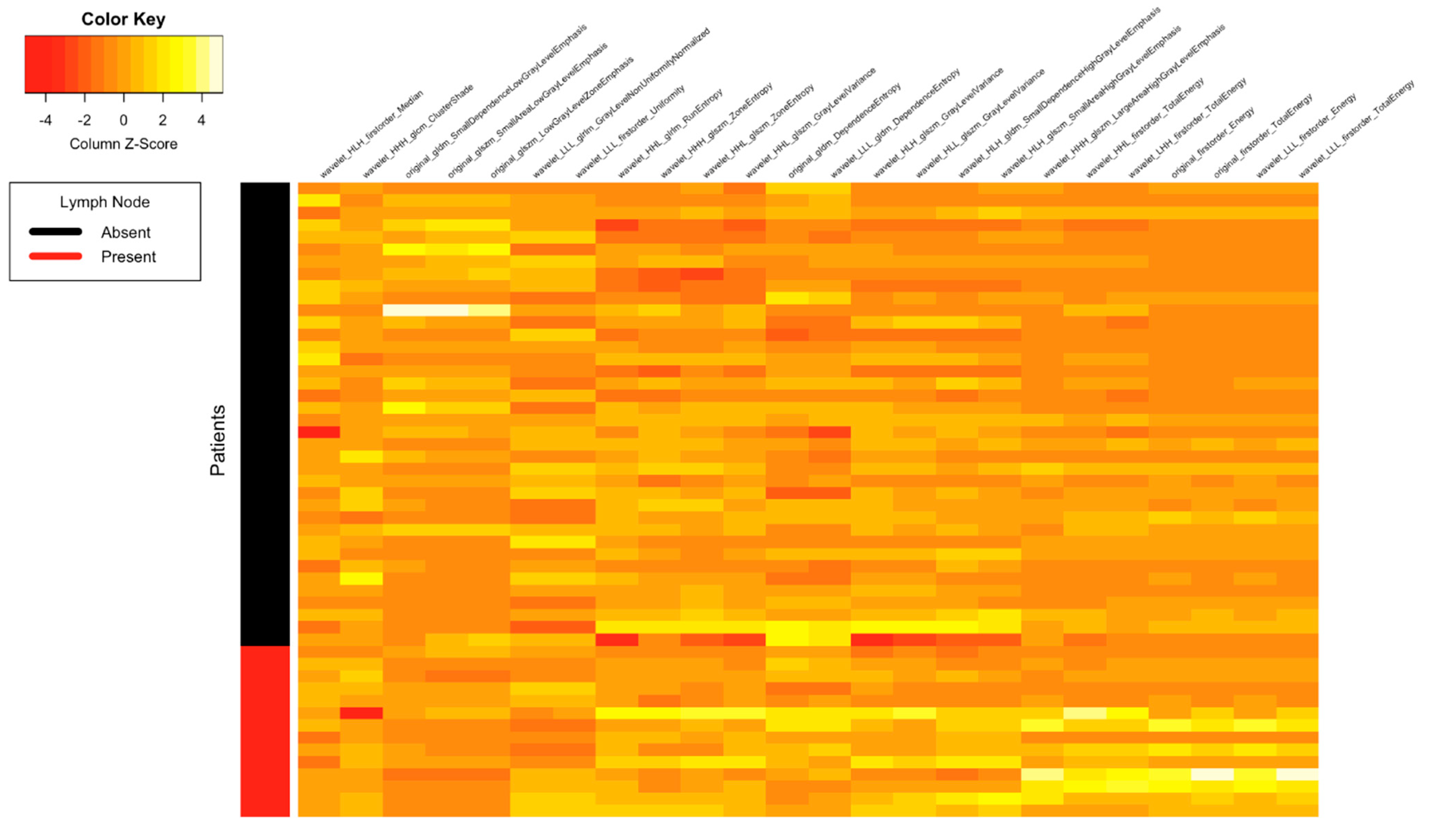
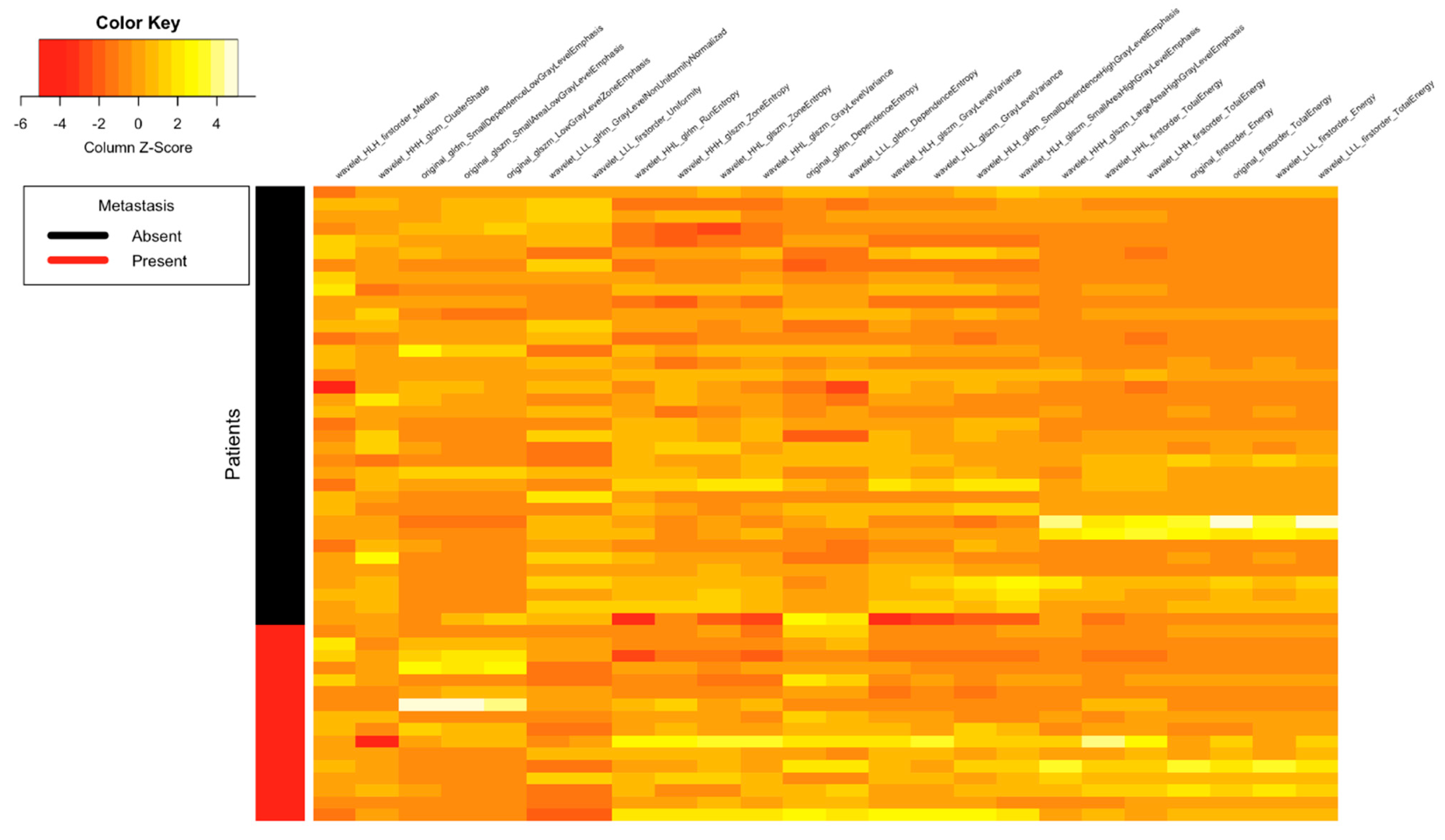
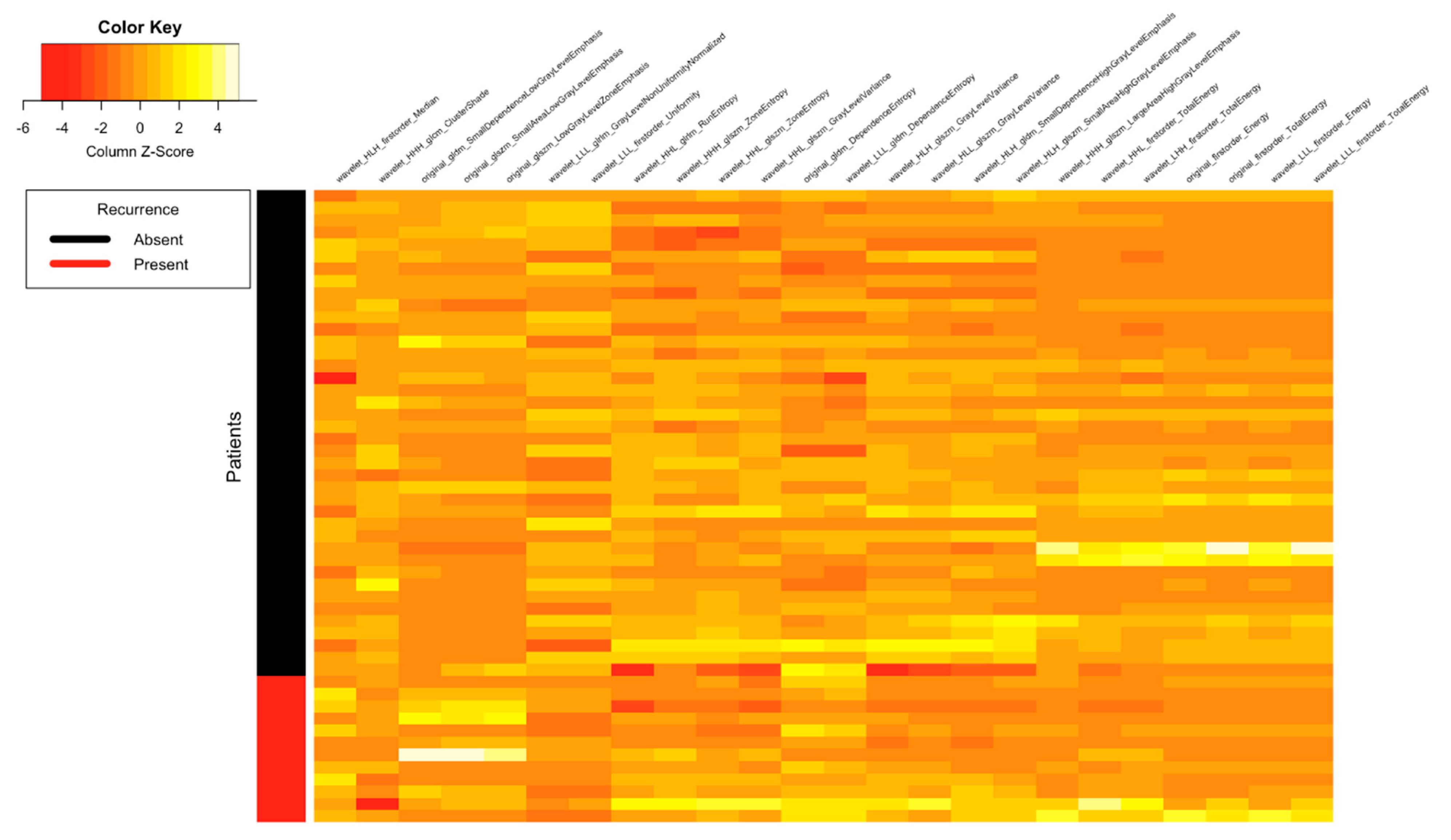
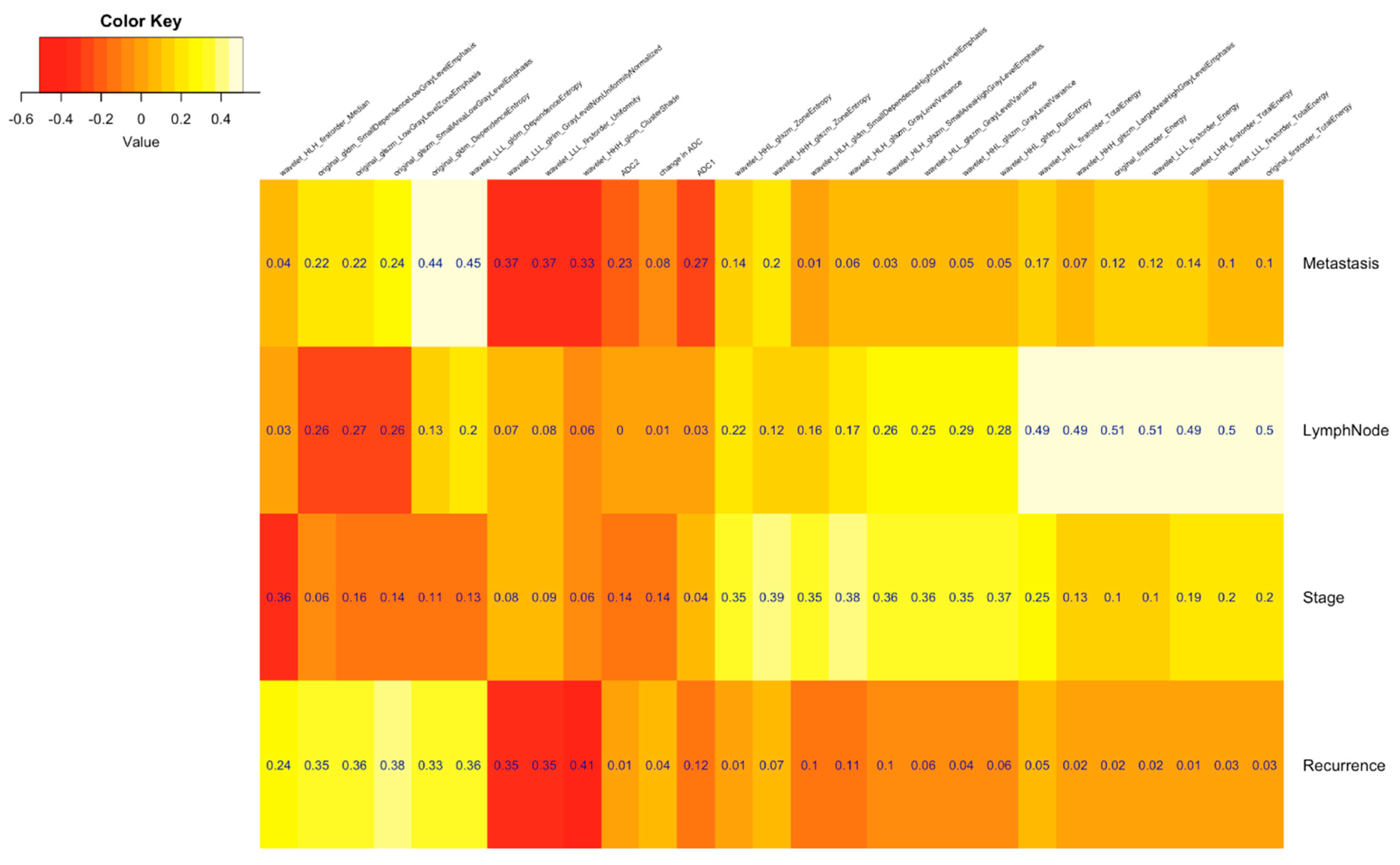
| Clinical Parameters | Total N = 52 (%) |
|---|---|
| Age range | 28–79 (Median = 53 years) |
| FIGO Stage | |
| IB2 | 3 (5.7%) |
| IIA | 8 (15.5%) |
| IIB | 16 (30.7%) |
| IIIA | 16 (30.7%) |
| IIIB | 4 (7.7%) |
| IVA | 3 (5.7%) |
| Clinical Outcomes/Variables | |
| Recurrence/No recurrence | 12/40 (23%/77%) |
| Distant Metastatic/Non metastatic | 15/37 (28%/72%) |
| Metastasis to Lung/Other sites | 5/10 (9%/19%) |
| Lymph node Present/Absent | 15/37 (28%/72%) |
| Paraaortic lymph node/Pelvic node | 2/13 (3.8%/25%) |
| Mean follow up | 29.9 months |
| Median follow up | 28.5 months |
| Mean recurrence interval | 18.5 months |
| Output | Features | Model | Metric | AUC | Kappa |
|---|---|---|---|---|---|
| Recurrence | Radiomics | pcaNNet (Neural Networks with Feature Extraction) | Kappa + AUC | 0.77 | 0.53 |
| Radiomics + ADC1 + ADC2 + Change ADC | svmLinearWeights (Linear Support Vector Machines with Class Weights) | Kappa + AUC | 0.76 | 0.49 | |
| Radiomics + ADC1 | Monmlp (Monotone Multi-Layer Perceptron Neural Network) | Kappa + AUC | 0.8 | 0.55 | |
| Radiomics + change ADC | RRFglobal (Regularized Random Forest) | Kappa | 0.74 | 0.5 | |
| Radiomics + change ADC | svmLinearWeights (Linear Support Vector Machines with Class Weights) | AUC | 0.77 | 0.48 | |
| ADC | FRBCS.W (Fuzzy Rules with Weight Factor) | Kappa + AUC | 0.57 | 0.17 |
| Output | Features | Model | Metric | AUC | Kappa |
|---|---|---|---|---|---|
| Metastasis | Radiomics | svmLinearWeights (Linear Support Vector Machines with Class Weights) | Kappa + AUC | 0.76 | 0.5 |
| Radiomics + ADC1 + ADC2 + Change ADC | pcaNNet (Neural Networks with Feature Extraction) | Kappa + AUC | 0.84 | 0.65 | |
| Radiomics + ADC1 | pcaNNet (Neural Networks with Feature Extraction) | Kappa + AUC | 0.79 | 0.59 | |
| Radiomics + change ADC | pcaNNet (Neural Networks with Feature Extraction) | Kappa + AUC | 0.73 | 0.46 | |
| ADC | Rocc (ROC-Based Classifier) | Kappa | 0.63 | 0.3 | |
| ADC | svmLinearWeights (Linear Support Vector Machines with Class Weights) | AUC | 0.67 | 0.27 |
| Output | Features | Model | Metric | AUC | Kappa |
|---|---|---|---|---|---|
| Stage | Radiomics | RRFglobal (Regularized Random Forest) | Kappa | 0.51 | 0.31 |
| Radiomics | Knn (k-Nearest Neighbors) | AUC | 0.71 | 0.25 | |
| Radiomics + ADC1 + ADC2 + Change ADC | Earth (Multivariate Adaptive Regression Spline) | Kappa | 0.64 | 0.3 | |
| Radiomics + ADC1 + ADC2 + Change ADC | Knn (k-Nearest Neighbors) | AUC | 0.71 | 0.25 | |
| Radiomics + ADC1 | Evtree (Tree Models from Genetic Algorithms) | Kappa | 0.63 | 0.33 | |
| Radiomics + ADC1 | Knn (k-Nearest Neighbors) | AUC | 0.71 | 0.25 | |
| Radiomics + change ADC | Earth (Multivariate Adaptive Regression Spline) | Kappa | 0.64 | 0.31 | |
| Radiomics + change ADC | Knn (k-Nearest Neighbors) | AUC | 0.71 | 0.25 | |
| ADC | RRFglobal (Regularized Random Forest) | Kappa | 0.57 | 0.19 | |
| ADC | LogitBoost | AUC | 0.66 | 0.06 |
| Output | Features | Model | Metric | AUC | Kappa |
|---|---|---|---|---|---|
| Lymph Node | Radiomics | evtree (Tree Models from Genetic Algorithms) | Kappa + AUC | 0.75 | 0.6 |
| Radiomics + ADC1 + ADC2 + Change ADC | evtree (Tree Models from Genetic Algorithms) | Kappa + AUC | 0.75 | 0.6 | |
| Radiomics + ADC1 | evtree (Tree Models from Genetic Algorithms) | Kappa + AUC | 0.75 | 0.6 | |
| Radiomics + change ADC | evtree (Tree Models from Genetic Algorithms) | Kappa + AUC | 0.75 | 0.6 | |
| ADC | evtree (Tree Models from Genetic Algorithms) | Kappa + AUC | 0.64 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jajodia, A.; Gupta, A.; Prosch, H.; Mayerhoefer, M.; Mitra, S.; Pasricha, S.; Mehta, A.; Puri, S.; Chaturvedi, A. Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer. Tomography 2021, 7, 344-357. https://doi.org/10.3390/tomography7030031
Jajodia A, Gupta A, Prosch H, Mayerhoefer M, Mitra S, Pasricha S, Mehta A, Puri S, Chaturvedi A. Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer. Tomography. 2021; 7(3):344-357. https://doi.org/10.3390/tomography7030031
Chicago/Turabian StyleJajodia, Ankush, Ayushi Gupta, Helmut Prosch, Marius Mayerhoefer, Swarupa Mitra, Sunil Pasricha, Anurag Mehta, Sunil Puri, and Arvind Chaturvedi. 2021. "Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer" Tomography 7, no. 3: 344-357. https://doi.org/10.3390/tomography7030031
APA StyleJajodia, A., Gupta, A., Prosch, H., Mayerhoefer, M., Mitra, S., Pasricha, S., Mehta, A., Puri, S., & Chaturvedi, A. (2021). Combination of Radiomics and Machine Learning with Diffusion-Weighted MR Imaging for Clinical Outcome Prognostication in Cervical Cancer. Tomography, 7(3), 344-357. https://doi.org/10.3390/tomography7030031







