Hyperpolarized Water for Coronary Artery Angiography and Whole-Heart Myocardial Perfusion Quantification
Abstract
1. Introduction
2. Methods
2.1. Sample Preparation
2.2. Hyperpolarization
2.3. Dissolution of Hyperpolarized Sample
2.4. Animal Protocol
2.5. Imaging Protocol
- 1H Multi-phase Anatomical Images: Acquired in a short axis view using a multi-slice 2D cine FIESTA (steady-state free precession type sequence) with breath hold. The sequence parameters are as follows: FOV = 400 400 mm2, resolution = 0.78 0.78 mm2, TE/TR = 1.5/3.4 ms, slice thickness = 10 mm, flip angle = 55°, cardiac phases = 30.
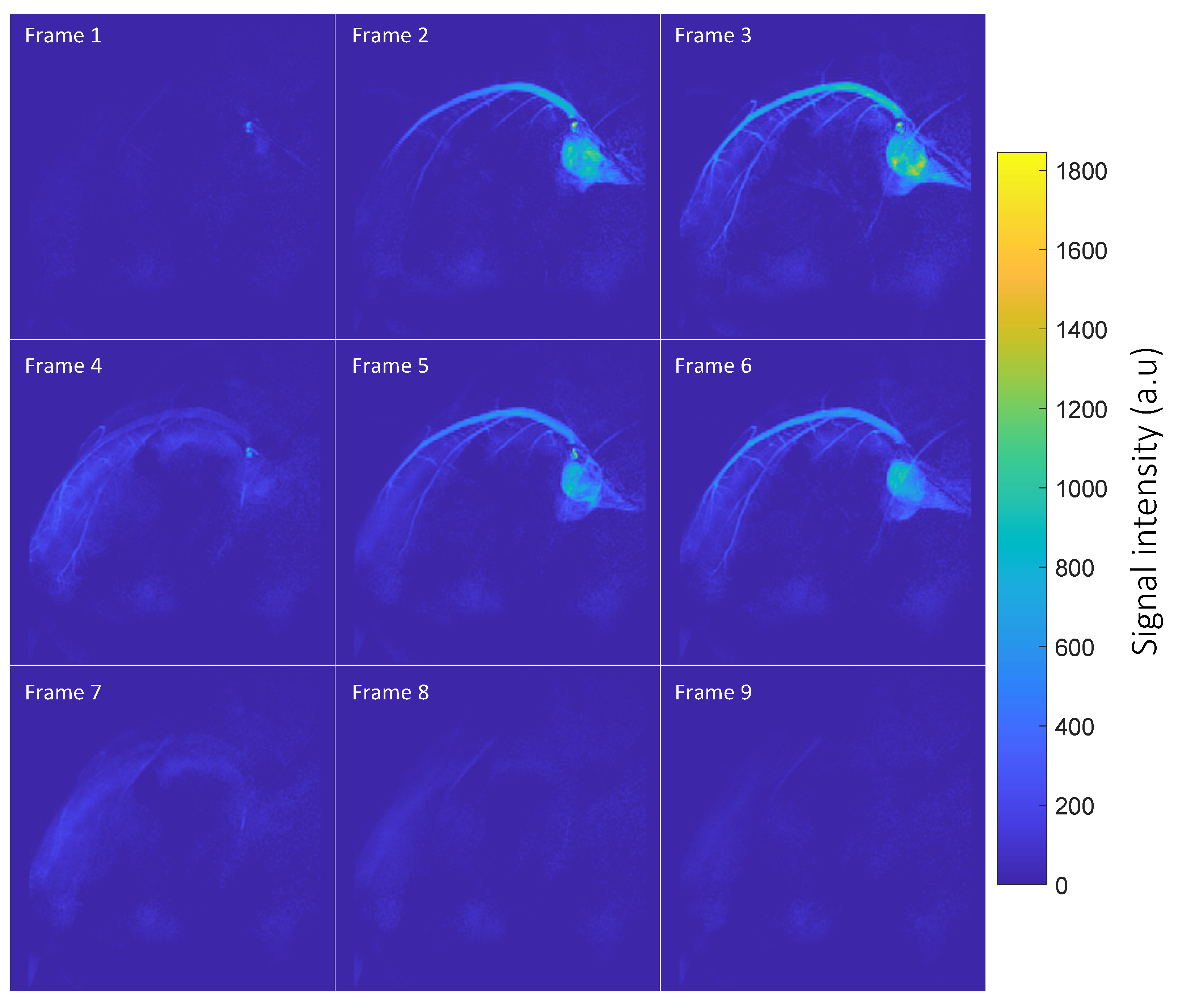
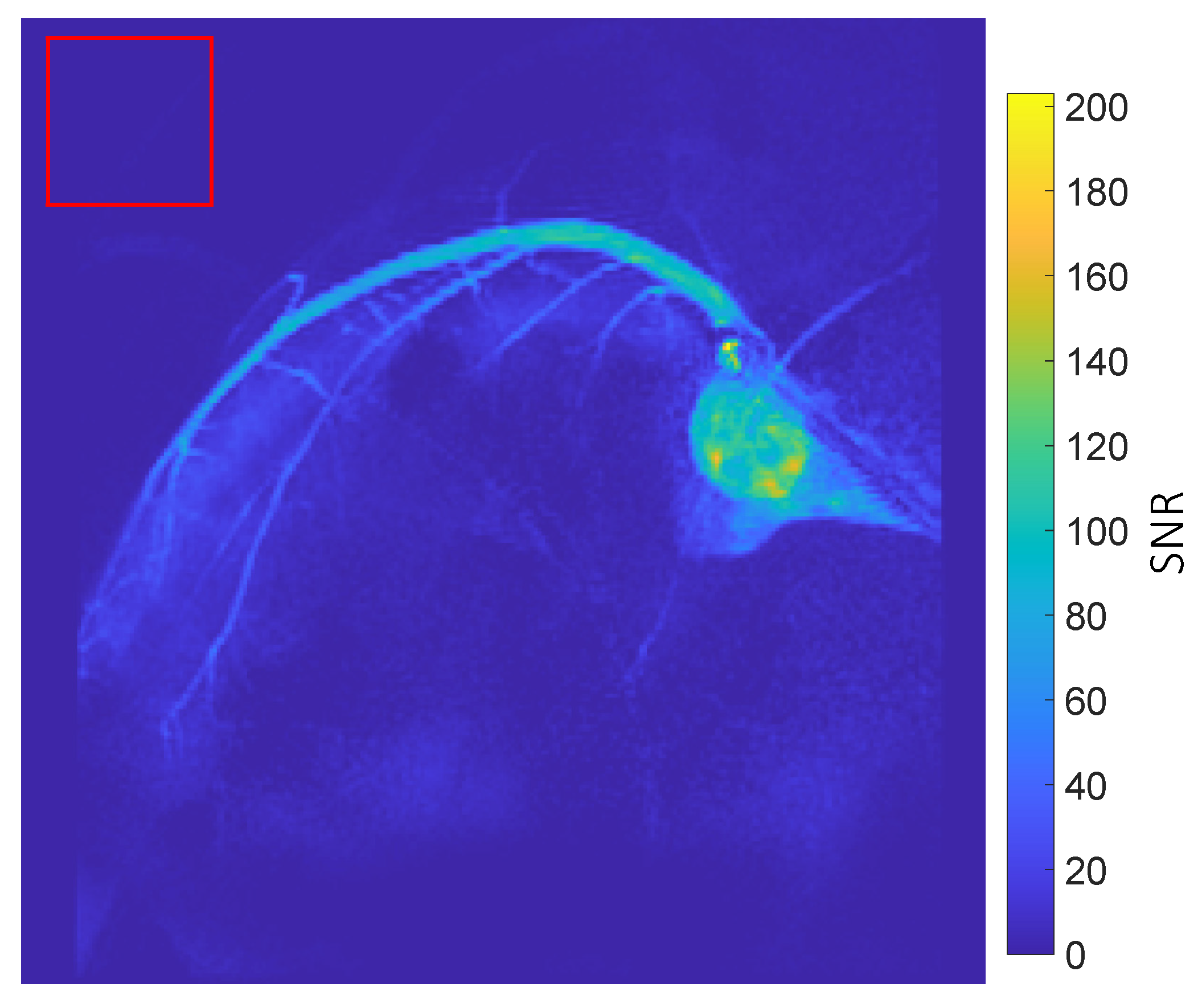
- Hyperpolarized 1H Multi-frame Coronary Artery Images: Acquired as a projection in the long axis plane of the heart with cardiac gating in diastole using a 2D gradient echo sequence with the following parameters: flip angle = 2°, TE = 2.2 ms, TR = 4.4 ms, slice thickness = 150 mm, FOV = 120 108 mm2, in-plane resolution = 0.6 0.6 mm2, acceleration = 2, heart rate = 51 bpm, frame rate = 1 frame per heartbeat.
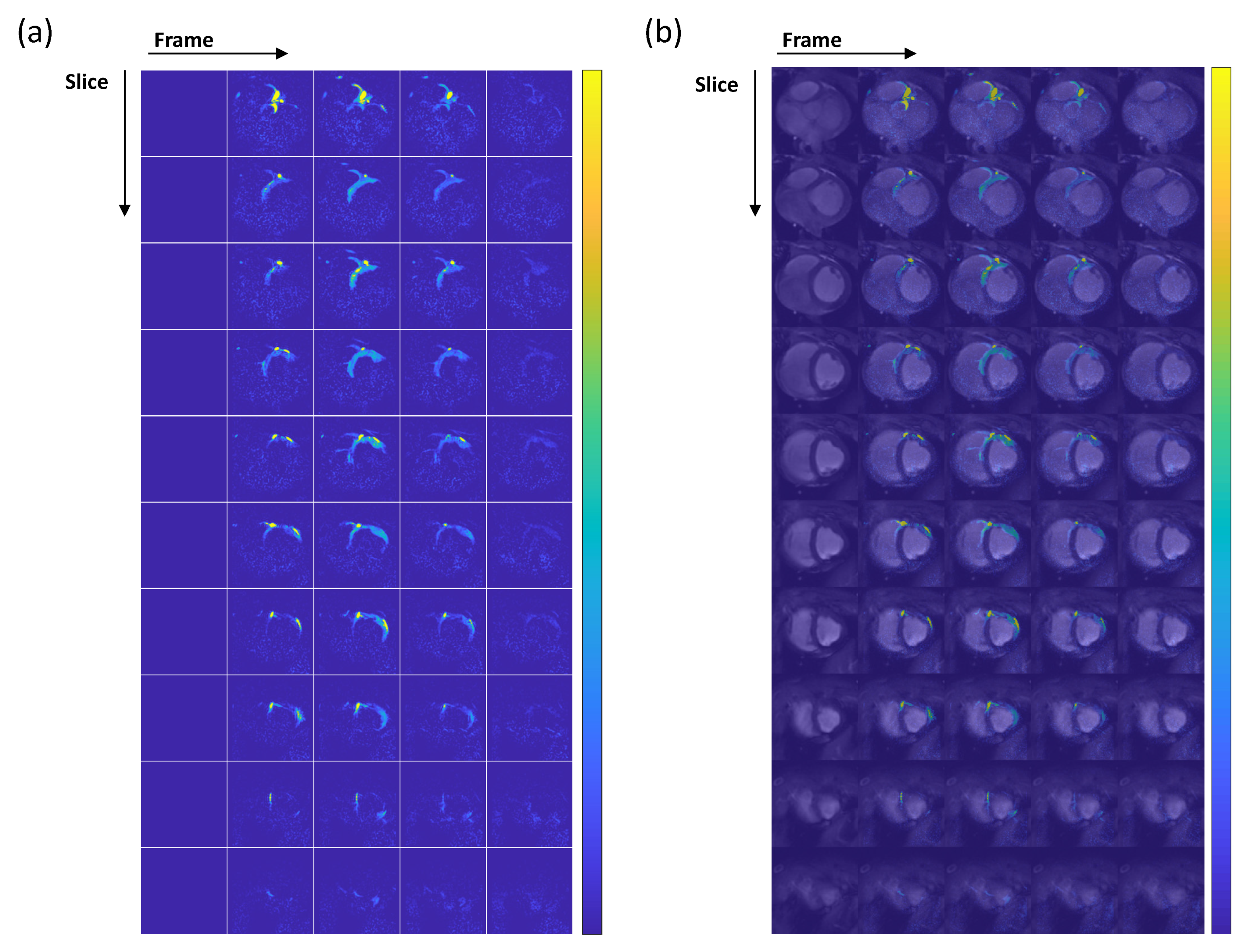
- Hyperpolarized 1H Myocardial Perfusion Images: Acquired to cover the whole heart in the short axis using a multi-slice 2D gradient echo sequence with the following parameters: flip angle = 2°, TE = 1.4 ms, TR = 2.7 ms, slice thickness = 8 mm, number of slices = 10, FOV = 120 108 mm2, in-plane resolution = 1.5 1.5 mm2, acceleration = 2, heart rate = 59 bpm, frame rate = 1 frame per heartbeat.
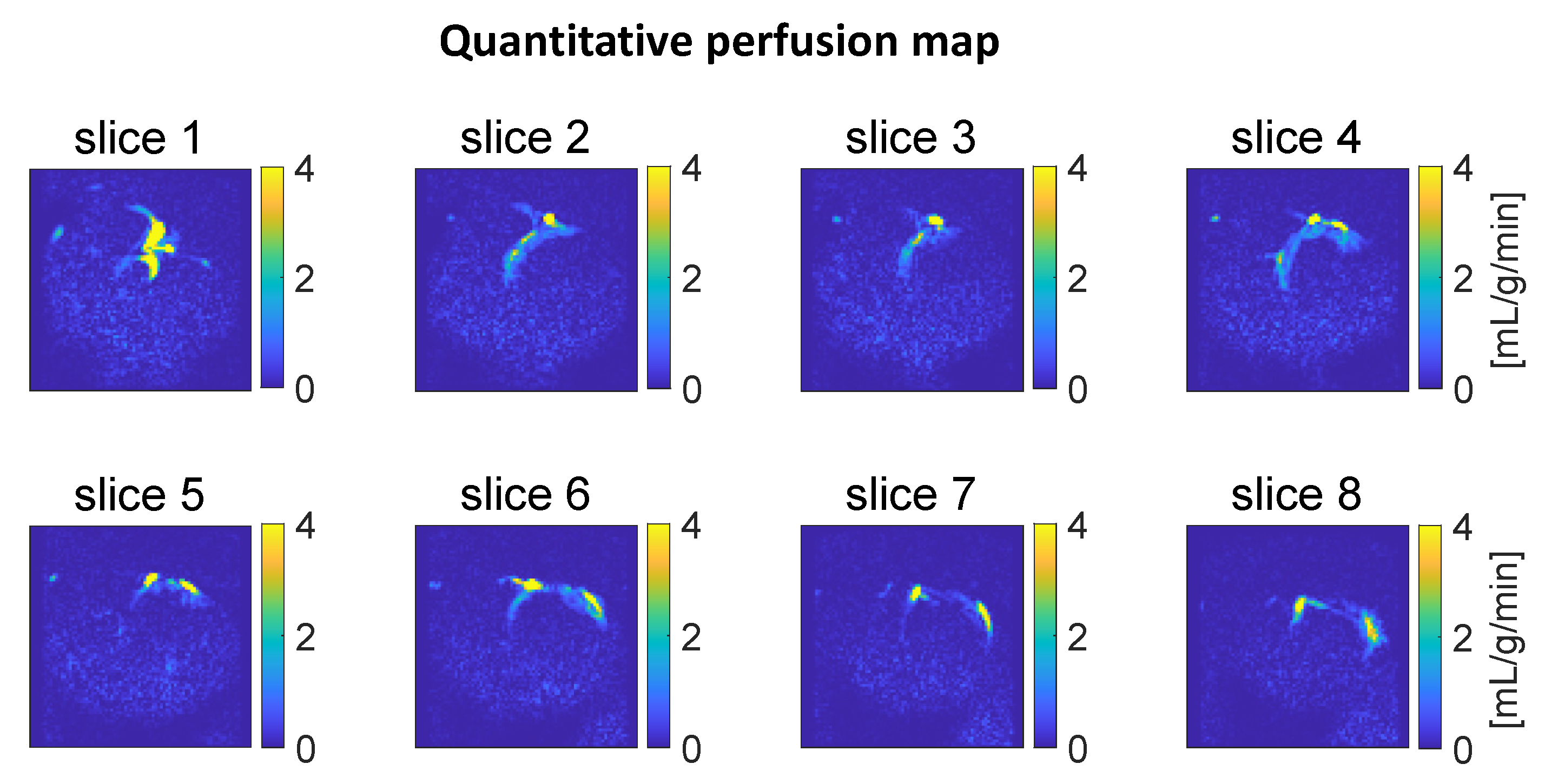
2.6. Perfusion Quantification
3. Results
3.1. Hyperpolarization
3.2. Coronary MRA
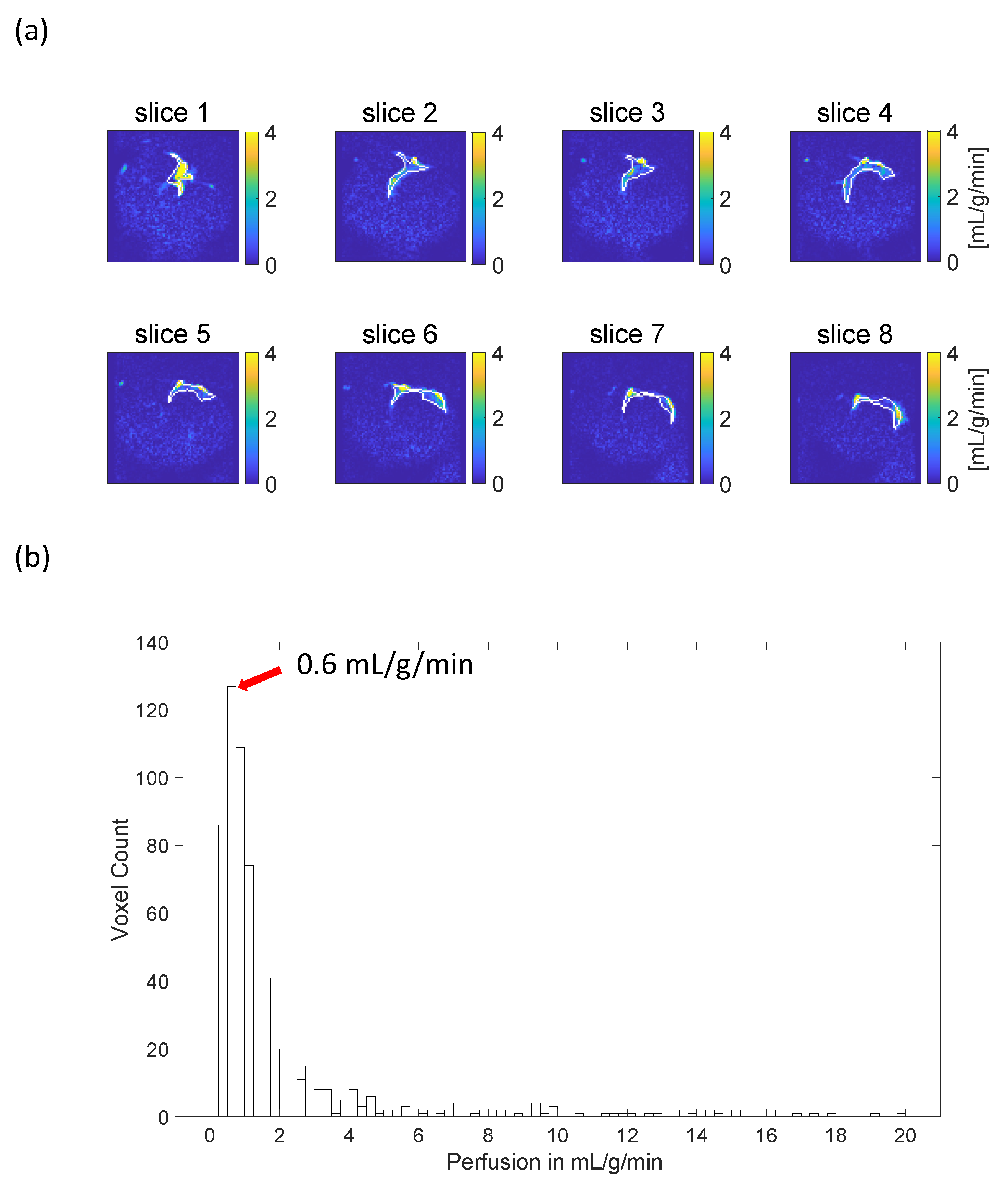
3.3. Myocardial Perfusion Quantification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs) (WHO). Published 7 November 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 26 February 2024).
- Heo, R.; Nakazato, R.; Kalra, D.; Min, J.K. Noninvasive Imaging in Coronary Artery Disease. Semin. Nucl. Med. 2014, 44, 398–409. [Google Scholar] [CrossRef]
- Webb, J.A.W.; Stacul, F.; Thomsen, H.S.; Morcos, S.K. Members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Late adverse reactions to intravascular iodinated contrast media. Eur. Radiol. 2003, 13, 181–184. [Google Scholar] [CrossRef]
- Nievelstein, R.A.J.; Quarles van Ufford, H.M.E.; Kwee, T.C.; Bierings, M.B.; Ludwig, I.; Beek, F.J.A.; de Klerk, J.M.H.; Mali, W.P.T.M.; de Bruin, P.W.; Geleijns, J. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur. Radiol. 2012, 22, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Chiribiri, A.; Botnar, R.M.; Nagel, E. Magnetic Resonance Coronary Angiography: Where Are We Today? Curr. Cardiol. Rep. 2013, 15, 328. [Google Scholar] [CrossRef]
- Khalifa, F.; Soliman, A.; El-Baz, A.; El-Ghar, M.A.; El-Diasty, T.; Gimel’Farb, G.; Ouseph, R.; Dwyer, A.C. Models and methods for analyzing DCE-MRI: A review. Med. Phys. 2014, 41, 124301. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Levine, D.; Weinreb, J.; Kanal, E.; Davenport, M.S.; Ellis, J.H.; Jacobs, P.M.; Lenkinski, R.E.; Maravilla, K.R.; Prince, M.R.; et al. Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology 2018, 289, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.S.; Shea, S.M.; Laub, G.; Simonetti, O.P.; Finn, J.P.; Li, D. 3D magnetization-prepared true-FISP: A new technique for imaging coronary arteries. Magn. Reson. Med. 2001, 46, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.M.; Tyler, D.J.; Rider, O. Clinical Cardiovascular Applications of Hyperpolarized Magnetic Resonance. Cardiovasc. Drugs Ther. 2020, 34, 231. [Google Scholar] [CrossRef]
- Fuetterer, M.; Busch, J.; Traechtler, J.; Wespi, P.; Peereboom, S.M.; Sauer, M.; Lipiski, M.; Fleischmann, T.; Cesarovic, N.; Stoeck, C.T.; et al. Quantitative myocardial first-pass cardiovascular magnetic resonance perfusion imaging using hyperpolarized [1-13C] pyruvate. J. Cardiovasc. Magn. Reson. 2018, 20, 73. [Google Scholar] [CrossRef]
- Fuetterer, M.; Busch, J.; Peereboom, S.M.; von Deuster, C.; Wissmann, L.; Lipiski, M.; Fleischmann, T.; Cesarovic, N.; Stoeck, C.T.; Kozerke, S. Hyperpolarized 13C urea myocardial first-pass perfusion imaging using velocity-selective excitation. J. Cardiovasc. Magn. Reson. 2017, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Tyler, D.J. Cardiovascular Applications of Hyperpolarized MRI. Curr. Cardiovasc. Imaging Rep. 2011, 4, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kajander, S.A.; Joutsiniemi, E.; Saraste, M.; Pietilä, M.; Ukkonen, H.; Saraste, A.; Sipilä, H.T.; Teräs, M.; Mäki, M.; Airaksinen, J.; et al. Clinical Value of Absolute Quantification of Myocardial Perfusion With 15O-Water in Coronary Artery Disease. Circ. Cardiovasc. Imaging 2011, 4, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Pinon, A.C.; Capozzi, A.; Ardenkjær-Larsen, J.H. Hyperpolarized water through dissolution dynamic nuclear polarization with UV-generated radicals. Commun. Chem. 2020, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Lipsø, K.W.; Bowen, S.; Rybalko, O.; Ardenkjær-Larsen, J.H. Large dose hyperpolarized water with dissolution-DNP at high magnetic field. J. Magn. Reson. 2017, 274, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, M.D.; Siaw, T.A.; Sailasuta, N.; Abulseoud, O.A.; Chan, H.R.; Ross, B.D.; Bhattacharya, P.; Han, S. Hyperpolarized Water as an MR Imaging Contrast Agent: Feasibility of in Vivo Imaging in a Rat Model. Radiology 2012, 265, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lipsø, K.W.; Hansen, E.S.S.; Tougaard, R.S.; Laustsen, C.; Ardenkjær-Larsen, J.H. Dynamic coronary MR angiography in a pig model with hyperpolarized water. Magn. Reson. Med. 2018, 80, 1165–1169. [Google Scholar] [CrossRef]
- Lipsø, K.W.; Hansen, E.S.S.; Tougaard, R.S.; Laustsen, C.; Ardenkjær-Larsen, J.H. Renal MR angiography and perfusion in the pig using hyperpolarized water. Magn. Reson. Med. 2017, 78, 1131–1135. [Google Scholar] [CrossRef]
- Capozzi, A.; Karlsson, M.; Petersen, J.R.; Lerche, M.H.; Ardenkjaer-Larsen, J.H. Liquid-State 13C Polarization of 30% through Photoinduced Nonpersistent Radicals. J. Phys. Chem. C 2018, 122, 7432–7443. [Google Scholar] [CrossRef]
- Tribuna, L.; Oliveira, P.B.; Iruela, A.; Marques, J.; Santos, P.; Teixeira, T. Reference Values of Native T1 at 3T Cardiac Magnetic Resonance-Standardization Considerations between Different Vendors. Diagnostics 2021, 11, 2334. [Google Scholar] [CrossRef]
- Hanson, L.G.; Lund, H.; Mikkelsen, I.K. Estimation of perfusion and other vascular parameters from first part of bolus passage. Proc. Intl. Soc. Mag. Reson. Med. 2009, 17, 1470. Available online: https://cds.ismrm.org/protected/09MProceedings/PDFfiles/01470.pdf (accessed on 8 July 2024).
- Lu, H.; Clingman, C.; Golay, X.; van Zijl, P.C. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn. Reson. Med. 2004, 52, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Husso, M.; Nissi, M.J.; Kuivanen, A.; Halonen, P.; Tarkia, M.; Teuho, J.; Saunavaara, V.; Vainio, P.; Sipola, P.; Manninen, H.; et al. Quantification of porcine myocardial perfusion with modified dual bolus MRI—A prospective study with a PET reference. BMC Med. Imaging 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Grönman, M.; Tarkia, M.; Stark, C.; Vähäsilta, T.; Kiviniemi, T.; Lubberink, M.; Halonen, P.; Kuivanen, A.; Saunavaara, V.; Tolvanen, T.; et al. Assessment of myocardial viability with [15O] water PET: A validation study in experimental myocardial infarction. J. Nucl. Cardiol. 2021, 28, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Lerche, M.H.; Karlsson, M.; Olin, R.B.; Hansen, E.S.S.; Aastrup, M.; Redda, M.; Laustsen, C.; Hanson, L.G.; Ardenkjær-Larsen, J.H. Hyperpolarized Water for Coronary Artery Angiography and Whole-Heart Myocardial Perfusion Quantification. Tomography 2024, 10, 1113-1122. https://doi.org/10.3390/tomography10070084
Zhao Y, Lerche MH, Karlsson M, Olin RB, Hansen ESS, Aastrup M, Redda M, Laustsen C, Hanson LG, Ardenkjær-Larsen JH. Hyperpolarized Water for Coronary Artery Angiography and Whole-Heart Myocardial Perfusion Quantification. Tomography. 2024; 10(7):1113-1122. https://doi.org/10.3390/tomography10070084
Chicago/Turabian StyleZhao, Yupeng, Mathilde Hauge Lerche, Magnus Karlsson, Rie Beck Olin, Esben Søvsø Szocska Hansen, Malene Aastrup, Mohsen Redda, Christoffer Laustsen, Lars G. Hanson, and Jan Henrik Ardenkjær-Larsen. 2024. "Hyperpolarized Water for Coronary Artery Angiography and Whole-Heart Myocardial Perfusion Quantification" Tomography 10, no. 7: 1113-1122. https://doi.org/10.3390/tomography10070084
APA StyleZhao, Y., Lerche, M. H., Karlsson, M., Olin, R. B., Hansen, E. S. S., Aastrup, M., Redda, M., Laustsen, C., Hanson, L. G., & Ardenkjær-Larsen, J. H. (2024). Hyperpolarized Water for Coronary Artery Angiography and Whole-Heart Myocardial Perfusion Quantification. Tomography, 10(7), 1113-1122. https://doi.org/10.3390/tomography10070084





