Assessment of Motor Dysfunction with Virtual Reality in Patients Undergoing [123I]FP-CIT SPECT/CT Brain Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluations
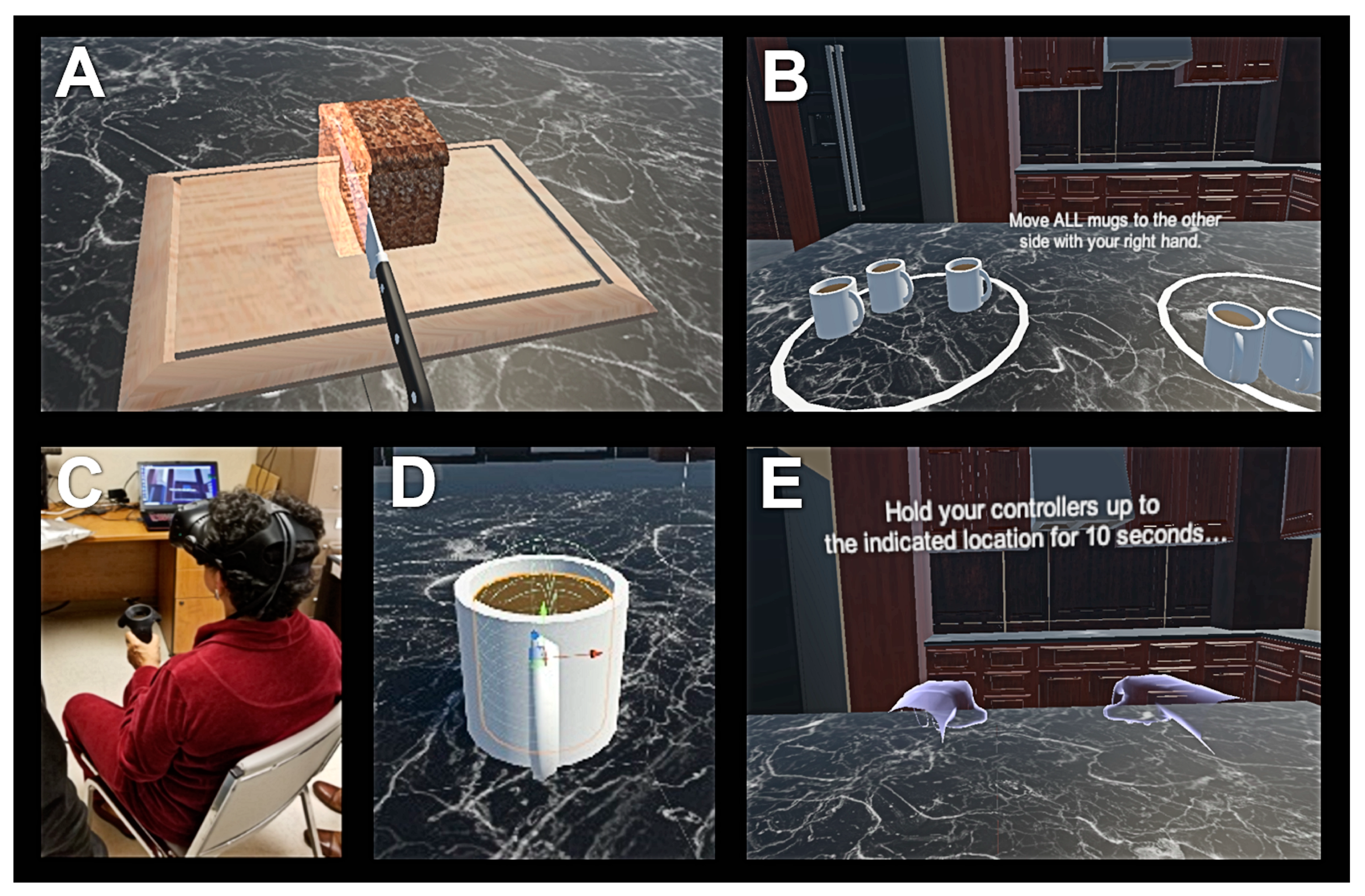
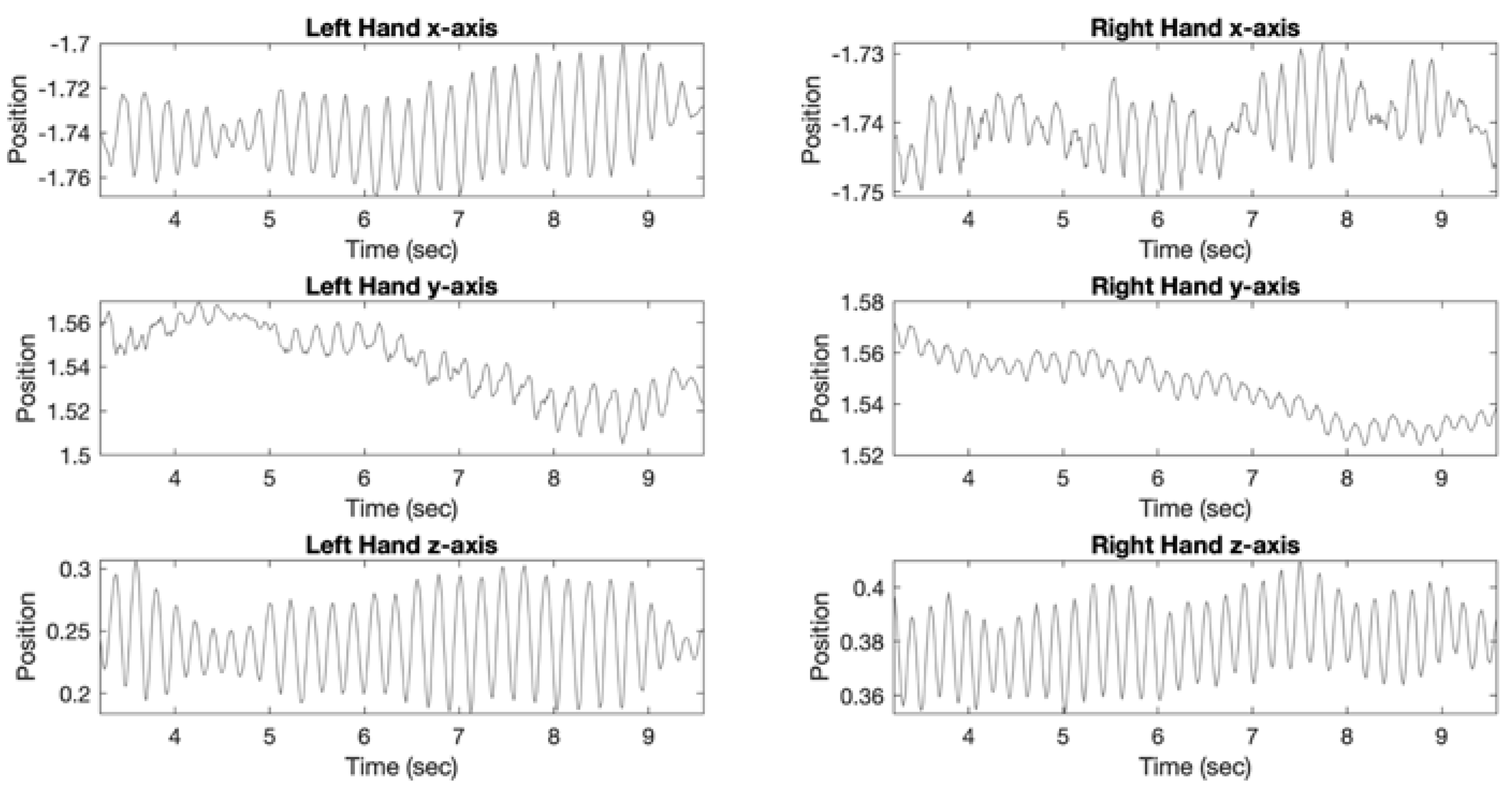
2.3. Virtual Reality
2.4. Brain SPECT/CT Imaging
2.5. Statistical Analysis
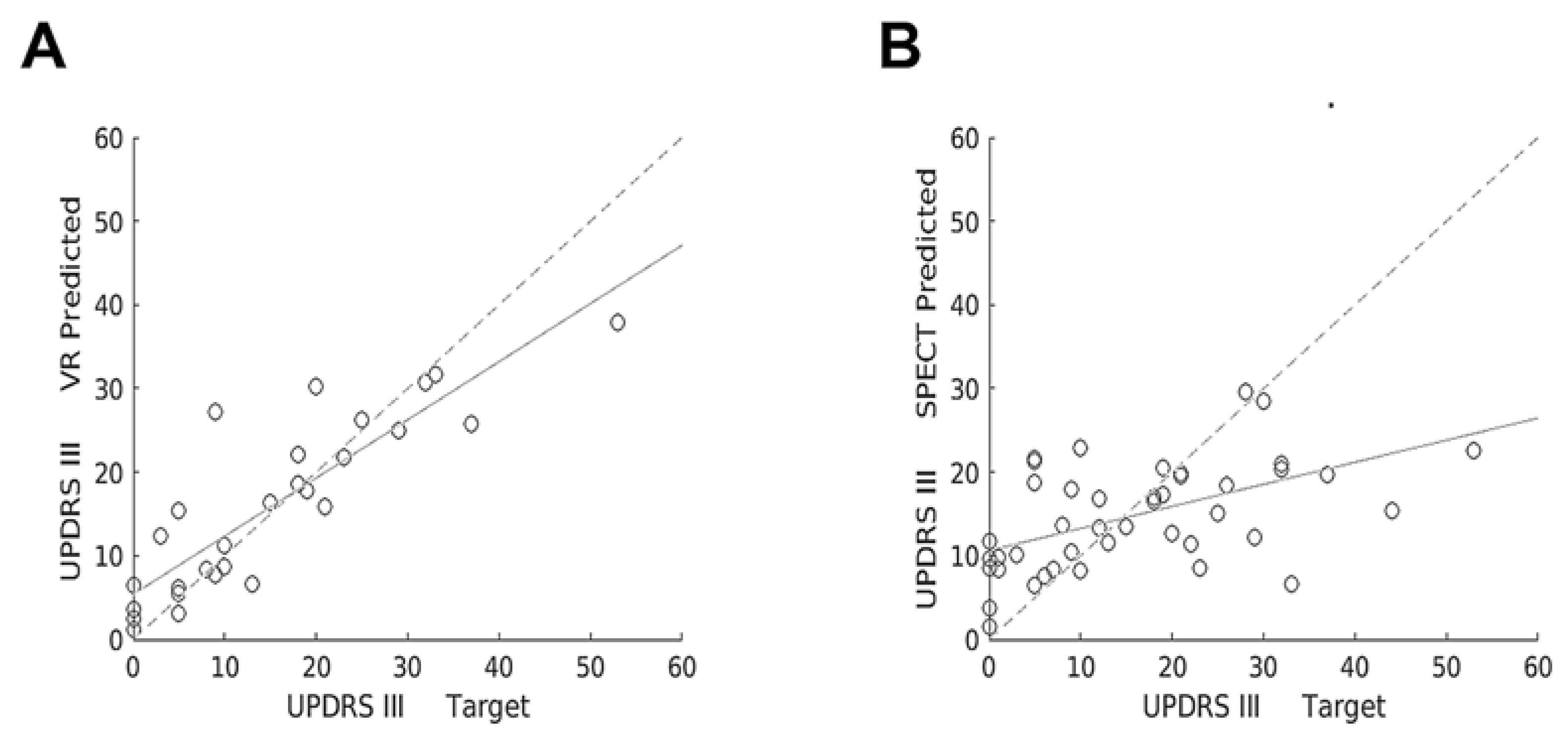
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. Cmaj 2016, 188, 1157–1165. [Google Scholar] [CrossRef]
- Visanji, N.P.; Brotchie, J.M.; Kalia, L.V.; Koprich, J.B.; Tandon, A.; Watts, J.C.; Lang, A.E. α-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016, 39, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef]
- Djang, D.S.W.; Janssen, M.J.R.; Bohnen, N.; Booij, J.; Henderson, T.A.; Herholz, K.; Minoshima, S.; Rowe, C.C.; Sabri, O.; Seibyl, J.; et al. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J. Nucl. Med. 2012, 53, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Martin, W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Park. Relat. Disord. 2015, 21, 87–94. [Google Scholar] [CrossRef]
- Benamer, H.T.S.; Patterson, J.; Wyper, D.J.; Hadley, D.M.; Macphee, G.J.A.; Grosset, D.G. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov. Disord. 2000, 15, 692–698. [Google Scholar] [CrossRef]
- Simuni, T.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C.; Jennings, D.; Tanner, C.M.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Longitudinal Change of Clinical and Biological Measures in Early Parkinson’s Disease: Parkinson’s Progression Markers Initiative Cohort. Mov. Disord. 2018, 33, 771–782. [Google Scholar] [CrossRef]
- Yang, Y.; Cheon, M.; Kwak, Y.T. 18F-FP-CIT Positron Emission Tomography for Correlating Motor and Cognitive Symptoms of Parkinson’s Disease. Dement. Neurocogn. Disord. 2017, 16, 57. [Google Scholar] [CrossRef]
- Riva, G.; Baños, R.M.; Botella, C.; Mantovani, F.; Gaggioli, A. Transforming experience: The potential of augmented reality and virtual reality for enhancing personal and clinical change. Front. Psychiatry 2016, 7, 164. [Google Scholar] [CrossRef]
- Robles-García, V.; Corral-Bergantiños, Y.; Espinosa, N.; García-Sancho, C.; Sanmartín, G.; Flores, J.; Cudeiro, J.; Arias, P. Effects of movement imitation training in Parkinson’s disease: A virtual reality pilot study. Park. Relat. Disord. 2016, 26, 17–23. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, D.K.; Song, H.S. Effect of virtual reality dance exercise on the balance, activities of daily living, And depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Yang, Y.R.; Cheng, S.J.; Wu, Y.R.; Fuh, J.L.; Wang, R.Y. Virtual Reality-Based Training to Improve Obstacle-Crossing Performance and Dynamic Balance in Patients with Parkinson’s Disease. Neurorehabil. Neural Repair 2015, 29, 658–667. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef]
- Mirelman, A.; Maidan, I.; Deutsch, J.E. Virtual reality and motor imagery: Promising tools for assessment and therapy in Parkinson’s disease. Mov. Disord. 2013, 28, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Mov. Disord. 2007, 22, 41–47. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Barrantes, S.; Sánchez Egea, A.J.; González Rojas, H.A.; Martí, M.J.; Compta, Y.; Valldeoriola, F.; Mezquita, E.S.; Tolosa, E.; Valls-Solè, J. Differential diagnosis between Parkinson’s disease and essential tremor using the smartphone’s accelerometer. PLoS ONE 2017, 12, e0183843. [Google Scholar] [CrossRef]
- Halliday, D.M.; Rosenberg, J.R.; Amjad, A.M.; Breeze, P.; Conway, B.A.; Farmer, S.F. A framework for the analysis of mixed time series/point process data-Theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog. Biophys. Mol. Biol. 1995, 64, 237–278. [Google Scholar] [CrossRef]
- Varrone, A.; Dickson, J.C.; Tossici-Bolt, L.; Sera, T.; Asenbaum, S.; Booij, J.; Kapucu, O.L.; Kluge, A.; Knudsen, G.M.; Koulibaly, P.M.; et al. European multicentre database of healthy controls for [123I]FP- CIT SPECT (ENC-DAT): Age-related effects, gender differences and evaluation of different methods of analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Chowdhury, S.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C.; Simuni, T.; Jennings, D.; Tanner, C.M.; Trojanowski, J.Q.; et al. The Parkinson’s progression markers initiative (PPMI)–establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 2018, 5, 1460–1477. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The clinically important difference on the unified parkinson’s disease rating scale. Arch. Neurol. 2010, 67, 64–70. [Google Scholar] [CrossRef]
- Metz, C.E.; Herman, B.A.; Shen, J.H. Maximum likelihood estimation of receiver operating characteristic (ROC) curves from continuously-distributed data. Stat. Med. 1998, 17, 1033–1053. [Google Scholar] [CrossRef]
- Metz, C.E.; Herman, B.A.; Roe, C.A. Statistical comparison of two ROC-curve estimates obtained from partially-paired datasets. Med. Decis. Mak. 1998, 18, 110–121. [Google Scholar] [CrossRef]
- Lee, A.; Hellmers, N.; Vo, M.; Wang, F.; Popa, P.; Barkan, S.; Patel, D.; Campbell, C.; Henchcliffe, C.; Sarva, H. Can google glassTM technology improve freezing of gait in parkinsonism? A pilot study. Disabil. Rehabil. Assist. Technol. 2020, 1–11, in press. [Google Scholar]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Baig, F.; Lawton, M.; Rolinski, M.; Ruffmann, C.; Nithi, K.; Evetts, S.G.; Fernandes, H.R.; Ben-Shlomo, Y.; Hu, M.T.M. Delineating nonmotor symptoms in early Parkinson’s disease and first-degree relatives. Mov. Disord. 2015, 30, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Wong, W.T.; Teh, T.A.; Lim, S.H.; Allen, J.C.; Quah, J.H.M.; Malhotra, R.; Tan, N.C. A Fully-Immersive and Automated Virtual Reality System to Assess the Six Domains of Cognition: Protocol for a Feasibility Study. Front. Aging Neurosci. 2021, 12, 604670. [Google Scholar] [CrossRef]


| N (%) or Mean ± Standard Deviation (Range) | |
|---|---|
| Male | 21/44 (48%) |
| Female | 23/44 (52%) |
| Age | 64.5 ± 12.4 (36–85) |
| UPDRS-III | 16.3 ± 13.0 (0–53) |
| UPDRS-III > 10 | 25/44 (57%) |
| PD Meds | 12/44 (27%) |
| H&Y | 1 ± 0.9 (0–3) |
| MoCA | 22.6 ± 6.1 (9–30) |
| Right | Left | p-Value | |
|---|---|---|---|
| Hand Dominance | 38 | 6 | NA |
| SPECT Striatum SBR | 1.6 ± 0.7 | 1.6 ± 0.7 | 1.000 |
| SPECT Caudate SBR | 1.9 ± 0.8 | 1.9 ± 0.7 | 1.000 |
| SPECT Putamen SBR | 1.5 ± 0.7 | 1.5 ± 0.7 | 1.000 |
| SPECT Putamen-To-Caudate Ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.000 |
| VR Time (s) | 72.1 ± 47.6 | 83.7 ± 60.1 | 0.318 |
| VR ADL Score | 8.5 ± 1.2 | 9.2 ± 0.9 | 0.003 * |
| VR Posture Tremor Frequency (Hz) | 2.1 ± 1.3 | 2.3 ± 1.8 | 0.552 |
| VR Posture Tremor Power (m2/Hz) | 4.86 × 10−6 ± 2.18 × 10−5 | 2.13 × 10−5 ± 1.23 × 10−4 | 0.385 |
| VR Rest Tremor Frequency (Hz) | 3.3 ± 2.0 | 3.4 ± 2.1 | 0.552 |
| VR Rest Tremor Power (m2/Hz) | 6.73 × 10−6 ± 2.99 × 10−5 | 1.38 × 10−6 ± 7.41 × 10−6 | 0.253 |
| VR # | VR | SPECT | SPECT + PD Meds | |
|---|---|---|---|---|
| AUC | 0.9133 | 0.8418 | 0.5357 | 0.5397 |
| AUC 95% CI | (0.6350–1.0000) | (0.6071–0.9617) | (0.3373–0.7357) | (0.3374–0.7345) |
| SE | 0.0577 | 0.0770 | 0.1038 | 0.1037 |
| p-value vs. VR AUC | NA | NA | 0.029 * | 0.042 * |
| Beta | SE | OR | p-Value | |
|---|---|---|---|---|
| Age | −0.01402 | 0.05022 | 0.9861 | 0.780 |
| Male Gender | −1.1185 | 1.2214 | 0.3268 | 0.360 |
| Right Hand Dominance | 1.2458 | 1.226 | 3.4756 | 0.319 |
| PD Meds | −0.13796 | 0.16178 | 0.8711 | 0.394 |
| VR SVM Score | 5.7881 | 2.172 | 326.4029 | 0.008 * |
| SPECT SVM Score | 7.353 | 53.781 | 1560.9 | 0.891 |
| VR | SPECT | SPECT + PD Meds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MSE | 41.6915 | 122.6062 | 122.4380 | ||||||
| N | R-Squared | p-Value | N | R-Squared | p-Value | N | R-Squared | p-Value | |
| All | 28 | 0.755 | 0.001 * | 44 | 0.272 | 0.001 * | 44 | 0.273 | 0.001 * |
| Train | 20 | 0.729 | 0.001 * | 41 | 0.254 | 0.004 * | 41 | 0.273 | 0.004 * |
| Test | 8 | 0.713 | 0.008 * | 13 | 0.0764 | 0.361 | 13 | 0.0676 | 0.391 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, J.P.; Yamin, G.; Reyes, Z.; Shin, A.; Young, A.; Litvan, I.; Xie, P.; Obrzut, S. Assessment of Motor Dysfunction with Virtual Reality in Patients Undergoing [123I]FP-CIT SPECT/CT Brain Imaging. Tomography 2021, 7, 95-106. https://doi.org/10.3390/tomography7020009
Vu JP, Yamin G, Reyes Z, Shin A, Young A, Litvan I, Xie P, Obrzut S. Assessment of Motor Dysfunction with Virtual Reality in Patients Undergoing [123I]FP-CIT SPECT/CT Brain Imaging. Tomography. 2021; 7(2):95-106. https://doi.org/10.3390/tomography7020009
Chicago/Turabian StyleVu, Jeanne P., Ghiam Yamin, Zabrina Reyes, Alex Shin, Alexander Young, Irene Litvan, Pengtao Xie, and Sebastian Obrzut. 2021. "Assessment of Motor Dysfunction with Virtual Reality in Patients Undergoing [123I]FP-CIT SPECT/CT Brain Imaging" Tomography 7, no. 2: 95-106. https://doi.org/10.3390/tomography7020009
APA StyleVu, J. P., Yamin, G., Reyes, Z., Shin, A., Young, A., Litvan, I., Xie, P., & Obrzut, S. (2021). Assessment of Motor Dysfunction with Virtual Reality in Patients Undergoing [123I]FP-CIT SPECT/CT Brain Imaging. Tomography, 7(2), 95-106. https://doi.org/10.3390/tomography7020009






