Collagen 1 Fiber Volume Predicts for Recurrence of Stage 1 Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Methods
2.1. Samples
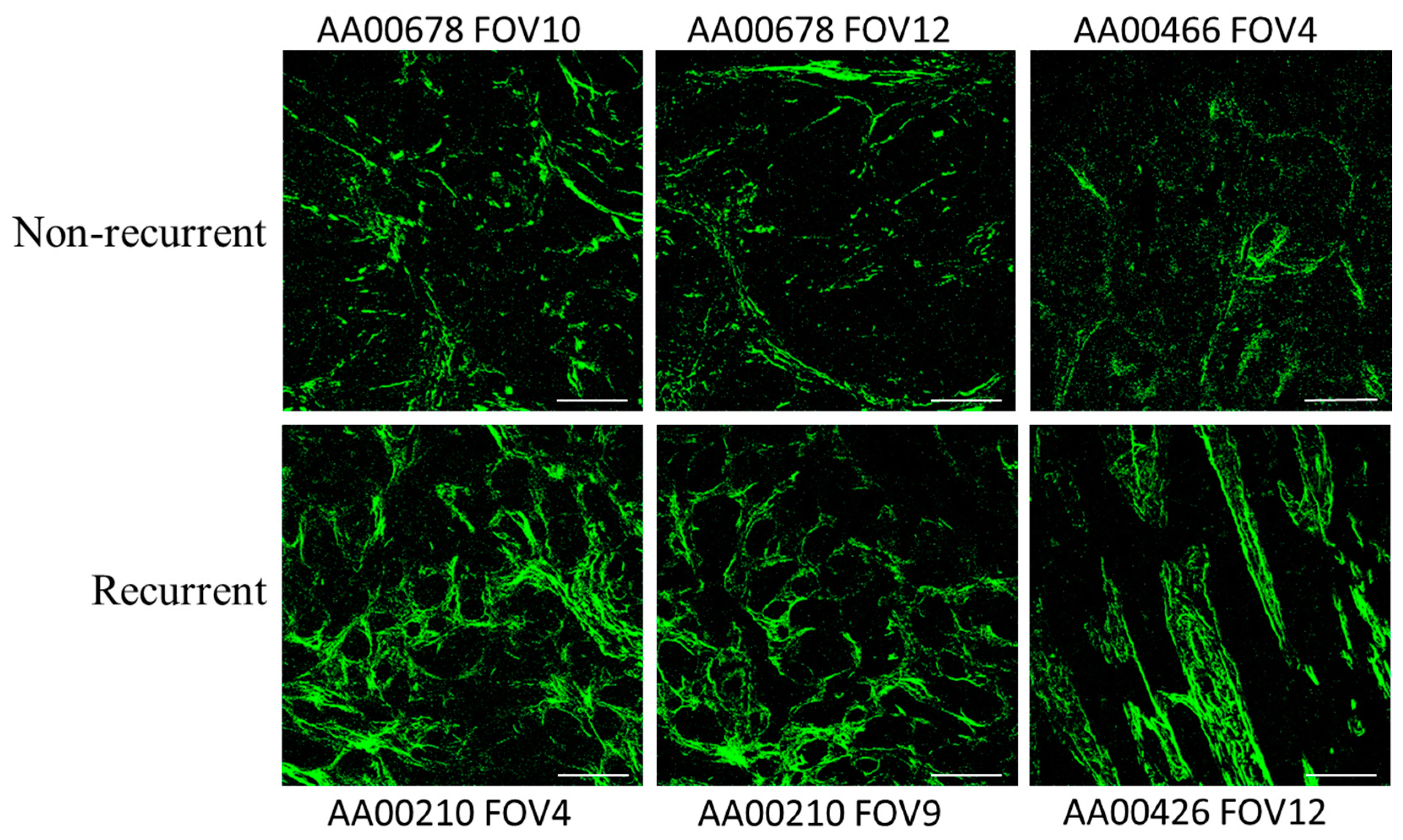
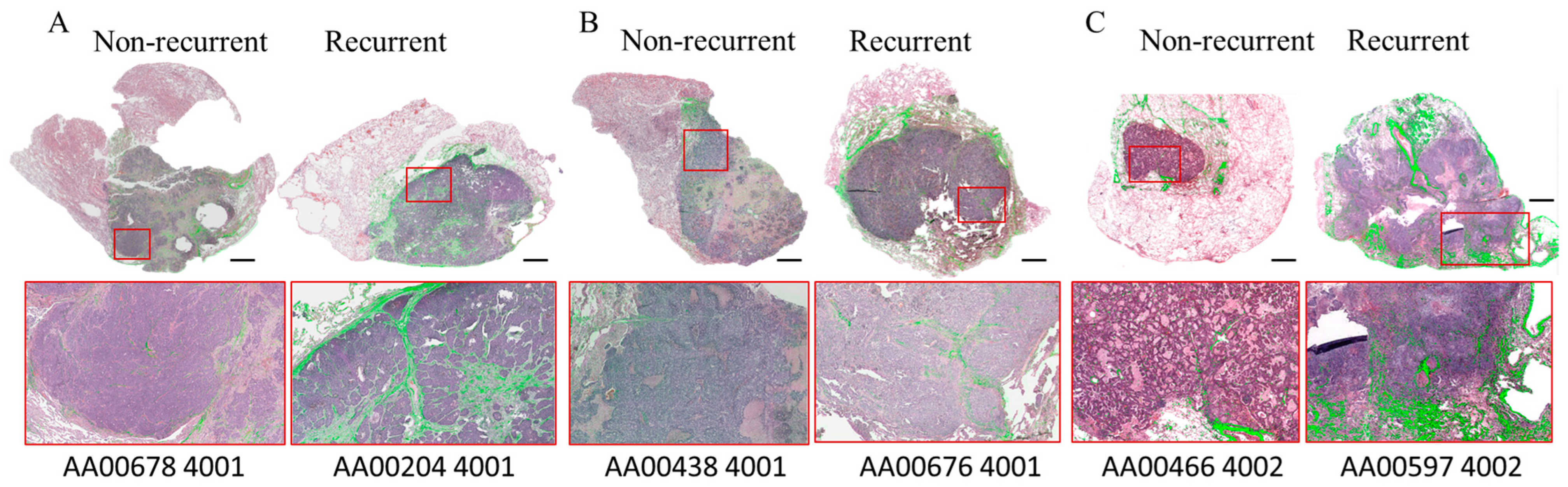
2.2. SHG Imaging and Analysis
2.3. Molecular Analysis of Noninvasive and Invasive Lung Adenocarcinoma
2.4. Statistical Analysis
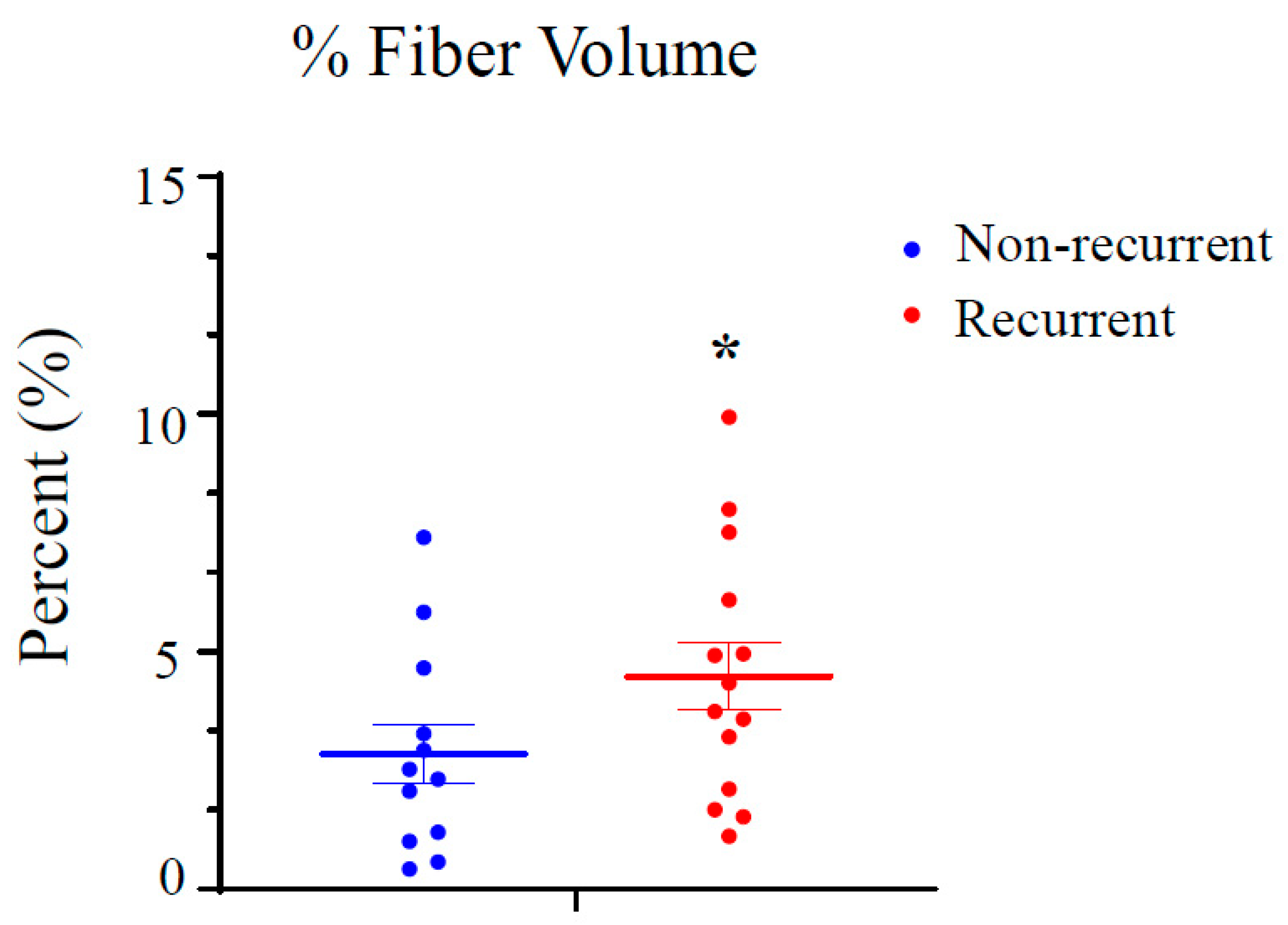
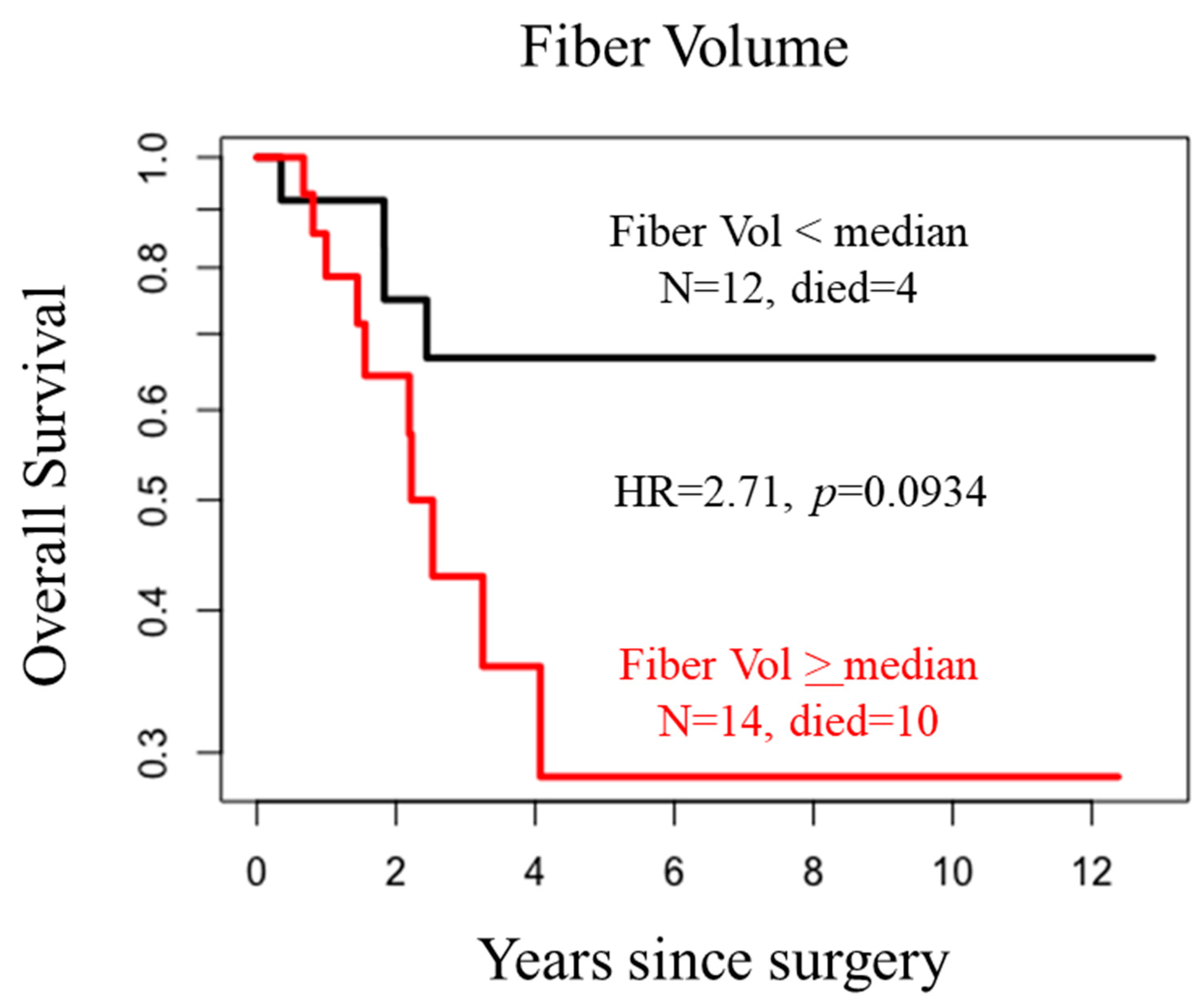
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAFs | Cancer-associated fibroblasts |
| Col1 | Collagen 1 |
| ECM | Extracellular matrix |
| FOVs | Fields of view |
| GEO | Gene expression omnibus |
| H&E | Hematoxylin and eosin |
| IRB | Institutional Review Board |
| NCBI | National Center for Biotechnology Information |
| NIH | National Institutes of Health |
| NLST | National Lung Screening Trial |
| NSCLC | Non-small cell lung carcinoma |
| SHG | Second harmonic generation |
References
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aberle, D.R.; DeMello, S.; Berg, C.D.; Black, W.C.; Brewer, B.; Church, T.R.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; et al. Results of the two incidence screenings in the National Lung Screening Trial. N. Engl. J. Med. 2013, 369, 920–931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Lung Screening Trial Research, T.; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgensztern, D.; Ng, S.H.; Gao, F.; Govindan, R. Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database survey. J. Thorac. Oncol. 2010, 5, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Fedor, D.; Johnson, W.R.; Singhal, S. Local recurrence following lung cancer surgery: Incidence, risk factors, and outcomes. Surg. Oncol. 2013, 22, 156–161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bille, A.; Ahmad, U.; Woo, K.M.; Suzuki, K.; Adusumilli, P.; Huang, J.; Jones, D.R.; Rizk, N.P. Detection of Recurrence Patterns After Wedge Resection for Early Stage Lung Cancer: Rationale for Radiologic Follow-Up. Ann. Thorac. Surg. 2016, 102, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, L.M.; Louie, B.E.; Jackson, N.; Farivar, A.S.; Aye, R.W.; Vallieres, E. Recurrence and Survival After Segmentectomy in Patients With Prior Lung Resection for Early-Stage Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2016, 102, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee; et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Scott, W.J.; Allen, M.S.; Darling, G.E.; Decker, P.A.; McKenna, R.J.; Meyers, B.F. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J. Thorac. Cardiovasc. Surg. 2014, 147, 747–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaft, J.E.; Rimner, A.; Weder, W.; Azzoli, C.G.; Kris, M.G.; Cascone, T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat. Rev. Clin. Oncol. 2021, 18, 547–557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, E.; McKee, T.; diTomaso, E.; Pluen, A.; Seed, B.; Boucher, Y.; Jain, R.K. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat. Med. 2003, 9, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penet, M.F.; Kakkad, S.; Pathak, A.P.; Krishnamachary, B.; Mironchik, Y.; Raman, V.; Solaiyappan, M.; Bhujwalla, Z.M. Structure and Function of a Prostate Cancer Dissemination-Permissive Extracellular Matrix. Clin. Cancer Res. 2017, 23, 2245–2254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, K.; Smid, M.; Dawes, R.P.; Timmermans, M.A.; Salzman, P.; van Deurzen, C.H.; Beer, D.G.; Foekens, J.A.; Brown, E. Using second harmonic generation to predict patient outcome in solid tumors. BMC Cancer 2015, 15, 929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, Y.; Lv, Z.; Huang, G.; Qin, J.; Li, H.; Nong, F.; Wen, B. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol. Rep. 2020, 44, 1671–1685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakkad, S.M.; Solaiyappan, M.; Argani, P.; Sukumar, S.; Jacobs, L.K.; Leibfritz, D.; Bhujwalla, Z.M.; Glunde, K. Collagen I fiber density increases in lymph node positive breast cancers: Pilot study. J. Biomed. Opt. 2012, 17, 116017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakkad, S.; Zhang, J.; Akhbardeh, A.; Jacob, D.; Krishnamachary, B.; Solaiyappan, M.; Jacobs, M.A.; Raman, V.; Leibfritz, D.; Glunde, K.; et al. Collagen fibers mediate MRI-detected water diffusion and anisotropy in breast cancers. Neoplasia 2016, 18, 585–593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakkad, S.M.; Penet, M.F.; Akhbardeh, A.; Pathak, A.P.; Solaiyappan, M.; Raman, V.; Leibfritz, D.; Glunde, K.; Bhujwalla, Z.M. Hypoxic tumor environments exhibit disrupted collagen I fibers and low macromolecular transport. PLoS ONE 2013, 8, e81869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sapudom, J.; Rubner, S.; Martin, S.; Kurth, T.; Riedel, S.; Mierke, C.T.; Pompe, T. The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 2015, 52, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.A.; Dawes, R.P.; Cheema, M.K.; Van Hove, A.; Benoit, D.S.; Perry, S.W.; Brown, E. Second-harmonic generation scattering directionality predicts tumor cell motility in collagen gels. J. Biomed. Opt. 2015, 20, 051024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Riching, K.M.; Cox, B.L.; Salick, M.R.; Pehlke, C.; Riching, A.S.; Ponik, S.M.; Bass, B.R.; Crone, W.C.; Jiang, Y.; Weaver, A.M.; et al. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014, 107, 2546–2558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Models Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohler, W.; Millard, A.C.; Campagnola, P.J. Second harmonic generation imaging of endogenous structural proteins. Methods 2003, 29, 97–109. [Google Scholar] [CrossRef]
- Xi, G.; Huang, C.; Lin, J.; Luo, T.; Kang, B.; Xu, M.; Xu, H.; Li, X.; Chen, J.; Qiu, L.; et al. Rapid label-free detection of early-stage lung adenocarcinoma and tumor boundary via multiphoton microscopy. J. Biophotonics 2023, 16, e202300172. [Google Scholar] [CrossRef] [PubMed]
- Kakkad, S.M.; Solaiyappan, M.; O'Rourke, B.; Stasinopoulos, I.; Ackerstaff, E.; Raman, V.; Bhujwalla, Z.M.; Glunde, K. Hypoxic tumor microenvironments reduce collagen I fiber density. Neoplasia 2010, 12, 608–617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goggins, E.; Kakkad, S.; Mironchik, Y.; Jacob, D.; Wildes, F.; Krishnamachary, B.; Bhujwalla, Z.M. Hypoxia Inducible Factors Modify Collagen I Fibers in MDA-MB-231 Triple Negative Breast Cancer Xenografts. Neoplasia 2018, 20, 131–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goggins, E.; Mironchik, Y.; Kakkad, S.; Jacob, D.; Wildes, F.; Bhujwalla, Z.M.; Krishnamachary, B. Reprogramming of VEGF-mediated extracellular matrix changes through autocrine signaling. Cancer Biol. Ther. 2023, 24, 2184145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuczek, D.E.; Larsen, A.M.H.; Thorseth, M.L.; Carretta, M.; Kalvisa, A.; Siersbaek, M.S.; Simoes, A.M.C.; Roslind, A.; Engelholm, L.H.; Noessner, E.; et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romer, A.M.A.; Thorseth, M.L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoo, S.; Sinha, A.; Yang, D.; Altorki, N.K.; Tandon, R.; Wang, W.; Chavez, D.; Lee, E.; Patel, A.S.; Sato, T.; et al. Integrative network analysis of early-stage lung adenocarcinoma identifies aurora kinase inhibition as interceptor of invasion and progression. Nat. Commun. 2022, 13, 1592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G.; Minguet, S.; Navarro-Lérida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibáñez, T.; Pellinen, T.; Echarri, A.; et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011, 146, 148–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vennin, C.; Mélénec, P.; Rouet, R.; Nobis, M.; Cazet, A.S.; Murphy, K.J.; Herrmann, D.; Reed, D.A.; Lucas, M.C.; Warren, S.C.; et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019, 10, 3637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grugan, K.D.; Miller, C.G.; Yao, Y.; Michaylira, C.Z.; Ohashi, S.; Klein-Szanto, A.J.; Diehl, J.A.; Herlyn, M.; Han, M.; Nakagawa, H.; et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc. Natl. Acad. Sci. USA 2010, 107, 11026–11031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, K.Y.; Cheung, A.H.; Chen, B.; Chan, W.N.; Yu, J.; Lo, K.W.; Kang, W.; To, K.F. Cancer-associated fibroblasts in nonsmall cell lung cancer: From molecular mechanisms to clinical implications. Int. J. Cancer 2022, 151, 1195–1215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patz, E.F., Jr.; Caporaso, N.E.; Dubinett, S.M.; Massion, P.P.; Hirsch, F.R.; Minna, J.D.; Gatsonis, C.; Duan, F.; Adams, A.; Apgar, C.; et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): Design, intent, and availability of specimens for validation of lung cancer biomarkers. J. Thorac. Oncol. 2010, 5, 1502–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghigh, A.; Bancelin, S.; Rivard, M.; Pinsard, M.; Ibrahim, H.; Legare, F. Second harmonic generation microscopy: A powerful tool for bio-imaging. Biophys. Rev. 2023, 15, 43–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, X.; Zhang, W.H.; Wang, W.Q.; Yu, X.J.; Liu, L. Cancer-associated fibroblasts in therapeutic resistance of pancreatic cancer: Present situation, predicaments, and perspectives. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188444. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, Y.; Wherry, E.J. Memory T-Cell Heterogeneity and Terminology. Cold Spring Harb. Perspect. Biol. 2021, 13, a037929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritzenthaler, J.D.; Han, S.; Roman, J. Stimulation of lung carcinoma cell growth by fibronectin-integrin signalling. Mol. Biosyst. 2008, 4, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cai, R.; Wang, T.; Yang, X.; Wang, M.; Kuang, Z.; Xie, Y.; Zhang, J.; Zheng, Y. LAMC2 promotes the proliferation of cancer cells and induce infiltration of macrophages in non-small cell lung cancer. Ann. Transl. Med. 2021, 9, 1392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, Y.W.; Rao, G.; Kim, J.J.; Shim, H.S.; Park, K.S.; An, S.S.; Kim, B.; Steeg, P.S.; Sarfaraz, S.; Changwoo Lee, L.; et al. LAMC2 enhances the metastatic potential of lung adenocarcinoma. Cell Death Differ. 2015, 22, 1341–1352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willumsen, N.; Bager, C.L.; Leeming, D.J.; Bay-Jensen, A.C.; Karsdal, M.A. Nidogen-1 Degraded by Cathepsin S can be Quantified in Serum and is Associated with Non-Small Cell Lung Cancer. Neoplasia 2017, 19, 271–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barkovskaya, A.; Buffone, A., Jr.; Zidek, M.; Weaver, V.M. Proteoglycans as Mediators of Cancer Tissue Mechanics. Front. Cell Dev. Biol. 2020, 8, 569377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanjore, H.; Kalluri, R. The role of type IV collagen and basement membranes in cancer progression and metastasis. Am. J. Pathol. 2006, 168, 715–717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miroshnikova, Y.A.; Rozenberg, G.I.; Cassereau, L.; Pickup, M.; Mouw, J.K.; Ou, G.; Templeman, K.L.; Hannachi, E.I.; Gooch, K.J.; Sarang-Sieminski, A.L.; et al. alpha5beta1-Integrin promotes tension-dependent mammary epithelial cell invasion by engaging the fibronectin synergy site. Mol. Biol. Cell 2017, 28, 2958–2977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, Y.; Wang, Z.; Ma, L.; Zhang, L.; Guo, Y.; Gu, M.; Wang, Z.; Wang, Y.; Yue, W. Prognostic significance of circulating laminin gamma2 for early-stage non-small-cell lung cancer. Onco Targets Ther. 2016, 9, 4151–4162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Mirzapoiazova, T.; Mambetsariev, N.; Lennon, F.E.; Mambetsariev, B.; Berlind, J.E.; Salgia, R.; Singleton, P.A. HABP2 is a Novel Regulator of Hyaluronan-Mediated Human Lung Cancer Progression. Front. Oncol. 2015, 5, 164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Della Corte, C.M.; Sen, T.; Gay, C.M.; Ramkumar, K.; Diao, L.; Cardnell, R.J.; Rodriguez, B.L.; Stewart, C.A.; Papadimitrakopoulou, V.A.; Gibson, L.; et al. STING Pathway Expression Identifies NSCLC with an Immune-Responsive Phenotype. J. Thorac. Oncol. 2020, 15, 777–791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benci, J.L.; Johnson, L.R.; Choa, R.; Xu, Y.; Qiu, J.; Zhou, Z.; Xu, B.; Ye, D.; Nathanson, K.L.; June, C.H.; et al. Opposing Functions of Interferon Coordinate Adaptive and Innate Immune Responses to Cancer Immune Checkpoint Blockade. Cell 2019, 178, 933–948.e914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| No Recurrence | * Recurrence | p Value | ||
|---|---|---|---|---|
| N | 12 | 14 | ||
| Female | N (%) | 4 (33%) | 7 (50%) | 1.0 |
| Age at surgery | Mean ± sd | 63.3 ± 4.4 | 66.5 ± 5.4 | 0.1141 |
| Current smoker | N (%) | 4 (33%) | 10 (71%) | 0.1131 |
| Years smoked | Mean ± sd | 42.0 ± 6.5 | 47.9 ± 6.6 | 0.0326 |
| Pack-years | Mean ± sd | 55.0 ± 17.0 | 83.3 ± 63.7 | 0.1308 |
| Family history of lung cancer | N (%) | 2 (17%) | 5 (36%) | 0.3913 |
| Description | Symbol | GeneID | log2Fold Change | p Value | padj | |
|---|---|---|---|---|---|---|
| ECM related proteins | Collagen type I alpha 1 chain | COL1A1 | 1277 | 2.63 | 5.27 × 10−19 | 0.0000 |
| Fibronectin type III domain containing 1 | FNDC1 | 84,624 | 1.97 | 2.44 × 10−11 | 0.0000 | |
| Collagen type I alpha 2 chain | COL1A2 | 1278 | 1.93 | 3.44 × 10−14 | 0.0000 | |
| Laminin subunit beta 3 | LAMB3 | 3914 | 1.64 | 4.20 × 10−10 | 0.0000 | |
| Laminin subunit gamma 2 | LAMC2 | 3918 | 1.62 | 5.88 × 10−07 | 0.0000 | |
| Nidogen 2 | NID2 | 22,795 | 1.51 | 2.04 × 10−13 | 0.0000 | |
| Aggrecan | ACAN | 176 | 1.50 | 3.76 × 10−05 | 0.0003 | |
| EGF-like, fibronectin type III and Laminin G domains | EGFLAM | 133,584 | 1.39 | 3.38 × 10−12 | 0.0000 | |
| Laminin subunit beta 4 | LAMB4 | 22,798 | 1.36 | 6.52 × 10−06 | 0.0001 | |
| Laminin subunit alpha 1 | LAMA1 | 284,217 | 0.75 | 7.09 × 10−03 | 0.0228 | |
| Laminin subunit beta 1 | LAMB1 | 3912 | 0.73 | 4.16 × 10−05 | 0.0003 | |
| Laminin subunit alpha 4 | LAMA4 | 3910 | 0.52 | 2.84 × 10−03 | 0.0108 | |
| Hyaluronan binding protein 2 | HABP2 | 3026 | −2.37 | 8.10 × 10−07 | 0.0000 | |
| CAFs | HHIP like 2 (myCAF) [45] | HHIPL2 | 79,802 | 3.27 | 1.44 × 10−14 | 0.0000 |
| Leucine-rich repeat containing 15 (myCAF) | LRRC15 | 131,578 | 2.37 | 6.07 × 10−10 | 0.0000 | |
| Thy-1 cell surface antigen (myCAF) [45,46] | THY1 | 7070 | 1.44 | 1.02 × 10−13 | 0.0000 | |
| Fibroblast activation protein alpha | FAP | 2191 | 1.40 | 5.33 × 10−09 | 0.0000 | |
| HHIP like 1 (myCAF) [45] | HHIPL1 | 84,439 | 0.85 | 2.06 × 10−05 | 0.0002 | |
| Platelet derived growth factor receptor beta (iCAF) [46] | PDGFRB | 5159 | 0.55 | 9.45 × 10−04 | 0.0044 | |
| Integrin subunit alpha 8 (apCAF) [46] | ITGA8 | 8516 | −0.82 | 3.29 × 10−05 | 0.0003 |
| Description | Symbol | GeneID | log2Fold Change | p Value | padj | |
|---|---|---|---|---|---|---|
| Immune checkpoints | Lymphocyte activating 3 | LAG3 | 3902 | 1.62 | 2.81 × 10−10 | 0.0000 |
| Indoleamine 2,3-dioxygenase 1 | IDO1 | 3620 | 1.60 | 2.73 × 10−06 | 0.0000 | |
| Sialic acid binding Ig like lectin 10 | SIGLEC10 | 89,790 | 1.35 | 4.07 × 10−07 | 0.0000 | |
| V-set domain containing T cell activation inhibitor 1 | VTCN1 | 79,679 | 1.33 | 1.30 × 10−02 | 0.0373 | |
| Programmed cell death 1 | PDCD1 | 5133 | 1.09 | 2.36 × 10−05 | 0.0002 | |
| CD274 molecule | CD274 | 29,126 | 0.68 | 1.55 × 10−02 | 0.0430 | |
| Immune cytokines/ * T-lymphocytes | Interferon gamma (Th1, Tc1) | IFNG | 3458 | 1.80 | 1.32 × 10−05 | 0.0001 |
| Transforming growth factor beta induced (Tregs) | TGFBI | 7045 | 1.11 | 1.06 × 10−08 | 0.0000 | |
| CD8b molecule (Tc) | CD8B | 926 | 0.85 | 1.69 × 10−03 | 0.0070 | |
| Interleukin 2 receptor subunit alpha (Tregs, Tcm) | IL2RA | 3559 | 0.84 | 3.54 × 10−03 | 0.0129 | |
| Integrin subunit alpha E (Trm, RTE) | ITGAE | 3682 | 0.82 | 4.69 × 10−07 | 0.0000 | |
| CD38 molecule (activated T cells) | CD38 | 952 | 0.71 | 2.01 × 10−02 | 0.0531 | |
| CD8a molecule (Tc) | CD8A | 925 | 0.69 | 7.49 × 10−03 | 0.0238 | |
| Interleukin 10 (Tregs, Trm, Th2, Tc9) | IL10 | 3586 | 0.68 | 6.95 × 10−03 | 0.0224 | |
| CD3 delta subunit of T-cell receptor complex (Pan T cells) | CD3D | 915 | 0.60 | 6.17 × 10−03 | 0.0204 | |
| Interleukin 2 receptor subunit beta (Tscm, Tcm, Tem, Teff) | IL2RB | 3560 | 0.59 | 1.21 × 10−02 | 0.0351 | |
| C-C motif chemokine receptor 6 (Th17, Tc17) | CCR6 | 1235 | −0.59 | 3.2 × 10−03 | 0.0119 | |
| prostaglandin D2 receptor 2 (Th2, Tc2) | PTGDR2 | 11,251 | −0.72 | 1.20 × 10−02 | 0.0348 | |
| CD69 molecule (Trm, Tcm, Tem, Teff) | CD69 | 969 | −0.72 | 2.08 × 10−03 | 0.0083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakkad, S.; Krishnamachary, B.; Fackche, N.; Garner, M.; Brock, M.; Huang, P.; Bhujwalla, Z.M. Collagen 1 Fiber Volume Predicts for Recurrence of Stage 1 Non-Small Cell Lung Cancer. Tomography 2024, 10, 1099-1112. https://doi.org/10.3390/tomography10070083
Kakkad S, Krishnamachary B, Fackche N, Garner M, Brock M, Huang P, Bhujwalla ZM. Collagen 1 Fiber Volume Predicts for Recurrence of Stage 1 Non-Small Cell Lung Cancer. Tomography. 2024; 10(7):1099-1112. https://doi.org/10.3390/tomography10070083
Chicago/Turabian StyleKakkad, Samata, Balaji Krishnamachary, Nadege Fackche, Matthew Garner, Malcom Brock, Peng Huang, and Zaver M. Bhujwalla. 2024. "Collagen 1 Fiber Volume Predicts for Recurrence of Stage 1 Non-Small Cell Lung Cancer" Tomography 10, no. 7: 1099-1112. https://doi.org/10.3390/tomography10070083
APA StyleKakkad, S., Krishnamachary, B., Fackche, N., Garner, M., Brock, M., Huang, P., & Bhujwalla, Z. M. (2024). Collagen 1 Fiber Volume Predicts for Recurrence of Stage 1 Non-Small Cell Lung Cancer. Tomography, 10(7), 1099-1112. https://doi.org/10.3390/tomography10070083







