Breast Tomographic Ultrasound: The Spectrum from Current Dense Breast Cancer Screenings to Future Theranostic Treatments
Abstract
1. Introduction
2. SoftVue—Breast Density, Risk, Screening and Specificity
2.1. The Density Risk
2.2. Specificity and DBT
2.3. Specificity and Breast Ultrasound
2.4. What about Other Imaging Modalities for Dense Breast Screening?
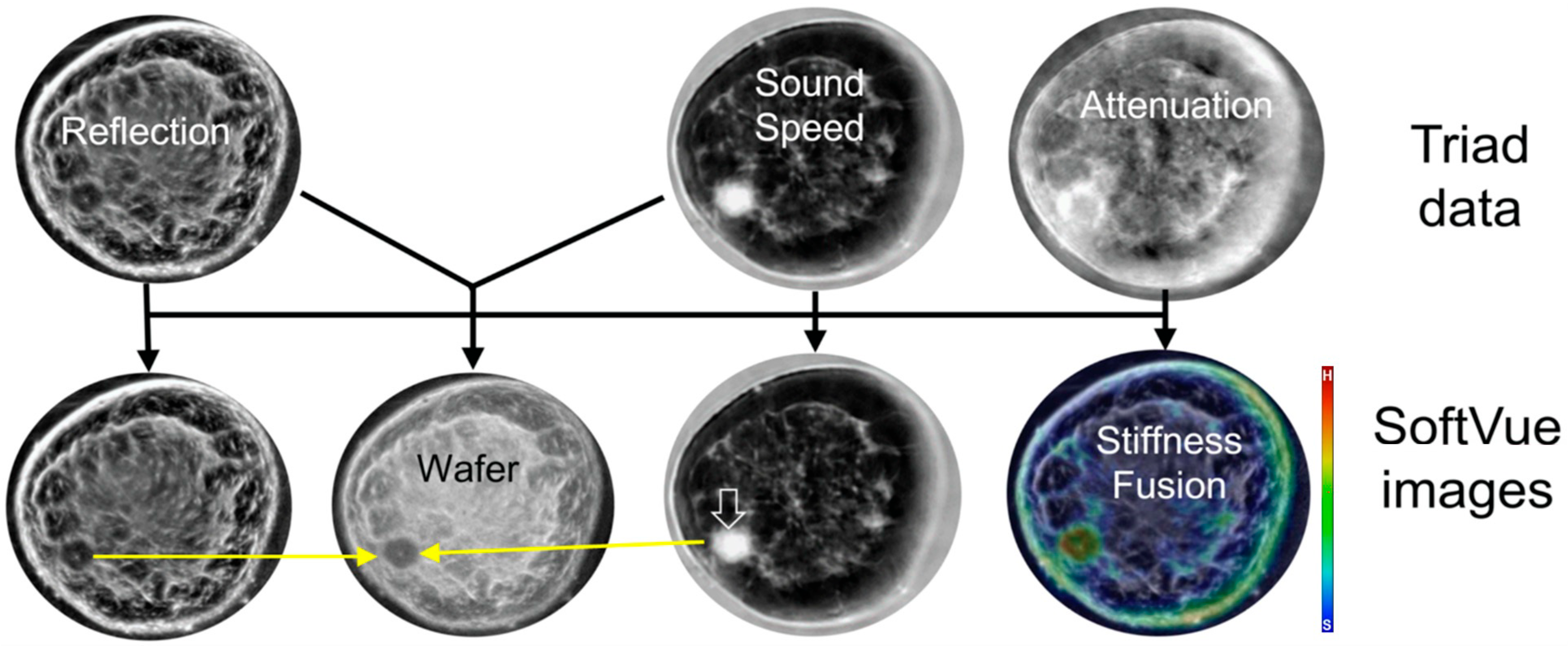
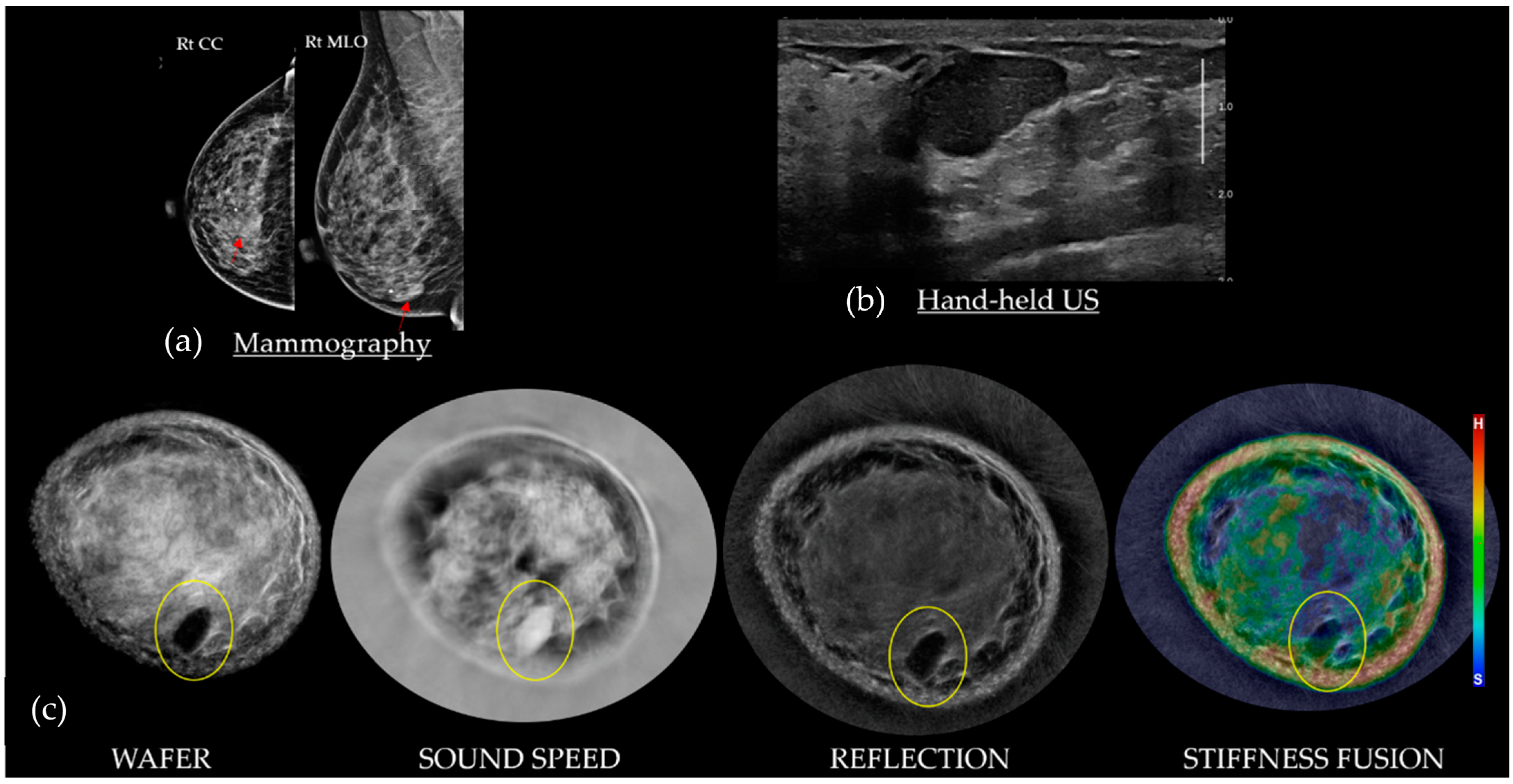
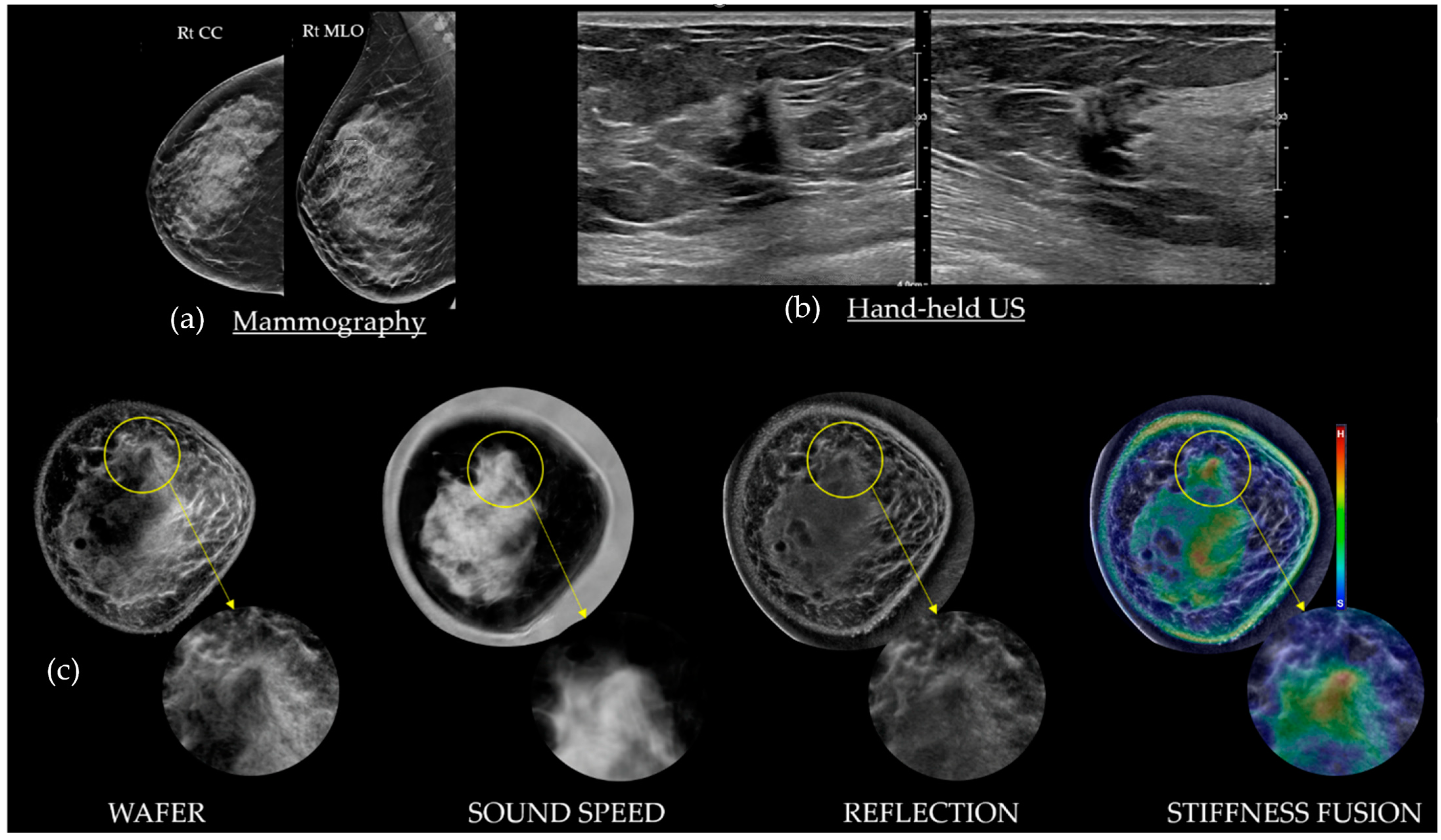
3. Understanding SoftVue Image Sequences: Tissue Acoustics and Clinical Imaging
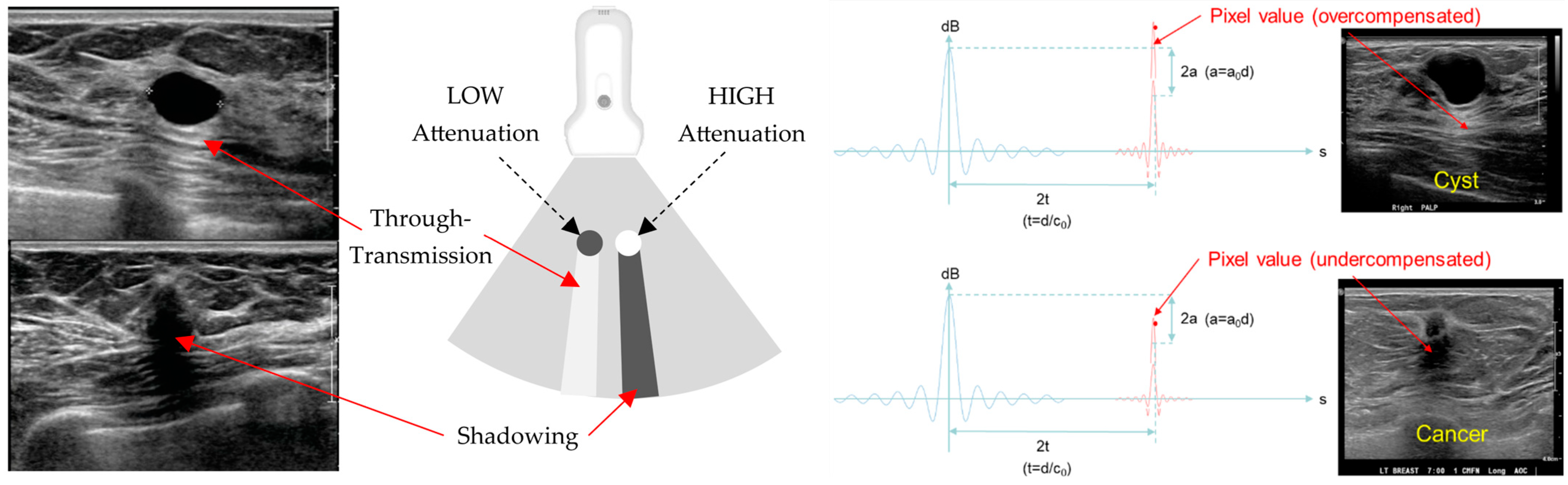
3.1. HHUS Reflection vs. SoftVue Reflection
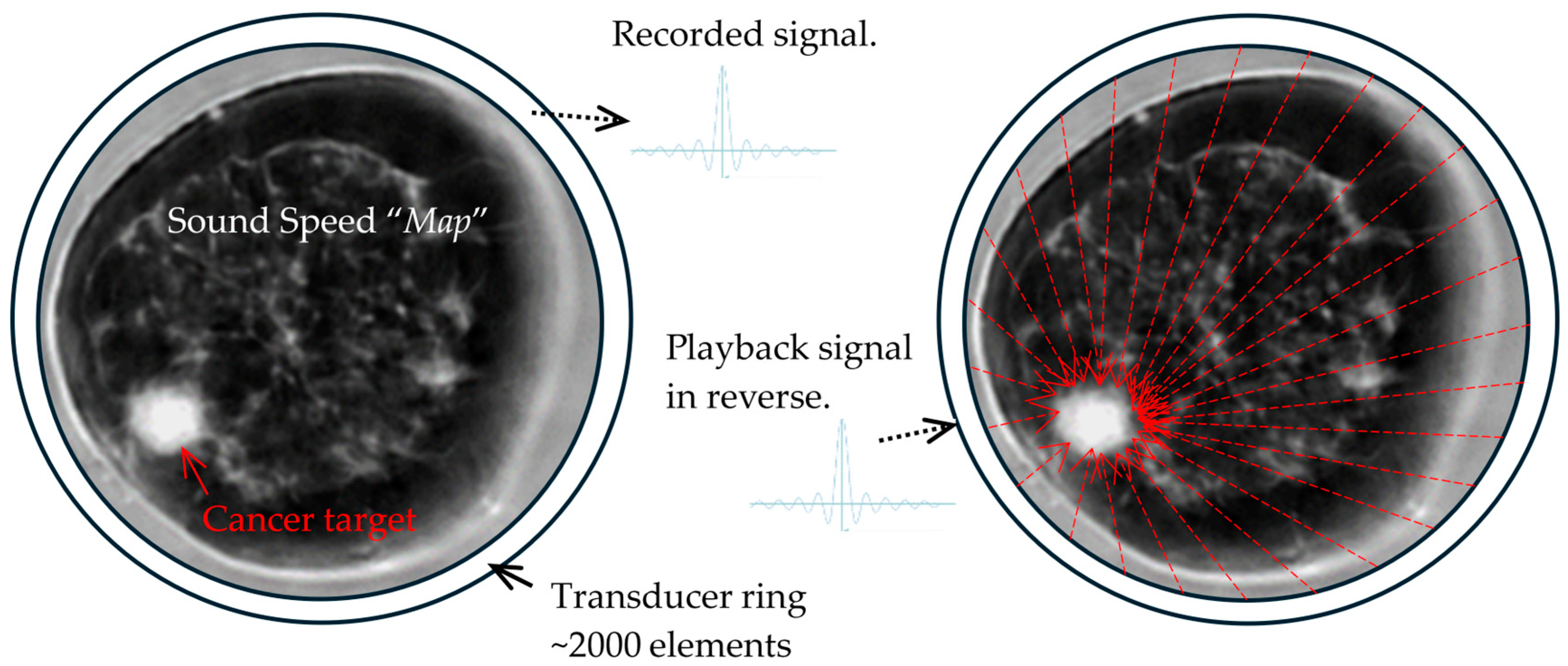
3.2. SoftVue Sound Speed
3.3. SoftVue WAveForm Enhanced Reflection (Wafer)
3.4. SoftVue Stiffness Fusion
4. SoftVue UST and US BI-RADS—Review of Common Breast Masses
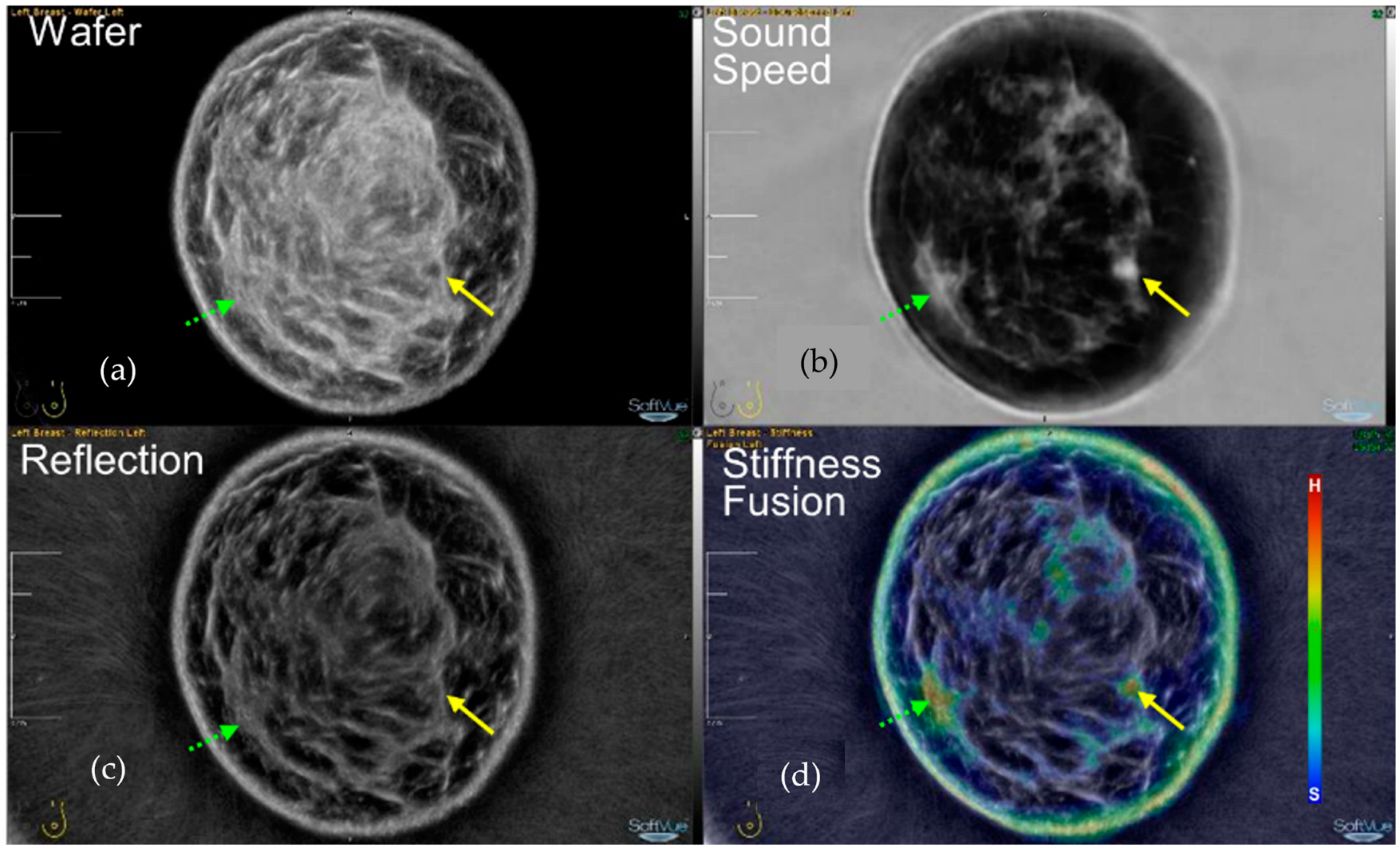
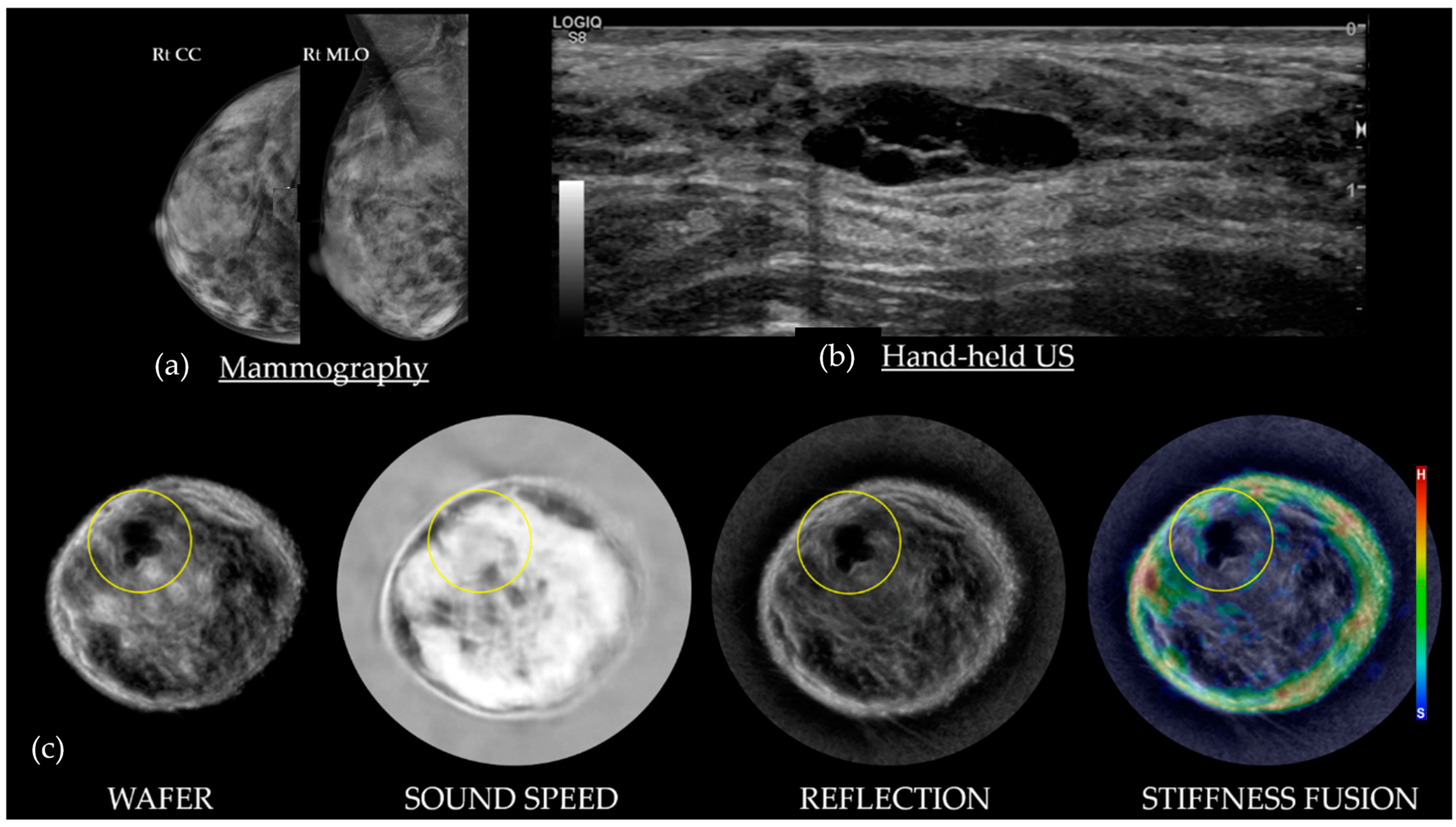
4.1. Cysts on SoftVue
4.2. Fibroadenomas on SoftVue
4.3. Cancers on SoftVue
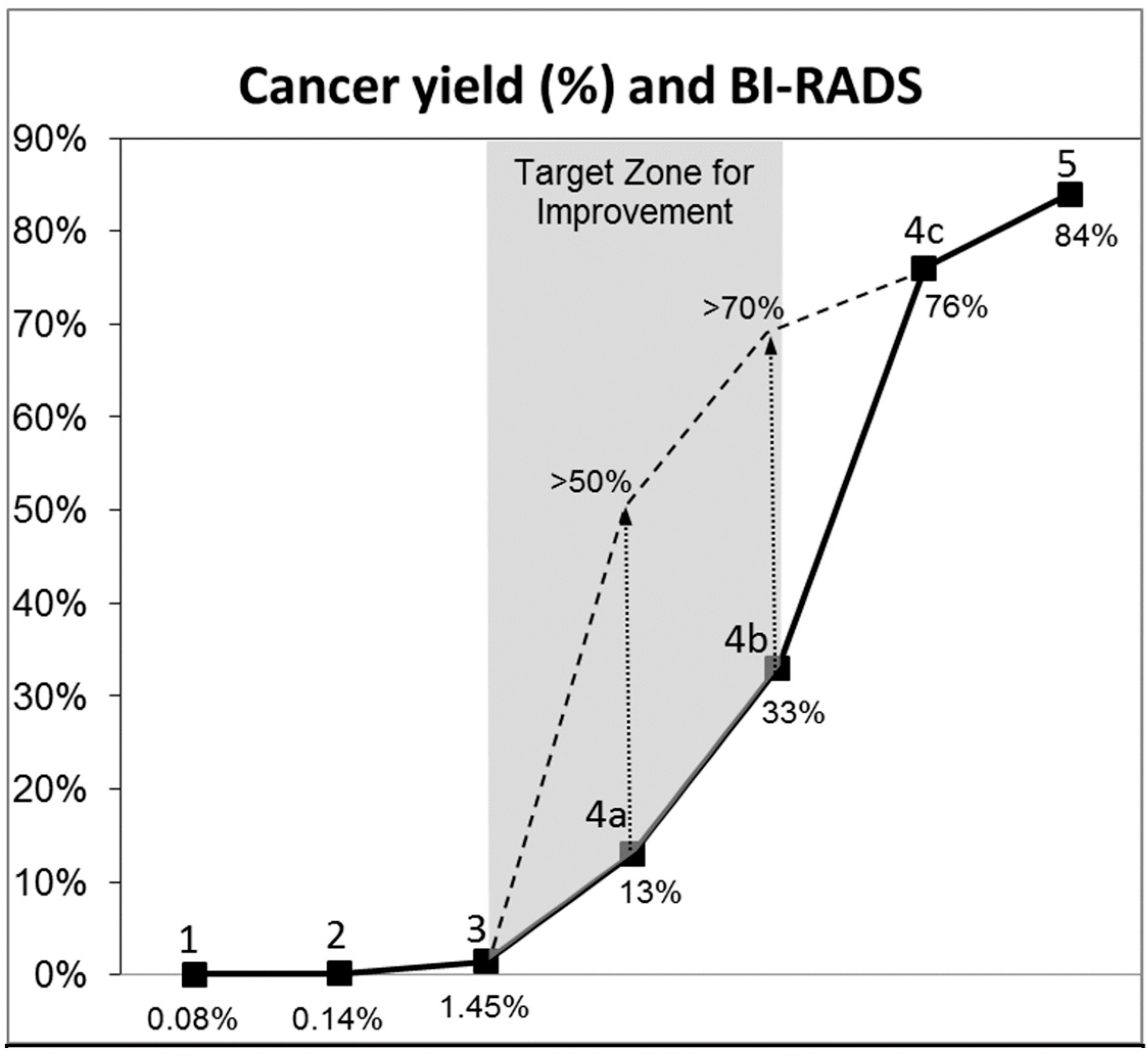
4.4. SoftVue and BI-RADS
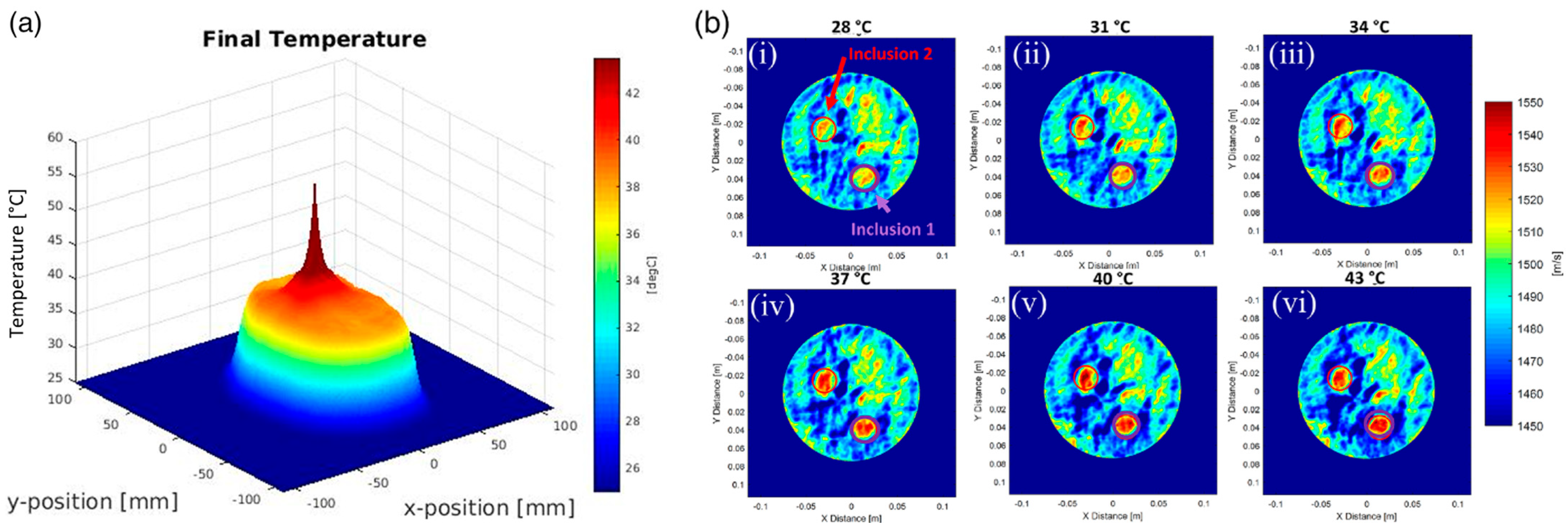
5. The Future of Dynamic Focusing for Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200040B.pdf (accessed on 3 April 2024).
- Rather, J.D.G.; Caulfield, H.J.; Doolittle, R.D.; Littrup, P.; Zeiders, G.W. Method and Apparatus for High-Resolution Detection and Characterization of Medical Pathologies. U.S. Patent 6385474, 7 May 2002. [Google Scholar]
- Rather, J.D.G.; Caulfield, H.J.; Doolittle, R.D.; Littrup, P.; Zeiders, G.W. Method and Apparatus for High-Resolution Detection and Characterization of Medical Pathologies. U.S. Patent 6728567, 27 April 2004. [Google Scholar]
- Geras, K.J.; Mann, R.M.; Moy, L. Artificial Intelligence for Mammography and Digital Breast Tomosynthesis: Current Concepts and Future Perspectives. Radiology 2019, 293, 246–259. [Google Scholar] [CrossRef]
- Jairam, M.P.; Ha, R. A review of artificial intelligence in mammography. Clin. Imaging 2022, 88, 36–44. [Google Scholar] [CrossRef]
- Littrup, P.J.; Duric, N.; Holsapple, E. Method Apparatus for Combined Diagnostic Therapeutic Ultrasound System Incorporating Noninvasive Thermometry Ablation Control Automation. U.S. Patent 8376946, 19 February 2013. (Submitted 16 May 2003). [Google Scholar]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS® fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef]
- Bodewes, F.T.H.; van Asselt, A.A.; Dorrius, M.D.; Greuter, M.J.W.; de Bock, G.H. Mammographic breast density and the risk of breast cancer: A systematic review and meta-analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef]
- Hussein, H.; Abbas, E.; Keshavarzi, S.; Fazelzad, R.; Bukhanov, K.; Kulkarni, S.; Au, F.; Ghai, S.; Alabousi, A.; Freitas, V. Supplemental Breast Cancer Screening in Women with Dense Breasts and Negative Mammography: A Systematic Review and Meta-Analysis. Radiology 2023, 306, e221785. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130020b.pdf (accessed on 3 April 2024).
- Littrup, P.J.; Goodman, A.C.; Mettlin, C.J. The benefit and cost of prostate cancer early detection. The Investigators of the American Cancer Society-National Prostate Cancer Detection Project. CA Cancer J. Clin. 1993, 43, 134–149. [Google Scholar] [CrossRef]
- Sankatsing, V.D.V.; Juraniec, K.; Grimm, S.E.; Joore, M.A.; Pijnappel, R.M.; de Koning, H.J.; van Ravesteyn, N.T. Cost-effectiveness of Digital Breast Tomosynthesis in Population-based Breast Cancer Screening: A Probabilistic Sensitivity Analysis. Radiology 2020, 297, 40–48. [Google Scholar] [CrossRef]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf11/P110006b.pdf (accessed on 3 April 2024).
- Berg, W.A.; Rafferty, E.A.; Friedewald, S.M.; Hruska, C.B.; Rahbar, H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2021, 216, 275–294. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Adams, A.M.; Jong, R.A.; Barr, R.G.; Lehrer, D.E.; Pisano, E.D.; Evans, W.P., 3rd; Mahoney, M.C.; Hovanessian Larsen, L.; et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology 2010, 254, 79–87. [Google Scholar] [CrossRef]
- Duric, N.; Littrup, P.; Poulo, L.; Babkin, A.; Pevzner, R.; Holsapple, E.; Rama, O.; Glide, C. Detection of breast cancer with ultrasound tomography: First results with the Computed Ultrasound Risk Evaluation (CURE) prototype. Med. Phys. 2007, 34, 773–785. [Google Scholar] [CrossRef]
- Duric, N.; Boyd, N.; Littrup, P.; Sak, M.; Myc, L.; Li, C.; West, E.; Minkin, S.; Martin, L.; Yaffe, M.; et al. Breast density measurements with ultrasound tomography: A comparison with film and digital mammography. Med. Phys. 2013, 40, 013501. [Google Scholar] [CrossRef]
- O’Flynn, E.A.M.; Fromageau, J.; Ledger, A.E.; Messa, A.; D’Aquino, A.; Schoemaker, M.J.; Schmidt, M.; Duric Swerdlow, A.J.; Bamber, J.C. Ultrasound tomography evaluation of breast density: A comparison with non-contrast magnetic resonance imaging. Investig. Radiol. 2017, 52, 343–348. [Google Scholar] [CrossRef]
- Duric, N.; Sak, M.; Fan, S.; Pfeiffer, R.M.; Littrup, P.J.; Simon, M.S.; Gorski, D.H.; Ali, H.; Purrington, K.S.; Brem, R.F.; et al. Using whole breast ultrasound tomography to improve breast cancer risk assessment: A novel risk factor based on the quantitative tissue property of sound speed. J. Clin. Med. 2020, 9, 367. [Google Scholar] [CrossRef]
- Sak, M.; Littrup, P.; Brem, R.; Duric, N. Whole Breast Sound Speed Measurement from US Tomography Correlates Strongly with Volumetric Breast Density from Mammography. J. Breast Imaging 2020, 2, 443–451. [Google Scholar] [CrossRef]
- Duric, N.; Littrup, P.; Sak, M.; Li, C.; Chen, D.; Roy, O.; Bey-Knight, L.; Brem, R. A Novel Marker, Based on Ultrasound Tomography, for Monitoring Early Response to Neoadjuvant Chemotherapy. J. Breast Imaging 2020, 2, 569–576. [Google Scholar] [CrossRef]
- Littrup, P.J.; Duric, N.; Sak, M.; Li, C.; Roy, O.; Brem, R.F.; Yamashita, M. The Fat-glandular Interface and Breast Tumor Locations: Appearances on Ultrasound Tomography Are Supported by Quantitative Peritumoral Analyses. J. Breast Imaging 2021, 3, 455–464. [Google Scholar] [CrossRef]
- Littrup, P.J.; Duric, N.; Sak, M.; Li, C.; Roy, O.; Brem, R.F.; Larsen, L.H.; Yamashita, M. Multicenter Study of Whole Breast Stiffness Imaging by Ultrasound Tomography (SoftVue) for Characterization of Breast Tissues and Masses. J. Clin. Med. 2021, 10, 5528. [Google Scholar] [CrossRef]
- Gierach, G.L.; Sak, M.; Fan, S.; Pfeiffer, R.M.; Palakal, M.; Ramin, C.; Bey-Knight, L.; Simon, M.S.; Gorski, D.; Ali, H.; et al. Rapid Reductions in Breast Density following Tamoxifen Therapy as Evaluated by Whole-Breast Ultrasound Tomography. J. Clin. Med. 2022, 11, 792. [Google Scholar] [CrossRef]
- Glover, G.H. Computerized time-of-flight ultrasonic tomography for breast examination. Ultrasound Med. Biol. 1977, 3, 117–127. [Google Scholar] [CrossRef]
- Greenleaf, J.F.; Johnson, S.A.; Lent, A.H. Measurement of spatial distribution of refractive index in tissues by ultrasonic computer assisted tomography. Ultrasound Med. Biol. 1978, 3, 327–339. [Google Scholar] [CrossRef]
- André, M.P.; Martin, P.J.; Otto, G.P.; Olson, L.K.; Barrett, T.K.; Spivey, B.A.; Palmer, D.A. A New Consideration of Diffraction Computed Tomography for Breast Imaging: Studies in Phantoms and Patients. In Acoustical Imaging. Acoustical Imaging; Jones, J.P., Ed.; Springer: Boston, MA, USA, 1995; Volume 21. [Google Scholar] [CrossRef]
- Jeong, J.W.; Shin, D.C.; Do, S.H.; Blanco, C.; Klipfel, N.E.; Holmes, D.R.; Hovanessian-Larsen, L.J.; Marmarelis, V.Z. Differentiation of cancerous lesions in excised human breast specimens using multiband attenuation profiles from ultrasonic transmission tomography. J. Ultrasound Med. 2008, 27, 435–451. [Google Scholar] [CrossRef]
- Ruiter, N.V.; Zapf, M.; Hopp, T.; Dapp, R.; Kretzek, E.; Birk, M.; Kohout, B.; Gemmeke, H. 3D ultrasound computer tomography of the breast: A new era? Eur. J. Radiol. 2012, 81 (Suppl. S1), S133–S134. [Google Scholar] [CrossRef]
- Malik, B.; Klock, J.; Wiskin, J.; Lenox, M. Objective breast tissue image classification using Quantitative Transmission ultrasound tomography. Sci. Rep. 2016, 6, 38857. [Google Scholar] [CrossRef]
- Iuanow, E.; Smith, K.; Obuchowski Bullen, J.; Klock, J. Accuracy of cyst versus solid diagnosis in the breast using quantitative transmission (QT) ultrasound. Acad. Radiol. 2017, 24, 1148–1153. [Google Scholar] [CrossRef]
- Malik, B.; Terry, R.; Wiskin, J.; Lenox, M. Quantitative transmission ultrasound tomography: Imaging and performance characteristics. Med. Phys. 2018, 45, 3063–3075. [Google Scholar] [CrossRef]
- Malik, B.; Klock, J. Breast cyst fluid analysis correlations with speed of sound using transmission ultrasound. Acad. Radiol. 2019, 26, 76–85. [Google Scholar] [CrossRef]
- Wiskin, J.; Malik, B.; Natesan, R.; Lenox, M. Quantitative assessment of breast density using transmission ultrasound tomography. Med. Phys. 2019, 46, 2610–2620. [Google Scholar] [CrossRef]
- Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J. Comparison of lesion detection in the transverse and coronal views on automated breast sonography. J. Ultrasound Med. 2015, 34, 125–135. [Google Scholar] [CrossRef]
- Van Zelst, J.C.; Platel, B.; Karssemeijer, N.; Mann, R.M. Multiplanar Reconstructions of 3D Automated Breast Ultrasound Improve Lesion Differentiation by Radiologists. Acad. Radiol. 2015, 22, 1489–1496. [Google Scholar] [CrossRef]
- Wang, K.; Matthews, T.; Anis, F.; Li, C.; Duric, N.; Anastasio, M.A. Waveform inversion with source encoding for breast sound speed reconstruction in ultrasound computed tomography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 475–493. [Google Scholar] [CrossRef]
- Kim, W.H.; Li, M.; Han, W.; Ryu, H.S.; Moon, W.K. The spatial relationship of malignant and benign breast lesions with respect to the fat-gland interface on magnetic resonance imaging. Nat. Sci. Rep. 2016, 6, 39085. [Google Scholar] [CrossRef]
- Zhu, W.; Harvey, S.; Macura, K.J.; Euhus, D.M.; Artemov, D. Invasive breast cancer preferably and predominantly occurs at the interface between fibroglandular and adipose tissue. Clin. Breast Cancer 2017, 17, e11–e18. [Google Scholar] [CrossRef]
- Boyd, N.F.; Li, Q.; Melnichouk, O.; Huszti, E.; Martin, L.J.; Gunasekara, A.; Mawdsley, G.; Yaffe, M.J.; Minkin, S. Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS ONE 2014, 9, e100937. [Google Scholar] [CrossRef]
- Jiang, Y.; Metz, C.E. BI-RADS data should not be used to estimate ROC curves. Radiology 2010, 256, 29–31. [Google Scholar] [CrossRef]
- Lazarus, E.; Mainiero, M.B.; Schepps, B.; Koelliker, S.L.; Livingston, L.S. BI-RADS lexicon for US and mammography: Interobserver variability and positive predictive value. Radiology 2006, 239, 385–391. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.J.; Moon, H.J.; Kwak, J.Y.; Kim, E.K. Subcategorization of ultrasonographic BI-RADS category 4: Positive predictive value and clinical factors affecting it. Ultrasound Med. Biol. 2011, 37, 693–699. [Google Scholar] [CrossRef]
- Jales, R.M.; Sarian, L.O.; Torresan, R.; Marussi, E.F.; Alvares, B.R.; Derchain, S. Simple rules for ultrasonographic subcategorization of BI-RADS®-US 4 breast masses. Eur. J. Radiol. 2013, 82, 1231–1235. [Google Scholar] [CrossRef]
- Thomas, J.L.; Roux, P.; Fink, M. Inverse scattering analysis with an acoustic time-reversal mirror. Phys. Rev. Lett. 1994, 72, 637–640. [Google Scholar] [CrossRef]
- Fink, M. Time-reversed acoustics. Sci. Am. 1999, 281, 91–97. [Google Scholar] [CrossRef]
- Pattyn, A.; Kratkiewicz, K.; Alijabbari, N.; Carson, P.L.; Littrup, P.; Fowlkes, J.B.; Duric, N.; Mehrmohammadi, M. Feasibility of ultrasound tomography-guided localized mild hyperthermia using a ring transducer: Ex vivo and in silico studies. Med. Phys. 2022, 49, 6120–6136. [Google Scholar] [CrossRef]
- Pattyn, A.; Kratkiewicz, K.; Alijabbari, N.; Mehrmohammadi, M.; Duric, N.; Carson, P.L. Mild-Hyperthermia Generation and Control with a Ring-based Ultrasound Tomography. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Kuo, L.-W.; Dong, G.-C.; Pan, C.-C.; Chen, S.-F.; Chen, G.S. An MRI-Guided Ring High-Intensity Focused Ultrasound System for Noninvasive Breast Ablation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1839–1847. [Google Scholar] [CrossRef]
- Lymperopoulos, G.; Lymperopoulos, P.; Alikari, V.; Dafogianni, C.; Zyga, S.; Margari, N. Application of Theranostics in Oncology. Adv. Exp. Med. Biol. 2017, 989, 119–128. [Google Scholar] [CrossRef]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Jokar, N.; Velikyan, I.; Ahmadzadehfar, H.; Rekabpour, S.J.; Jafari, E.; Ting, H.H.; Biersack, H.J.; Assadi, M. Theranostic Approach in Breast Cancer: A Treasured Tailor for Future Oncology. Clin. Nucl. Med. 2021, 46, e410–e420. [Google Scholar] [CrossRef]
- Zulkifli, D.; Manan, H.A.; Yahya, N.; Hamid, H.A. The Applications of High-Intensity Focused Ultrasound (HIFU) Ablative Therapy in the Treatment of Primary Breast Cancer: A Systematic Review. Diagnostics 2023, 13, 2595. [Google Scholar] [CrossRef]
- Ghoshal, G.; Luchies, A.C.; Blue, J.P.; Oelze, M.L. Temperature dependent ultrasonic characterization of biological media. J. Acoust. Soc. Am. 2011, 130, 2203–2211. [Google Scholar] [CrossRef]
- Choi, M.J.; Guntur, S.R.; Lee, J.M.; Paeng, D.G.; Lee, K.I.; Coleman, A. Changes in ultrasonic properties of liver tissue in vitro during heating-cooling cycle concomitant with thermal coagulation. Ultrasound Med. Biol. 2011, 37, 2000–2012. [Google Scholar] [CrossRef]
- Borden, M.A.; Shakya, G.; Upadhyay, A.; Song, K.H. Acoustic Nanodrops for Biomedical Applications. Curr. Opin. Colloid Interface Sci. 2020, 50, 101383. [Google Scholar] [CrossRef]
- Spatarelu, C.P.; Jandhyala, S.; Luke, G.P. Dual-drug loaded ultrasound-responsive nanodroplets for on-demand combination chemotherapy. Ultrasonics 2023, 133, 107056. [Google Scholar] [CrossRef]
- Meltzer, R.S. Food and Drug Administration ultrasound device regulation: The output display standard, the “mechanical index,” and ultrasound safety. J. Am. Soc. Echocardiogr. 1996, 9, 216–220. [Google Scholar] [CrossRef]
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114. (In English) [Google Scholar] [CrossRef]
- Crezee, J.; Franken, N.A.P.; Oei, A.L. Hyperthermia-Based Anti-Cancer Treatments. Cancers 2021, 13, 1240. (In English) [Google Scholar] [CrossRef]
- Institute, N.C. Hyperthermia to Treat Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/types/hyperthermia (accessed on 3 April 2024).
- Priester, M.I.; Curto, S.; van Rhoon, G.C.; Ten Hagen, T.L.M. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 2021, 13, 4628. (In English) [Google Scholar] [CrossRef]
- Partanen, A.; Tillander, M.; Yarmolenko, P.S.; Wood, B.J.; Dreher, M.R.; Köhler, M.O. Reduction of peak acoustic pressure and shaping of heated region by use of multifoci sonications in MR-guided high-intensity focused ultrasound mediated mild hyperthermia. Med. Phys. 2013, 40, 013301. [Google Scholar] [CrossRef]
- Cho, C.W.; Liu, Y.; Cobb, W.N.; Henthorn, T.K.; Lillehei, K.; Christians, U.; Ng, K.Y. Ultrasound-induced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharm. Res. 2002, 19, 1123–1129. (In English) [Google Scholar] [CrossRef]
- Copelan, A.; Hartman, J.; Chehab, M.; Venkatesan, A.M. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Semin. Intervent. Radiol. 2015, 32, 398–415. (In English) [Google Scholar] [CrossRef]
- Toraya-Brown, S.; Fiering, S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int. J. Hyperth. 2014, 30, 531–539. [Google Scholar] [CrossRef]
- Zhou, Y.F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8–27. (In English) [Google Scholar] [CrossRef]
- Littrup, P.J.; Jallad, B.; Chandiwala-Mody, P.; D’Agostini, M.; Adam, B.A.; Bouwman, D. Cryotherapy for breast cancer: A feasibility study without excision. J. Vasc. Interv. Radiol. 2009, 20, 1329–1341. [Google Scholar] [CrossRef]
- Littrup, P.J.; Jallad, B.; Vorugu, V.; Littrup, G.; Currier, B.; George, M.; Herring, D. Lethal isotherms of cryoablation in a phantom study: Effects of heat load, probe size, and number. J. Vasc. Interv. Radiol. 2009, 20, 1343–1351. [Google Scholar] [CrossRef]


| Material | Sound Speed [mm × µs−1] | Attenuation Coefficient at 2.5 MHz [dB/cm] |
|---|---|---|
| Fat | 1.467 | 0.48 |
| Parenchymal tissue | 1.552 | 0.89 |
| Cancer | 1.563 | 1.20 |
| Fibroadenoma | 1.552 | 0.52 |
| Gelatin cyst | 1.585 | 0.16 |
| ULTRASOUND BI-RADSs * | BTUS BI-RADSs * (Under Development) | |||||
|---|---|---|---|---|---|---|
| Tissue Composition (screening only) | a. Homogeneous—fat | Volume Averaged Sound Speed | a. Fatty | |||
| b. Homogeneous—fibroglandular | Like Mammography | b. Scattered | ||||
| c. Heterogeneous echotexture | c. Heterogenously Dense | |||||
| d. Extremely Dense | ||||||
| Masses | Shape | Oval | Oval | |||
| Round | Round | |||||
| Irregular | Irregular | |||||
| Orientation | Parallel | |||||
| Not parallel | ||||||
| Margin | Circumscribed | Circumscribed | ||||
| Not circumscribed | Indistinct | Not circumscribed | Indistinct | |||
| Angular | ||||||
| Microlobulated | ||||||
| Spiculated | Spiculated | |||||
| Echo pattern | Anechoic | Black (Reflection) | ||||
| Hyperechoic | Bright/White (Reflection) | |||||
| Complex cystic and solid | ||||||
| Hypoechoic | Dark (Reflection) | |||||
| Isoechoic | Gray (Reflection) | |||||
| Heterogeneous | ||||||
| Posterior features | No posterior features | |||||
| Enhancement | Blue * (Stiffness Fusion contains low attenuation) | |||||
| Shadowing * | Red * (Stiffness Fusion contains high attenuation) | |||||
| Combined pattern | ||||||
| Calcifications | In a mass | (Resolution limited) | ||||
| Outside a mass | ||||||
| Intraductal calcifications | ||||||
| Associated features | Architectural distortion | Prominent in Coronal (REF and SS) | ||||
| Duct changes | (Resolution limited) | |||||
| Skin changes | Skin-thickening | (Angle dependent) | ||||
| Skin retraction | Prominent in Coronal (REF and SS) | |||||
| Edema | Seen in REF and SS | |||||
| Vascularity | Absent | (Not currently available) | ||||
| Internal vascularity | ||||||
| Vessels in rim | ||||||
| Elasticity Assessment | Soft | Black-Blue (Stiffness Fusion) | ||||
| Intermediate | Green-Yellow (Stiffness Fusion) | |||||
| Hard | Orange-Red (Stiffness Fusion) | |||||
| SoftVue Findings | BTUS * BI-RADSs | |
|---|---|---|
| Negative | 1 | Negative |
| Circumscribed + Blue | 2 | Benign |
| Circumscribed + Green | 3 | Probably Benign |
| Circumscribed + Orange/Red | 4a | Low Suspicion for Malignancy |
| Indistinct + Green | 4b | Moderate Suspicion |
| Indistinct/Irregular + Orange/Red | 4c | High Suspicion |
| Spiculations/AD + Orange/Red | 5 | High Suspicion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Littrup, P.J.; Mehrmohammadi, M.; Duric, N. Breast Tomographic Ultrasound: The Spectrum from Current Dense Breast Cancer Screenings to Future Theranostic Treatments. Tomography 2024, 10, 554-573. https://doi.org/10.3390/tomography10040044
Littrup PJ, Mehrmohammadi M, Duric N. Breast Tomographic Ultrasound: The Spectrum from Current Dense Breast Cancer Screenings to Future Theranostic Treatments. Tomography. 2024; 10(4):554-573. https://doi.org/10.3390/tomography10040044
Chicago/Turabian StyleLittrup, Peter J., Mohammad Mehrmohammadi, and Nebojsa Duric. 2024. "Breast Tomographic Ultrasound: The Spectrum from Current Dense Breast Cancer Screenings to Future Theranostic Treatments" Tomography 10, no. 4: 554-573. https://doi.org/10.3390/tomography10040044
APA StyleLittrup, P. J., Mehrmohammadi, M., & Duric, N. (2024). Breast Tomographic Ultrasound: The Spectrum from Current Dense Breast Cancer Screenings to Future Theranostic Treatments. Tomography, 10(4), 554-573. https://doi.org/10.3390/tomography10040044







