Computed Tomography and Coronary Plaque Analysis
Abstract
1. Introduction
1.1. Types of CT Imaging Modalities
1.1.1. Dual-Layer Spectral CT Angiography (DL-SCTA)
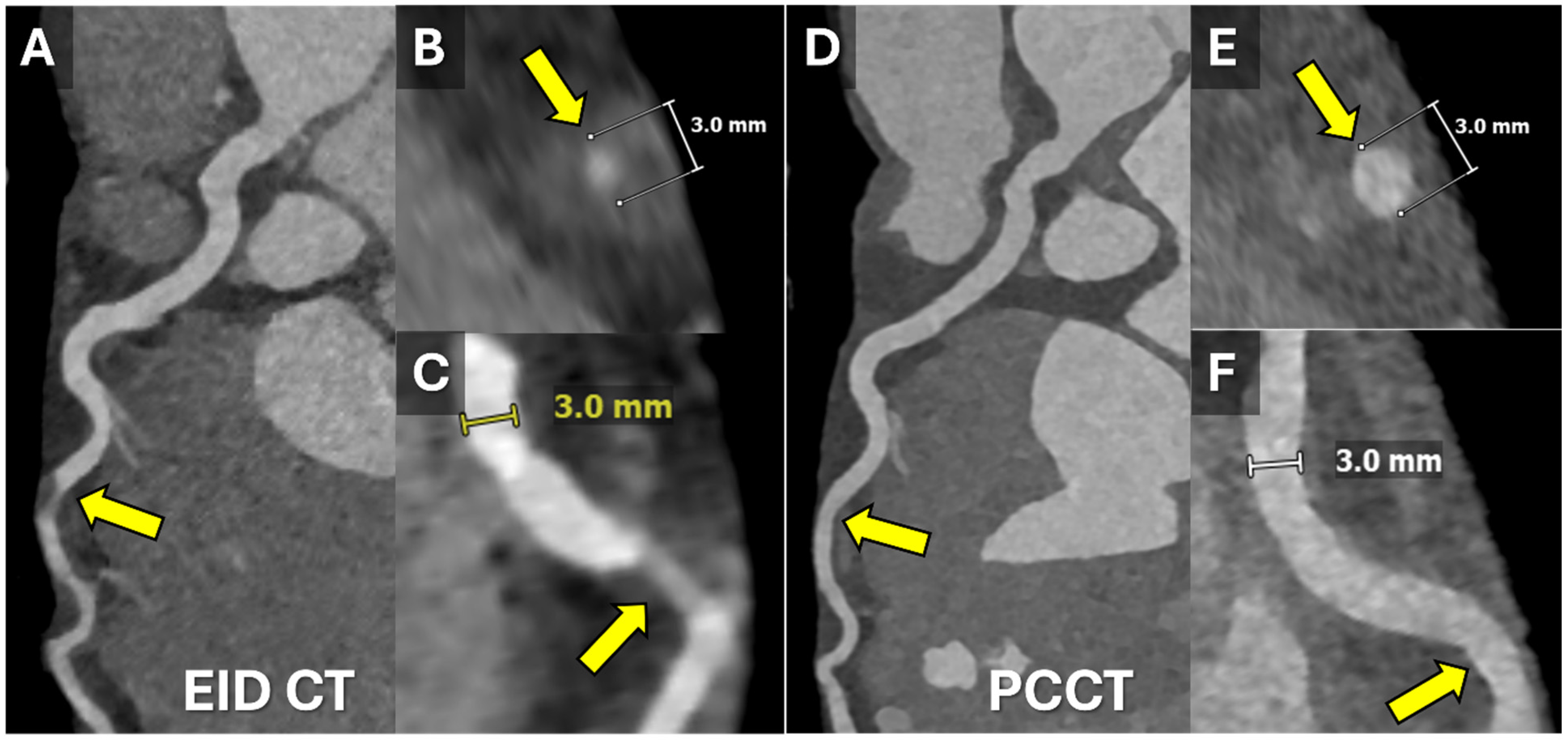
1.1.2. Photon-Counting CT (PCCT)
1.1.3. Dual-Energy CT (DECT)
1.1.4. CT-Derived Fractional Flow Reserve (CT-FFR)
1.2. Significance of Plaque Burden
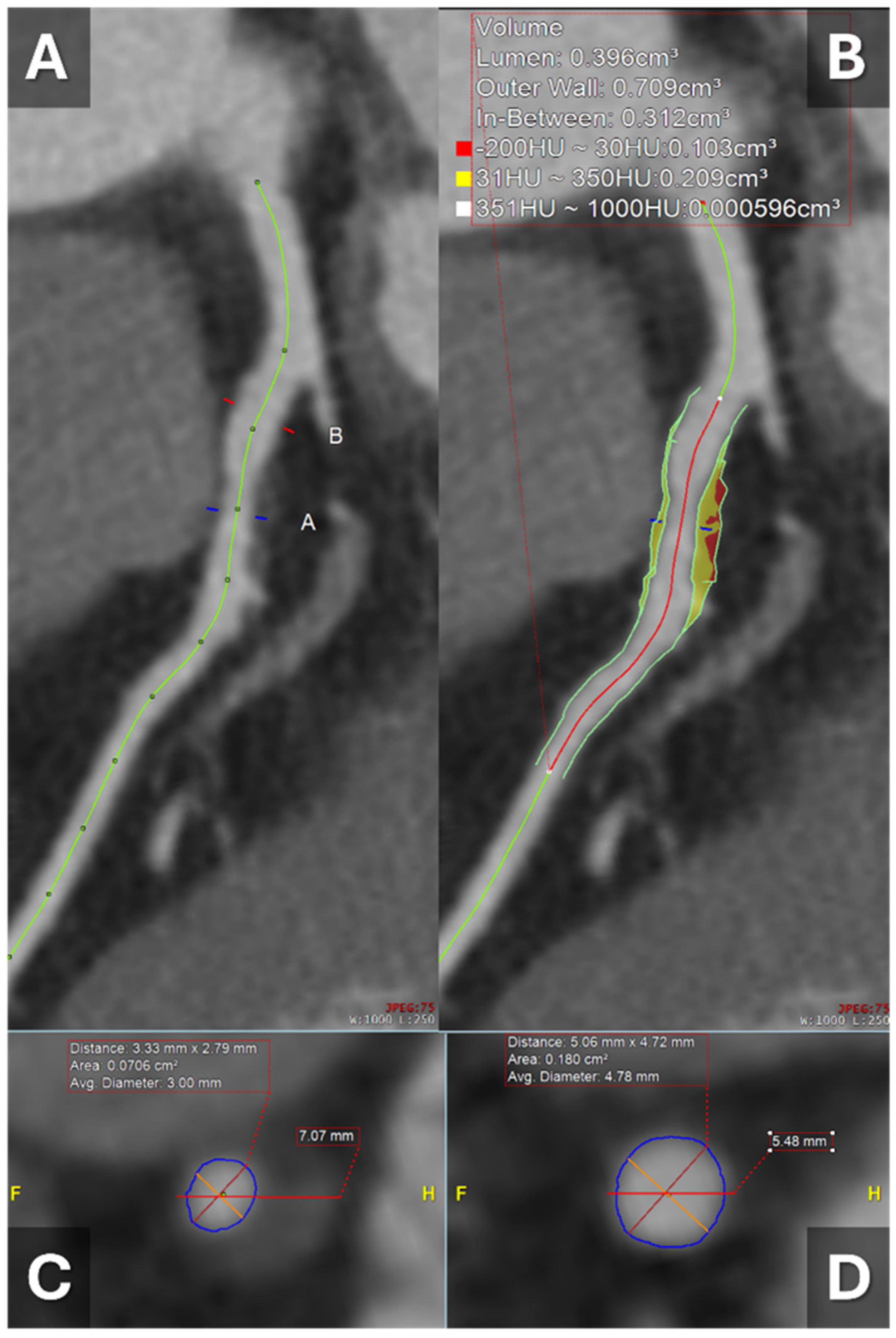
1.3. Artificial Intelligence and CT-Derived Plaque Analysis
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Glover, D.K.; Lanza, G.M.; Fayad, Z.A.; Johnson, L.L. Imaging Atherosclerosis and Vulnerable Plaque. J. Nucl. Med. 2010, 51, 51S–65S. [Google Scholar] [CrossRef]
- Gać, P.; Jakubowska-Martyniuk, A.; Żórawik, A.; Hajdusianek, W.; Żytkowski, D.; Matys, T.; Poręba, R. Diagnostic Methods of Atherosclerotic Plaque and the Assessment of Its Prognostic Significance—A Narrative Review. J. Cardiovasc. Dev. Dis. 2024, 11, 343. [Google Scholar] [CrossRef]
- Nadjiri, J.; Koppara, T.; Kafka, A.; Weis, F.; Rasper, M.; Gassert, F.G.; von Schacky, C.E.; Pfeiffer, D.; Laugwitz, K.-L.; Makowski, M.R.; et al. Coronary plaque characterization assessed by delayed enhancement dual-layer spectral CT angiography and optical coherence tomography. Int. J. Cardiovasc. Imaging 2022, 38, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Lacaita, P.G.; Luger, A.; Troger, F.; Widmann, G.; Feuchtner, G.M. Photon-Counting Detector Computed Tomography (PCD-CT): A New Era for Cardiovascular Imaging? Current Status and Future Outlooks. J. Cardiovasc. Dev. Dis. 2024, 11, 127. [Google Scholar] [CrossRef]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Rau, A.; Taron, J.; Weiss, J.; Stein, T.; Faby, S.; von Zur Muehlen, C.; Ruile, P.; et al. Accuracy of Ultrahigh-Resolution Photon-counting CT for Detecting Coronary Artery Disease in a High-Risk Population. Radiology 2023, 307, e223305. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Molina-Fuentes, M.F.; Weiss, J.; Rau, A.; Schuppert, C.; Ruile, P.; Faby, S.; Schibilsky, D.; et al. Ultra-high-resolution photon-counting detector CT in evaluating coronary stent patency: A comparison to invasive coronary angiography. Eur. Radiol. 2024, 34, 4273–4283. [Google Scholar] [CrossRef]
- Ding, H.; Wang, C.; Malkasian, S.; Johnson, T.; Molloi, S. Characterization of arterial plaque composition with dual energy computed tomography: A simulation study. Int. J. Cardiovasc. Imaging 2021, 37, 331–341. [Google Scholar] [CrossRef]
- Yuenyongsinchai, K.; Tan, C.O.; Vranic, J.; Flores, E.; Silverman, S.; Gupta, R. Carotid Plaque Characterization Using Dual-Energy Computed Tomography: Predicting Imminent Ipsilateral Ischemic Stroke in 30 Days. Stroke: Vasc. Interv. Neurol. 2022, 2, e000313. [Google Scholar] [CrossRef]
- Li, Z.; Xu, T.; Wang, Z.; Ding, Y.; Zhang, Y.; Lin, L.; Wang, M.; Xu, L.; Zeng, Y. Prognostic Significance of Computed Tomography-Derived Fractional Flow Reserve for Long-Term Outcomes in Individuals with Coronary Artery Disease. J. Am. Heart Assoc. 2025, 14, e037988. [Google Scholar] [CrossRef]
- Tesche, C.; Otani, K.; De Cecco, C.N.; Coenen, A.; De Geer, J.; Kruk, M.; Kim, Y.H.; Albrecht, M.H.; Baumann, S.; Renker, M.; et al. Influence of Coronary Calcium on Diagnostic Performance of Machine Learning CT-FFR: Results from MACHINE Registry. Cardiovasc. Imaging 2020, 13, 760–770. [Google Scholar] [CrossRef]
- Ma, Z.; Tu, C.; Zhang, B.; Zhang, D.; Song, X.; Zhang, H. A meta-analysis comparing the diagnostic performance of computed tomography-derived fractional flow reserve and coronary computed tomography angiography at different levels of coronary artery calcium score. Eur. Radiol. 2024, 34, 5621–5632. [Google Scholar] [CrossRef]
- Tsuda, T.; Ishii, H.; Ichimiya, S.; Kanashiro, M.; Watanabe, J.; Takefuji, M.; Aoyama, T.; Suzuki, S.; Tanaka, A.; Matsubara, T.; et al. Assessment of in-stent restenosis using high-definition computed tomography with a new gemstone detector. Circ. J. 2015, 79, 1542–1548. [Google Scholar] [CrossRef]
- Tang, C.X.; Guo, B.J.; Schoepf, J.U.; Bayer, R.R.; Liu, C.Y.; Qiao, H.Y.; Zhou, F.; Lu, G.M.; Zhou, C.S.; Zhang, L.J. Feasibility and prognostic role of machine learning-based FFRCT in patients with stent implantation. Eur. Radiol. 2021, 31, 6592–6604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, M.; Li, W.; Lu, Z.; Wei, M.; Zhang, J. Third generation dual-source CT enables accurate diagnosis of coronary restenosis in all size stents with low radiation dose and preserved image quality. Eur. Radiol. 2018, 28, 2647–2654. [Google Scholar] [CrossRef]
- Szilveszter, B.; Celeng, C.; Maurovich-Horvat, P. Plaque assessment by coronary CT. Int. J. Cardiovasc. Imaging 2016, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Martinez Vives, P.; Ramos-Cano, P.; Monteagudo-Ruiz, J.M.; Piris, A.; Carrion-Sanchez, I.; Garcia-Sebastian, C.; Antonana, S.; Garcia-Martin, A.; Gonzalez, A.; Hinojar, R.; et al. Coronary atherosclerotic plaque burden and characterization by spectral computed tomography scan in patients with high lipoprotein a and non-hemodynamically significant stenosis. Eur. Heart J. 2024, 45, ehae666-210. [Google Scholar] [CrossRef]
- Flohr, T.; Schmidt, B.; Ulzheimer, S.; Alkadhi, H. Cardiac imaging with photon counting CT. Br. J. Radiol. 2023, 96, 20230407. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Richards, T.; Nikpanah, M.; Sahbaee, P.; Shanbhag, S.M.; Bandettini, W.P.; Saboury, B.; Malayeri, A.A.; Pritchard, W.F.; et al. Evaluation of Coronary Plaques and Stents with Conventional and Photon-counting CT: Benefits of High-Resolution Photon-counting CT. Radiol. Cardiothorac. Imaging 2021, 3, e210102. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography from Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Dual-Energy CT. Radiology Reference Article. Available online: https://radiopaedia.org/articles/dual-energy-ct-4?lang=us (accessed on 19 January 2025).
- Halfmann, M.C.; Bockius, S.; Emrich, T.; Hell, M.; Schoepf, U.J.; Laux, G.S.; Kavermann, L.; Graafen, D.; Gori, T.; Yang, Y.; et al. Ultrahigh-Spatial-Resolution Photon-counting Detector CT Angiography of Coronary Artery Disease for Stenosis Assessment. Radiology 2024, 310, e231956. [Google Scholar] [CrossRef]
- Sheta, H.M.; Precht, H.; Busk, C.A.G.R.; Heinsen, L.J.; Nieman, K.; Egstrup, K.; Lambrechtsen, J. Dual-energy CT plaque characteristics of post mortem thin-cap fibroatheroma in comparison to infarct-related culprit lesions. Heart Vessel. 2022, 37, 400–410. [Google Scholar] [CrossRef]
- Shami, A.; Eisa, M.; Klink, T.; Albrecht, M.H.; Lenga, L.; Borggrefe, J.; Köhler, T.; Vogl, T.J.; Booz, C. Atherosclerotic plaque features relevant to rupture-risk detected by clinical photon-counting CT ex vivo: A proof-of-concept study. Eur. Radiol. Exp. 2024, 8, 14. [Google Scholar] [CrossRef]
- Dang, Y.; Chen, X.; Ma, S.; Ma, Y.; Ma, Q.; Zhou, K.; Liu, T.; Wang, K.; Hou, Y. Association of Pericoronary Adipose Tissue Quality Determined by Dual-Layer Spectral Detector CT with Severity of Coronary Artery Disease: A Preliminary Study. Front. Cardiovasc. Med. 2021, 8, 720127. [Google Scholar] [CrossRef]
- van Veelen, A.; van der Sangen, N.M.R.; Delewi, R.; Beijk, M.A.M.; Henriques, J.P.S.; Claessen, B.E.P.M. Detection of Vulnerable Coronary Plaques Using Invasive and Non-Invasive Imaging Modalities. J. Clin. Med. 2022, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bartykowszki, A.; Celeng, C.; Károlyi, M.; Maurovich-Horvat, P. High Risk Plaque Features on Coronary CT Angiography. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9279. [Google Scholar] [CrossRef]
- Channon, K.M.; Newby, D.E.; Nicol, E.D.; Deanfield, J. Cardiovascular computed tomography imaging for coronary artery disease risk: Plaque, flow and fat. Heart 2022, 108, 1510–1515. [Google Scholar] [CrossRef]
- Chan, C.; Wang, M.; Kong, L.; Li, L.; Chi Chan, L.W. Clinical Applications of Fractional Flow Reserve Derived from Computed Tomography in Coronary Artery Disease. Mayo Clin. Proc. Digit. Health 2025, 3, 100187. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Xian, H.; Luo, X.; Zhang, D.; Hou, S.; Qu, C.; Zhang, R.; Qu, X. Prognostic value of computed tomography-derived fractional flow reserve in patients with diabetes mellitus and unstable angina. Cardiovasc. Diabetol. 2024, 23, 404. [Google Scholar] [CrossRef]
- Lan, Z.; Ding, X.; Yu, Y.; Yu, L.; Yang, W.; Dai, X.; Ling, R.; Wang, Y.; Yang, W.; Zhang, J. CT-derived fractional flow reserve for prediction of major adverse cardiovascular events in diabetic patients. Cardiovasc. Diabetol. 2023, 22, 65. [Google Scholar] [CrossRef]
- Madsen, K.T.; Nørgaard, B.L.; Øvrehus, K.A.; Jensen, J.M.; Parner, E.; Grove, E.L.; Fairbairn, T.A.; Nieman, K.; Patel, M.R.; Rogers, C.; et al. Prognostic Value of Coronary CT Angiography–derived Fractional Flow Reserve on 3-year Outcomes in Patients with Stable Angina. Radiology 2023, 308, e230524. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Li, J.H.; Schoepf, U.J.; Bayer, R.R., 2nd; Tinnefeld, F.C.; Di Jiang, M.; Yang, F.; Guo, B.J.; Zhou, C.S.; Ge, Y.Q.; et al. Prognostic implication of CT-FFR based functional SYNTAX score in patients with de novo three-vessel disease. Eur. Heart J.—Cardiovasc. Imaging Oxf. Acad. 2021, 22, 1434–1442. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Tzimas, G.; Holmes, K.-R.; Takagi, H.; Sellers, S.L.; Blanke, P.; Koweek, L.M.H.; Nørgaard, B.L.; Jensen, J.; Rabbat, M.G.; et al. Impact of Coronary CT Angiography–derived Fractional Flow Reserve on Downstream Management and Clinical Outcomes in Individuals with and without Diabetes. Radiol. Cardiothorac. Imaging 2023, 5, e220276. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Norgaard, B.L.; Gaur, S.; Leipsic, J.; Nerlekar, N.; Osawa, K.; Miyoshi, T.; Jensen, J.M.; Kimura, T.; Shiomi, H.; et al. Prognostic Value and Risk Continuum of Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. Radiology 2019, 292, 343–351. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Dou, G.; Wang, X.; Shan, D.; He, B.; Jing, J.; Li, T.; Chen, Y.; Yang, J. Global trans-lesional computed tomography-derived fractional flow reserve gradient is associated with clinical outcomes in diabetic patients with non-obstructive coronary artery disease. Cardiovasc. Diabetol. 2023, 22, 186. [Google Scholar] [CrossRef]
- Han, H.; Liu, M.; Yu, Y.; Chen, Y.; Xu, Y. Predictive value of coronary artery computed tomography-derived fractional flow reserve for cardiovascular events in patients with coronary artery disease. Herz 2024, 49, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.M.; Peper, J.; Verhappen, B.J.L.A.; Swart, L.A.; Dedic, A.; van Dockum, W.G.; van der Ent, M.; Royaards, K.-J.; Niezen, A.; Hensen, J.-H.J.; et al. Real world impact of added FFR-CT to coronary CT angiography on clinical decision-making and patient prognosis—IMPACT FFR study. Eur. Radiol. 2023, 33, 5465–5475. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shan, D.; Wang, X.; Sun, X.; Shao, M.; Wang, K.; Pan, Y.; Wang, Z.; Schoepf, U.J.; Savage, R.H.; et al. On-Site Computed Tomography-Derived Fractional Flow Reserve to Guide Management of Patients with Stable Coronary Artery Disease: The TARGET Randomized Trial. Circulation 2023, 147, 1369–1381. [Google Scholar] [CrossRef]
- Liu, X.; Mo, X.; Zhang, H.; Yang, G.; Shi, C.; Hau, W.K. A 2-year investigation of the impact of the computed tomography-derived fractional flow reserve calculated using a deep learning algorithm on routine decision-making for coronary artery disease management. Eur. Radiol. 2021, 31, 7039–7046. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.; Becker, L.M.; van den Berg, H.; Bor, W.L.; Brouwer, J.; Nijenhuis, V.J.; van Ginkel, D.-J.; Rensing, B.J.M.W.; Ten Berg, J.M.; Timmers, L.; et al. Diagnostic Performance of CCTA and CT-FFR for the Detection of CAD in TAVR Work-Up. JACC Cardiovasc. Interv. 2022, 15, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Tzolos, E.; Williams, M.C.; Dey, D.; Berman, D.; Slomka, P.; Newby, D.E.; Dweck, M.R. Noninvasive Coronary Atherosclerotic Plaque Imaging. JACC Cardiovasc. Imaging 2023, 16, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, D. Noninvasive imaging modalities to visualize atherosclerotic plaques. Cardiovasc. Diagn. Ther. 2016, 6, 34053–34353. [Google Scholar] [CrossRef]
- Giangregorio, F.; Mosconi, E.; Debellis, M.G.; Provini, S.; Esposito, C.; Garolfi, M.; Oraka, S.; Kaloudi, O.; Mustafazade, G.; Marín-Baselga, R.; et al. A Systematic Review of Metabolic Syndrome: Key Correlated Pathologies and Non-Invasive Diagnostic Approaches. J. Clin. Med. 2024, 13, 5880. [Google Scholar] [CrossRef]
- Gaur, S.; Taylor, C.A.; Jensen, J.M.; Bøtker, H.E.; Christiansen, E.H.; Kaltoft, A.K.; Holm, N.R.; Leipsic, J.; Zarins, C.K.; Achenbach, S.; et al. FFR Derived from Coronary CT Angiography in Nonculprit Lesions of Patients with Recent STEMI. JACC Cardiovasc. Imaging 2017, 10, 424–433. [Google Scholar] [CrossRef]
- Cuculi, F.; De Maria, G.L.; Meier, P.; Dall’Armellina, E.; de Caterina, A.R.; Channon, K.M.; Prendergast, B.D.; Choudhury, R.C.; Forfar, J.C.; Kharbanda, R.K.; et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1894–1904. [Google Scholar] [CrossRef]
- Pontone, G.; Weir-McCall, J.R.; Baggiano, A.; Del Torto, A.; Fusini, L.; Guglielmo, M.; Muscogiuri, G.; Guaricci, A.I.; Andreini, D.; Patel, M.; et al. Determinants of rejection rate for coronary CT angiography fractional flow reserve analysis. Radiology 2019, 292, 597–605. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Steffensen, F.H.; Bøtker, H.E.; Jensen, J.M.; Rønnow Sand, N.P.; Kragholm, K.H.; Sørensen, H.T.; Leipsic, J.; Mæng, M.; et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients with Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2020, 76, 2803–2813. [Google Scholar] [CrossRef]
- Tzolos, E.; Williams, M.C.; McElhinney, P.; Lin, A.; Grodecki, K.; Flores Tomasino, G.; Cadet, S.; Kwiecinski, J.; Doris, M.; Adamson, P.D.; et al. Pericoronary Adipose Tissue Attenuation, Low-Attenuation Plaque Burden, and 5-Year Risk of Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, M.; Szilveszter, B.; Merkely, B.; Maurovich-Horvat, P. Plaque imaging with CT—A comprehensive review on coronary CT angiography based risk assessment. Cardiovasc. Diagn. Ther. 2017, 7, 489. [Google Scholar] [CrossRef]
- van Assen, M.; von Knebel Doeberitz, P.; Quyyumi, A.A.; De Cecco, C.N. Artificial intelligence for advanced analysis of coronary plaque. Eur. Heart J. Suppl. 2023, 25, C112–C117. [Google Scholar] [CrossRef]
- Petersen, K.; Schaap, M.; Mirza, S.; Ng, N.; Maehara, A.; Matsumura, M.; Safian, R. 452 Quantitative Assessment Of AI-based CCTA Plaque Volume Compared with IVUS. J. Cardiovasc. Comput. Tomogr. 2022, 16, S24. [Google Scholar] [CrossRef]
- Lee, H.; Emrich, T.; Schoepf, U.; Leonard, T.; Gray, H.; Giovagnoli, V.; Dargis, D.; Burt, J.; Tesche, C. Artificial Intelligence in Cardiac CT: Automated Calcium Scoring and Plaque Analysis. Curr. Cardiovasc. Imaging Rep. 2020, 13, 29. [Google Scholar] [CrossRef]
- Jie, P.; Fan, M.; Zhang, H.; Wang, O.; Lv, J.; Liu, Y.; Zhang, C.; Liu, Y.; Zhao, J. Diagnostic value of artificial intelligence-assisted CTA for the assessment of atherosclerosis plaque: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1398963. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, S.; Raible, S.J.; Ng, N.; Mullen, S.; Huey, W.; Rogers, C.; Pursnani, A. Utility of Artificial Intelligence Plaque Quantification: Results of the DECODE Study. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101296. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Stuckey, T.; Nakazawa, G.; Maehara, A.; Matsumura, M.; Petersen, K.; Mirza, S.; Ng, N.; Mullen, S.; Schaap, M.; et al. Primary Results of The REVEALPLAQUE Study: A Prospective Quantitative Assessment of AI-based CCTA Plaque Volume Compared with IVUS. J. Cardiovasc. Comput. Tomogr. 2023, 17, S39. [Google Scholar] [CrossRef]

| Imaging Modality | Key Features | Clinical Utility | Diagnostic Performance | Limitations |
|---|---|---|---|---|
| Dual-Layer Spectral CT Angiography (DL-SCTA) | Differentiates calcified, non-calcified, and mixed plaques; identifies high-risk plaques using low attenuation values (<30 HU); assesses plaque burden and arterial remodeling. | Risk stratification, high-risk plaque identification, improved assessment of plaque burden. | Sensitivity 77%, Specificity 56% for high-risk plaques [4]; enhanced identification of lipid-rich plaques and neovascularization. | Lower specificity; limited data in certain populations; motion artifacts can impact image quality. |
| Photon-Counting CT (PCCT) | Measures individual photon interactions; improves spatial resolution and reduces noise; detects microcalcifications and thin-cap fibroatheromas with high sensitivity and specificity. | Early detection of vulnerable plaques, superior stent visualization, monitoring of disease progression. | Sensitivity 94%, Specificity 89% for lipid-rich plaques [5]; AUC 0.93 for CAD detection [6]; 100% sensitivity for stent patency [7]. | Cost and availability; motion artifacts still a concern; limited widespread use. |
| Dual-Energy CT (DECT) | Utilizes X-rays at two distinct energy levels; enables material decomposition for precise plaque characterization; correlates plaque composition with myocardial infarction risk. | Identification of rupture-prone plaques, prediction of ischemic events, accurate tissue characterization. | Strong correlation with MI risk; RMSE < 5% for plaque component quantification [8]; ORs up to 20.0 for stroke prediction based on plaque features [9]. | Radiation exposure; complexity in interpretation; limited by image noise and patient motion. |
| CT-Derived Fractional Flow Reserve (CT-FFR) | Combines anatomical and hemodynamic assessment; calculates functional significance of stenoses; improves specificity of CCTA and reduces unnecessary invasive angiography. | Improves clinical decision-making, guides revascularization, predicts major adverse cardiac events (MACE). | HR up to 5.05 for MACE with CT-FFR 0.80 [10]; improves specificity of CCTA; diagnostic accuracy reduced in high calcium scores or stents [11,12,13,14,15]. | Accuracy impacted by high calcium or metallic stents; manual editing needed in complex cases; poor image quality reduces utility. |
| AI Application | Key Benefits | Study Findings |
|---|---|---|
| Plaque Detection and Classification | High sensitivity (90%) and specificity (93%) in detecting high-risk plaques. | Meta-analysis (1484 patients) reported AUROC of 0.96 for detecting high-risk plaques [53]. |
| Quantification of Plaque Burden | Reduces inter-observer variability, aligns with IVUS standards. | AI-QCPA altered management in 66% of cases in DECODE study [54]. |
| CT-FFR Calculation | Machine learning accelerates CT-FFR calculations, improving workflow efficiency. | AI-QCPA significantly changed decisions for <50% stenosis plaques [54]. |
| Calcium Scoring | Automates and enhances calcium score accuracy, reducing reader variability. | AI achieved intra-class correlation coefficient of 0.98 with expert readings [52,54]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammouri, H.; Ibrahim, R.; Alasmar, R.; Abdelnabi, M.; Habib, E.; Allam, M.; Pham, H.N.; Elbenawi, H.; Farina, J.; Tamarappoo, B.; et al. Computed Tomography and Coronary Plaque Analysis. Tomography 2025, 11, 85. https://doi.org/10.3390/tomography11080085
Alhammouri H, Ibrahim R, Alasmar R, Abdelnabi M, Habib E, Allam M, Pham HN, Elbenawi H, Farina J, Tamarappoo B, et al. Computed Tomography and Coronary Plaque Analysis. Tomography. 2025; 11(8):85. https://doi.org/10.3390/tomography11080085
Chicago/Turabian StyleAlhammouri, Hashim, Ramzi Ibrahim, Rahmeh Alasmar, Mahmoud Abdelnabi, Eiad Habib, Mohamed Allam, Hoang Nhat Pham, Hossam Elbenawi, Juan Farina, Balaji Tamarappoo, and et al. 2025. "Computed Tomography and Coronary Plaque Analysis" Tomography 11, no. 8: 85. https://doi.org/10.3390/tomography11080085
APA StyleAlhammouri, H., Ibrahim, R., Alasmar, R., Abdelnabi, M., Habib, E., Allam, M., Pham, H. N., Elbenawi, H., Farina, J., Tamarappoo, B., Jokerst, C., Lee, K., Ayoub, C., & Arsanjani, R. (2025). Computed Tomography and Coronary Plaque Analysis. Tomography, 11(8), 85. https://doi.org/10.3390/tomography11080085






