The Significance of Echo Time in fMRI BOLD Contrast: A Clinical Study during Motor and Visual Activation Tasks at 1.5 T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli and Tasks
2.3. MR Image Acquisition
2.4. Anatomical/Diagnostic
2.5. Relaxometry
2.6. Functional
2.7. Preprocessing
2.8. Strength of Activation
2.9. Extent of Activation within Each Activated Anatomical Region
2.10. Quantitative T2* Analysis
2.11. Statistical Analyses
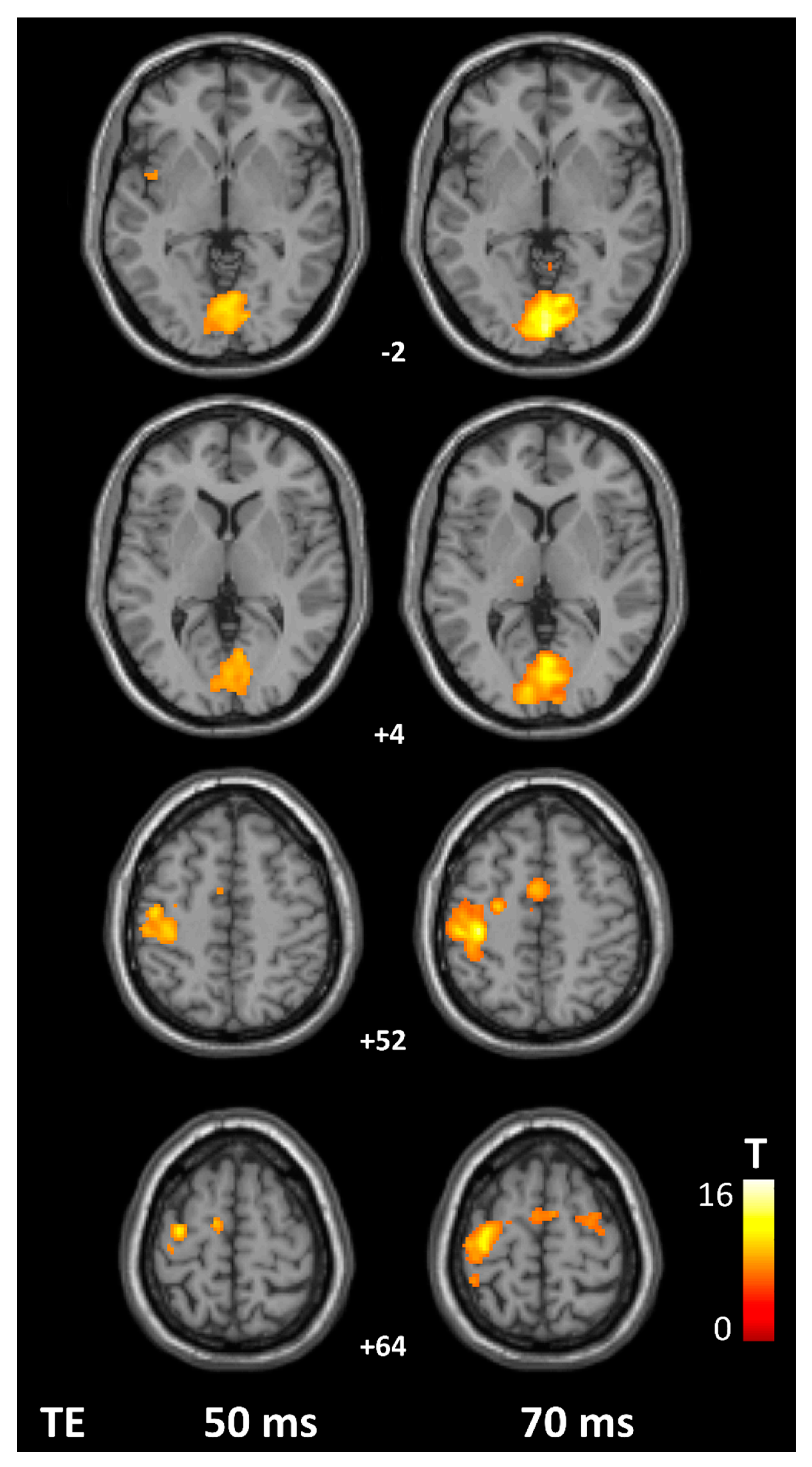
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogawa, S.; Lee, T.-M.; Nayak, A.S.; Glynn, P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990, 14, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gati, J.S.; Menon, R.S.; Uǧurbil, K.; Rutt, B.K. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn. Reson. Med. 1997, 38, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Krasnow, B.; Tamm, L.; Greicius, M.D.; Yang, T.T.; Glover, G.H.; Reiss, A.L.; Menon, V. Comparison of fMRI activation at 3 and 1.5 T during perceptual, cognitive, and affective processing. Neuroimage 2003, 18, 813–826. [Google Scholar] [CrossRef]
- Triantafyllou, C.; Wald, L.L.; Hoge, R.D. Echo-time and field strength dependence of BOLD reactivity in veins and parenchyma using flow-normalized hypercapnic manipulation. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zwaag, W.; Francis, S.; Head, K.; Peters, A.; Gowland, P.; Morris, P.; Bowtell, R. fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. Neuroimage 2009, 47, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.J.; Stanchev, P. Criteria for Analysis of Multicomponent Tissue. Magn. Reson. Med. 1996, 35, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.J.; Hoogduin, H.; van Zijl, P.C.M.; Jezzard, P.; Luijten, P.R.; Hendrikse, J. Blood oxygenation level-dependent (BOLD) total and extravascular signal changes and ΔR2* in human visual cortex at 1.5, 3.0 and 7.0 T. NMR Biomed. 2011, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fera, F.; Yongbi, M.N.; van Gelderen, P.; Frank, J.A.; Mattay, V.S.; Duyn, J.H. EPI-BOLD fMRI of human motor cortex at 1.5 T and 3.0 T: Sensitivity dependence on echo time and acquisition bandwidth. J. Magn. Reson. Imaging 2004, 19, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Krüger, G.; Glover, G.H. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 2001, 46, 631–637. [Google Scholar] [CrossRef]
- Schenck, J.F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef] [PubMed]
- Olsrud, J.; Nilsson, A.; Mannfolk, P.; Waites, A.; Ståhlberg, F. A two-compartment gel phantom for optimization and quality assurance in clinical BOLD fMRI. Magn. Reson. Imaging 2008, 26, 279–286. [Google Scholar] [CrossRef]
- Clare, S.; Francis, S.; Morris, P.G.; Bowtell, R. Single-shot T2(*) measurement to establish optimum echo time for fMRI: Studies of the visual, motor, and auditory cortices at 3.0 T. Magn. Reson. Med. 2001, 45, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Goksan, S.; Hartley, C.; Hurley, S.A.; Winkler, A.M.; Duff, E.P.; Jenkinson, M.; Rogers, R.; Clare, S.; Slater, R. Optimal echo time for functional MRI of the infant brain identified in response to noxious stimulation. Magn. Reson. Med. 2017, 78, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Markuerkiaga, I.; Marques, J.P.; Bains, L.J.; Norris, D.G. An in-vivo study of BOLD laminar responses as a function of echo time and static magnetic field strength. Sci. Rep. 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Holmes, A.P.; Worsley, K.J.; Poline, J.-P.; Frith, C.D.; Frackowiak, R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994, 2, 189–210. [Google Scholar] [CrossRef]
- Gao, J.H.; Liu, H.L. Inflow effects on functional MRI. Neuroimage 2012, 62, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.M.; Brookes, M.J.; Hoogenraad, F.G.; Gowland, P.A.; Francis, S.T.; Morris, P.G.; Bowtell, R. T2* measurements in human brain at 1.5, 3 and 7 T. Magn. Reson. Imaging 2007, 25, 748–753. [Google Scholar] [CrossRef]
- Li, T.-Q.; Yao, B.; van Gelderen, P.; Merkle, H.; Dodd, S.; Talagala, L.; Koretsky, A.P.; Duyn, J. Characterization of T(2)* heterogeneity in human brain white matter. Magn. Reson. Med. 2009, 62, 1652–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Parameters | 2D TSE-FLAIR | 2D T2*w MEGRE | 3D T1w MPRAGE | 2D GRE EPI (BOLD) |
|---|---|---|---|---|
| Field Of View (mm2) | 192 × 192 | 192 × 192 | 192 × 192 | 192 × 192 |
| Acquisition Matrix | 192 × 192 | 128 × 128 | 192 × 192 | 64 × 64 |
| Slice orientation | Oblique axial AC-PC line | Oblique axial AC-PC line | Oblique axial AC-PC line | Oblique axial AC-PC line |
| Slice thickness (mm) | 3 | 3 | 3 | 3 |
| Flip angle (°) | 150 | 25 | 15 | 90 |
| Pixel Bandwidth (Hz/Pixel) | 200 | 450 | 230 | 3255 |
| Partial Fourier | Phase: off | Phase: 6/8 | Phase: 7/8, slice: off | Phase: off |
| Repetition Time (ms) | 10,000 | 1300 | 778 | 4130 |
| Echo Time (ms) | 92 | 2.4 × (n + 1) n = 0 to 11 | 272 | 50/70 |
| Inversion Time (ms) | 2300 | Non applicable | 600 | Non applicable |
| Number of Slices | 36 | 36 | 36 | 36 |
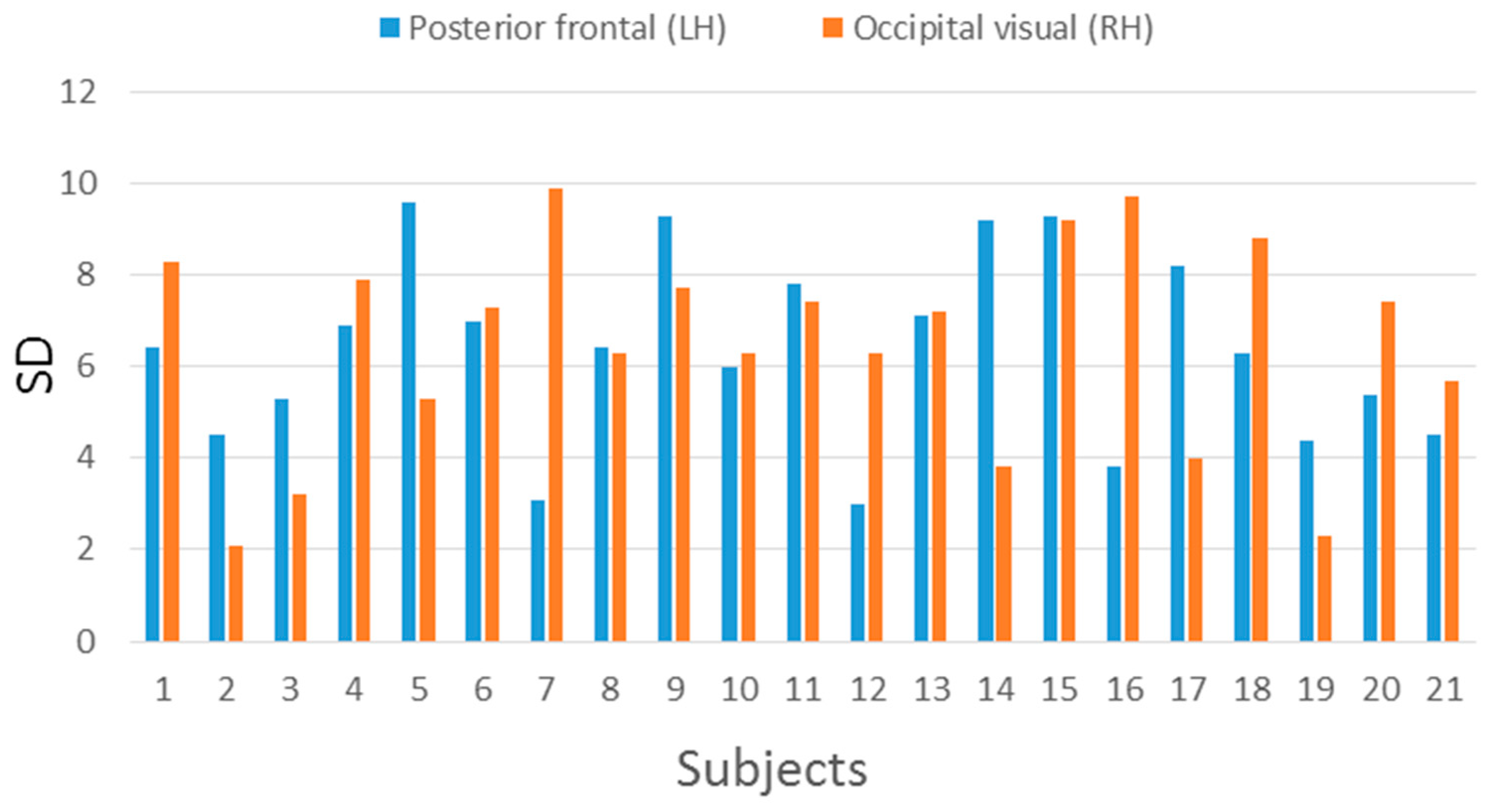
| Posterior Frontal (LH) | Occipital Visual (RH) | |||
|---|---|---|---|---|
| Subject | Mean | SD | Mean | SD |
| 1 | 62.35 | 6.4 | 68.63 | 8.3 |
| 2 | 61.45 | 4.5 | 53.10 | 2.1 |
| 3 | 73.65 | 5.3 | 67.10 | 3.2 |
| 4 | 75.00 | 6.9 | 74.33 | 7.9 |
| 5 | 73.85 | 9.6 | 70.07 | 5.3 |
| 6 | 76.40 | 7.0 | 76.93 | 7.3 |
| 7 | 79.70 | 3.1 | 72.65 | 9.9 |
| 8 | 70.70 | 6.4 | 65.53 | 6.3 |
| 9 | 78.00 | 9.3 | 65.00 | 7.7 |
| 10 | 73.35 | 6 | 69.47 | 6.3 |
| 11 | 71.10 | 7.8 | 64.50 | 7.4 |
| 12 | 70.90 | 3.0 | 70.40 | 6.3 |
| 13 | 68.95 | 7.1 | 67.35 | 7.2 |
| 14 | 70.50 | 9.2 | 71.50 | 3.8 |
| 15 | 72.25 | 9.3 | 71.15 | 9.2 |
| 16 | 76.50 | 3.8 | 69.45 | 9.7 |
| 17 | 70.10 | 8.2 | 75.10 | 4.0 |
| 18 | 75.25 | 6.3 | 65.20 | 8.8 |
| 19 | 66.90 | 4.4 | 63.35 | 2.3 |
| 20 | 64.00 | 5.4 | 79.75 | 7.4 |
| 21 | 70.50 | 4.5 | 75.50 | 5.7 |
| Average | 71.50 | 6.36 | 69.34 | 6.48 |
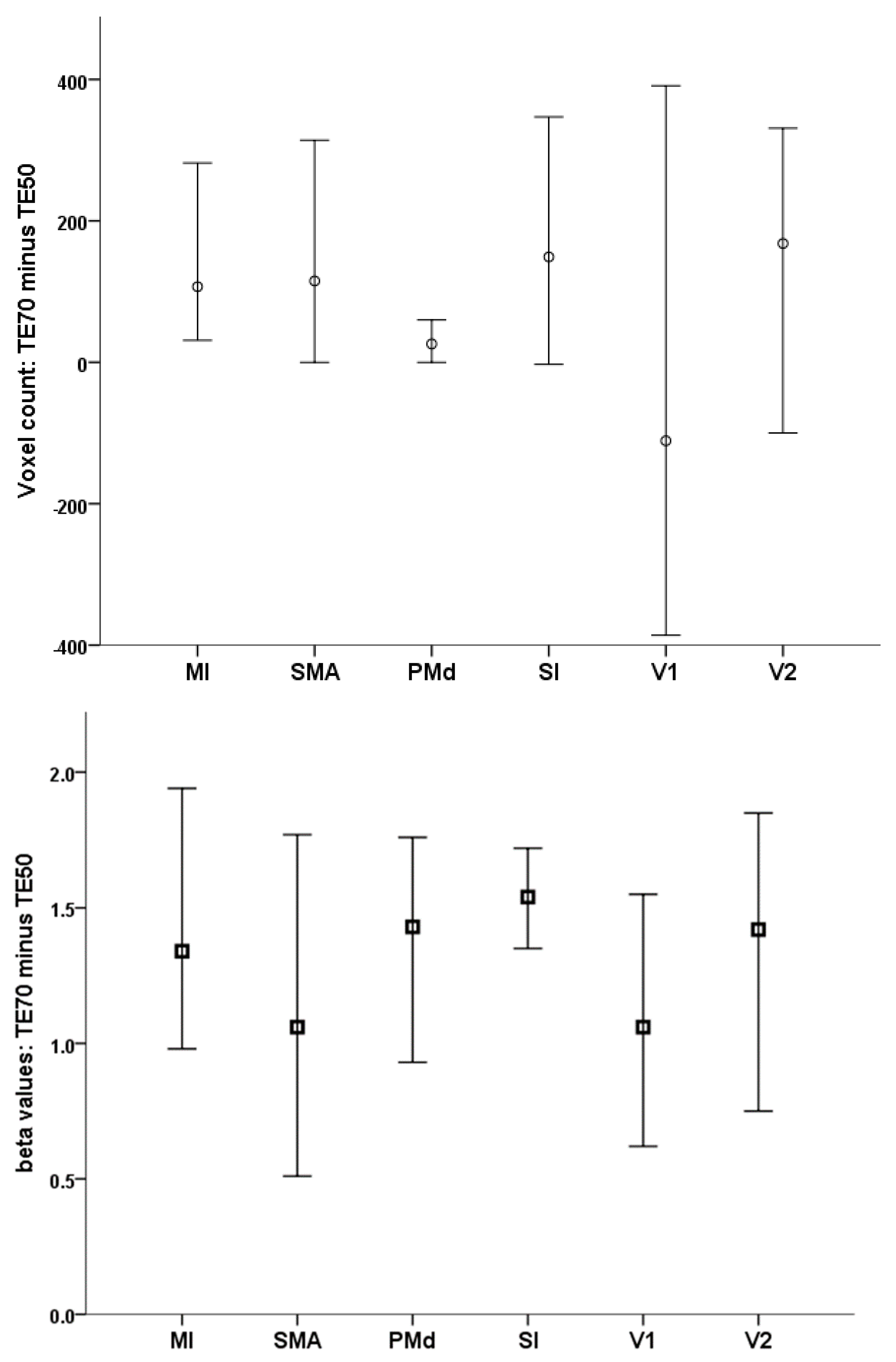
| TE 50 ms | TE 70 ms | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | |
| Number of active voxels | |||||
| MI (LH) | 529.3 | 380.4 | 735.9 | 341.8 | 0.002 |
| PMd (LH) | 111.4 | 117.4 | 142.1 | 121.8 | 0.1 |
| SMA | 415.1 | 390.7 | 606.3 | 409.9 | 0.003 |
| SI (LH) | 744.2 | 598.3 | 946.5 | 634.1 | 0.007 |
| V1 (RH) | 1356.5 | 824.8 | 1400.0 | 601.0 | 0.7 |
| V2 (RH) | 977.9 | 568.7 | 1102.9 | 530.5 | 0.2 |
| Peak beta value | |||||
| MI (LH) | 1.82 | 1.06 | 3.40 | 1.30 | <0.001 |
| PMd (LH) | 2.33 | 1.27 | 3.92 | 1.40 | <0.001 |
| SMA | 2.24 | 1.06 | 3.53 | 1.36 | <0.001 |
| SI (LH) | 2.15 | 1.08 | 3.84 | 1.42 | <0.001 |
| V1 (RH) | 3.55 | 1.65 | 4.85 | 1.54 | <0.001 |
| V2 (RH) | 3.18 | 1.87 | 4.61 | 1.66 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boursianis, T.; Kalaitzakis, G.; Nikiforaki, K.; Kosteletou, E.; Antypa, D.; Gourzoulidis, G.A.; Karantanas, A.; Papadaki, E.; Simos, P.; Maris, T.G.; et al. The Significance of Echo Time in fMRI BOLD Contrast: A Clinical Study during Motor and Visual Activation Tasks at 1.5 T. Tomography 2021, 7, 333-343. https://doi.org/10.3390/tomography7030030
Boursianis T, Kalaitzakis G, Nikiforaki K, Kosteletou E, Antypa D, Gourzoulidis GA, Karantanas A, Papadaki E, Simos P, Maris TG, et al. The Significance of Echo Time in fMRI BOLD Contrast: A Clinical Study during Motor and Visual Activation Tasks at 1.5 T. Tomography. 2021; 7(3):333-343. https://doi.org/10.3390/tomography7030030
Chicago/Turabian StyleBoursianis, Themistoklis, Georgios Kalaitzakis, Katerina Nikiforaki, Emmanouela Kosteletou, Despina Antypa, George A. Gourzoulidis, Apostolos Karantanas, Efrosini Papadaki, Panagiotis Simos, Thomas G. Maris, and et al. 2021. "The Significance of Echo Time in fMRI BOLD Contrast: A Clinical Study during Motor and Visual Activation Tasks at 1.5 T" Tomography 7, no. 3: 333-343. https://doi.org/10.3390/tomography7030030
APA StyleBoursianis, T., Kalaitzakis, G., Nikiforaki, K., Kosteletou, E., Antypa, D., Gourzoulidis, G. A., Karantanas, A., Papadaki, E., Simos, P., Maris, T. G., & Marias, K. (2021). The Significance of Echo Time in fMRI BOLD Contrast: A Clinical Study during Motor and Visual Activation Tasks at 1.5 T. Tomography, 7(3), 333-343. https://doi.org/10.3390/tomography7030030








