The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Stereotactic Biopsy
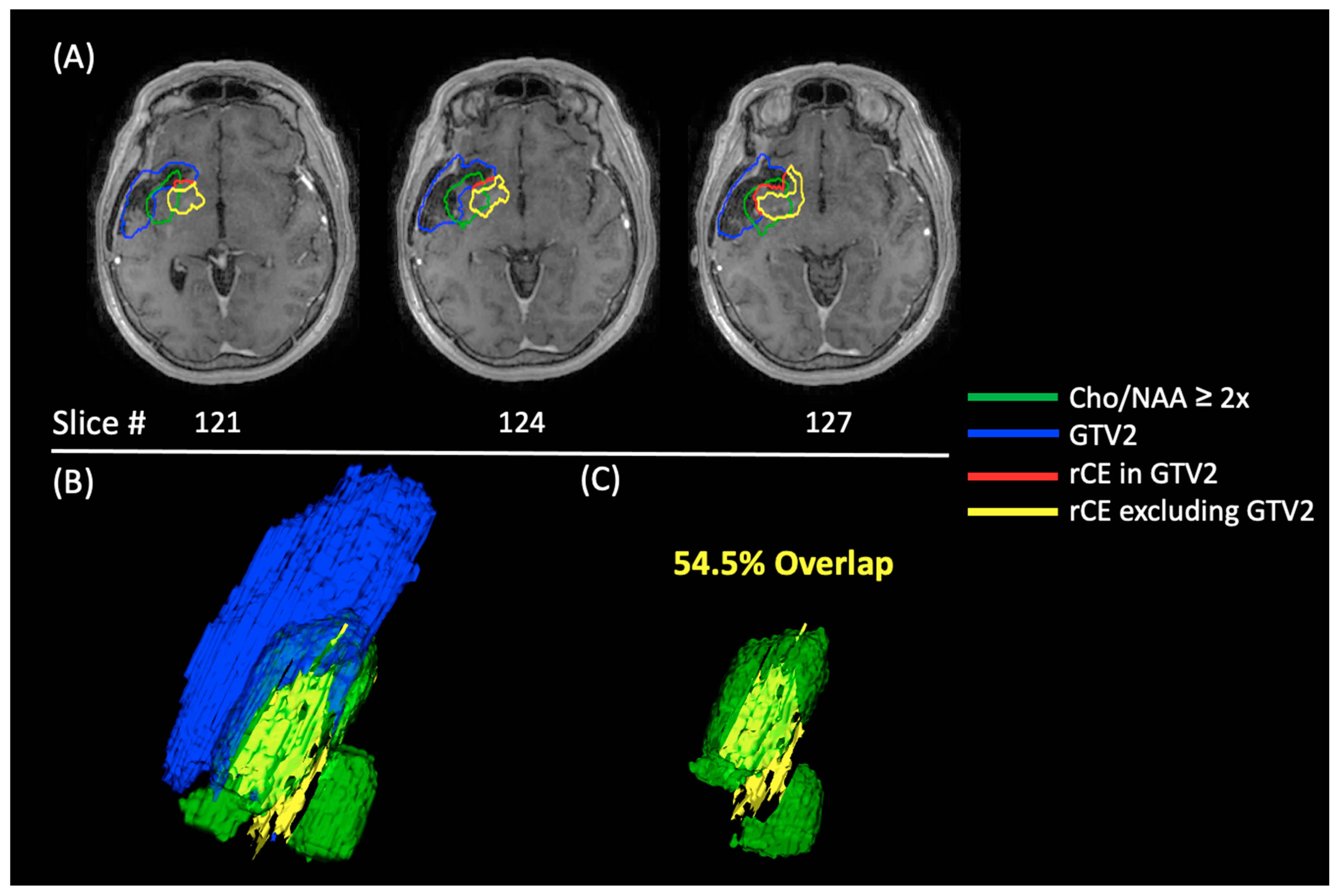
2.2. Tumor Volume Determination (NCT02137759)
2.3. Statistical Analysis/Survival Analysis
3. Results
3.1. Stereotactic Biopsy
3.2. NCT02137759 Treatment Volumes
3.3. NCT02137759 Recurrence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krieger, M.D.; Chandrasoma, P.T.; Zee, C.S.; Apuzzo, M.L. Role of stereotactic biopsy in the diagnosis and management of brain tumors. Semin. Surg. Oncol. 1998, 14, 13–25. [Google Scholar] [CrossRef]
- Scott, J.N.; Brasher, P.M.; Sevick, R.J.; Rewcastle, N.B.; Forsyth, P.A. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002, 59, 947–949. [Google Scholar] [CrossRef]
- Kondziolka, D.; Lunsford, L.D.; Martinez, A.J. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J. Neurosurg. 1993, 79, 533–536. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Mizutani, Y.; Hachiya, J. Preliminary evaluation of fluid-attenuated inversion-recovery MR in the diagnosis of intracranial tumors. AJNR Am. J. Neuroradiol. 1996, 17, 1081–1086. [Google Scholar]
- Pope, W.B.; Young, J.R.; Ellingson, B.M. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr. Neurol. Neurosci. Rep. 2011, 11, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Scarabino, T.; Trojsi, F.; Giannatempo, G.M.; Popolizio, T.; Catapano, D.; Bonavita, S.; Maggialetti, N.; Tosetti, M.; Salvolini, U.; et al. Multiparametric 3T MR approach to the assessment of cerebral gliomas: Tumor extent and malignancy. Neuroradiology 2006, 48, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Law, M. MR Spectroscopy of brain tumors. Top. Magn. Reson. Imaging 2004, 15, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Bluml, S.; Mamelak, A.N. Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J. Neuro-Oncol. 1999, 45, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, H.; Bachert, P.; Herfarth, K.K.; Zuna, I.; Debus, J.; van Kaick, G. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am. J. Neuroradiol. 2001, 22, 1316–1324. [Google Scholar] [PubMed]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Shu, H.-K.G.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.D.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro-Oncology 2016, 18, 1180–1189. [Google Scholar] [CrossRef]
- Go, K.G.; Kamman, R.L.; Mooyaart, E.L.; Heesters, M.A.A.M.; Pruim, J.; Vaalburg, W.; Paans, A.M.J. Localised proton spectroscopy and spectroscopic imaging in cerebral gliomas, with comparison to positron emission tomography. Neuroradiology 1995, 37, 198–206. [Google Scholar] [CrossRef]
- Chang, J.; Thakur, S.; Perera, G.; Kowalski, A.; Huang, W.; Karimi, S.; Hunt, M.; Koutcher, J.; Fuks, Z.; Amols, H.; et al. Image-fusion of MR spectroscopic images for treatment planning of gliomas. Med. Phys. 2006, 33, 32–40. [Google Scholar] [CrossRef]
- Graves, E.E.; Pirzkall, A.; Nelson, S.J.; Larson, D.; Verhey, L. Registration of magnetic resonance spectroscopic imaging to computed tomography for radiotherapy treatment planning. Med. Phys. 2001, 28, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Narayana, A.; Chang, J.; Thakur, S.; Huang, W.; Karimi, S.; Hou, B.; Kowalski, A.; Perera, G.; Holodny, A.; Gutin, P.H. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br. J. Radiol. 2007, 80, 347–354. [Google Scholar] [CrossRef]
- Nelson, S.J.; Vigneron, D.B.; Dillon, W.P. Serial evaluation of patients with brain tumors using volume MRI and 3D1H MRSI. NMR Biomed. 1999, 12, 123–138. [Google Scholar] [CrossRef]
- Pirzkall, A.; Li, X.; Oh, J.; Chang, S.; Berger, M.S.; Larson, D.A.; Verhey, L.J.; Dillon, W.P.; Nelson, S.J. 3D MRSI for resected high-grade gliomas before RT: Tumor extent according to metabolic activity in relation to MRI. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 126–137. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, V.; Gurbani, S.S.; Ramesh, K.; Cordova, J.S.; Schreibmann, E.; Shu, H.-K.G.; Olson, J.; Han, H.; Giuffrida, A.; et al. 3D whole-brain metabolite imaging to improve characterization of low-to-intermediate grade gliomas. J. Neuro-Oncol. 2021, 153, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.; Weinberg, B.; Cooper, L.; Mellon, E.; Schreibmann, E.; Sheriff, S.; Maudsley, A.; Goryawala, M.; Shu, H.-K.; Shim, H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019, 5, 184–191. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Cohen, K.J.; Buxton, A.; Krailo, M.D.; Burger, P.C.; Rosenblum, M.K.; Brat, D.J.; Hamilton, R.L.; Eckel, S.P.; Zhou, T.; et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: A report of the Children’s Oncology Group ACNS0423 study. Neuro-Oncology 2016, 18, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Mellon, E.A.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; Sheriff, S.A.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac006. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Krol, S.K.; Rotili, D.; Mai, A.; Wojtas, B.; Kaminska, B.; Maleszewska, M. Histone deacetylase inhibitors exert anti-tumor effects on human adherent and stem-like glioma cells. Clin. Epigenet. 2019, 11, 11. [Google Scholar] [CrossRef]
- Wang, C.; Eessalu, T.E.; Barth, V.N.; Mitch, C.H.; Wagner, F.F.; Hong, Y.; Neelamegam, R.; Schroeder, F.A.; Holson, E.B.; Haggarty, S.J.; et al. Design, synthesis, and evaluation of hydroxamic acid-based molecular probes for in vivo imaging of histone deacetylase (HDAC) in brain. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 29–38. [Google Scholar]
- Xu, K.; Ramesh, K.; Huang, V.; Gurbani, S.S.; Cordova, J.S.; Schreibmann, E.; Weinberg, B.D.; Sengupta, S.; Voloschin, A.D.; Holdhoff, M.; et al. Final Report on Clinical Outcomes and Tumor Recurrence Patterns of a Pilot Study Assessing Efficacy of Belinostat (PXD-101) with Chemoradiation for Newly Diagnosed Glioblastoma. Tomography 2022, 8, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Sabati, M.; Sheriff, S.; Gu, M.; Wei, J.; Zhu, H.; Barker, P.B.; Spielman, D.M.; Alger, J.R.; Maudsley, A.A. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn. Reson. Med. 2015, 74, 1209–1220. [Google Scholar] [CrossRef]
- Maudsley, A.; Darkazanli, A.; Alger, J.; Hall, L.; Schuff, N.; Studholme, C.; Yu, Y.; Ebel, A.; Frew, A.; Goldgof, D. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006, 19, 492–503. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Beare, R.; Lowekamp, B.; Yaniv, Z. Image Segmentation, Registration and Characterization in R with SimpleITK. J. Stat. Softw. 2018, 86, 8. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Z.; Lowekamp, B.C.; Johnson, H.J.; Beare, R. SimpleITK Image-Analysis Notebooks: A Collaborative Environment for Education and Reproducible Research. J. Digit. Imaging 2018, 31, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Lowekamp, B.C.; Chen, D.T.; Ibáñez, L.; Blezek, D. The Design of SimpleITK. Front. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Pilon, C. lifelines: Survival analysis in Python. J. Open Source Softw. 2019, 4, 1317. [Google Scholar] [CrossRef]
- Jackson, R.J.; Fuller, G.N.; Abi-Said, D.; Lang, F.F.; Gokaslan, Z.L.; Shi, W.M.; Wildrick, D.M.; Sawaya, R. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-Oncology 2001, 3, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gates, E.D.H.; Lin, J.S.; Weinberg, J.S.; Hamilton, J.; Prabhu, S.S.; Hazle, J.D.; Fuller, G.N.; Baladandayuthapani, V.; Fuentes, D.; Schellingerhout, D. Guiding the first biopsy in glioma patients using estimated Ki-67 maps derived from MRI: Conventional versus advanced imaging. Neuro-Oncology 2019, 21, 527–536. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Tesileanu, C.M.S.; Wick, W.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Erridge, S.; Vogelbaum, M.A.; Nowak, A.K.; Baurain, J.F.; et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): Second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 813–823. [Google Scholar] [CrossRef]
- Gurbani, S.S.; Yoon, Y.; Weinberg, B.D.; Salgado, E.; Press, R.H.; Cordova, J.S.; Ramesh, K.K.; Liang, Z.; Vega, J.V.; Voloschin, A.; et al. Assessing Treatment Response of Glioblastoma to an HDAC Inhibitor Using Whole-Brain Spectroscopic MRI. Tomography 2019, 5, 53–60. [Google Scholar] [CrossRef]
- Everix, L.; Seane, E.N.; Ebenhan, T.; Goethals, I.; Bolcaen, J. Introducing HDAC-Targeting Radiopharmaceuticals for Glioblastoma Imaging and Therapy. Pharmaceuticals 2023, 16, 227. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Miller, C.R.; Sarkaria, J.N.; Jaeckle, K.; Buckner, J.C.; Ligon, K.L.; Ballman, K.V.; Moore, D.F.; Nebozhyn, M.; et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro-Oncology 2018, 20, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Weis, J.A.; Luo, M.; Simpson, A.L.; Thompson, R.C.; Miga, M.I. Accounting for intraoperative brain shift ascribable to cavity collapse during intracranial tumor resection. J. Med. Imaging 2020, 7, 031506. [Google Scholar] [CrossRef] [PubMed]
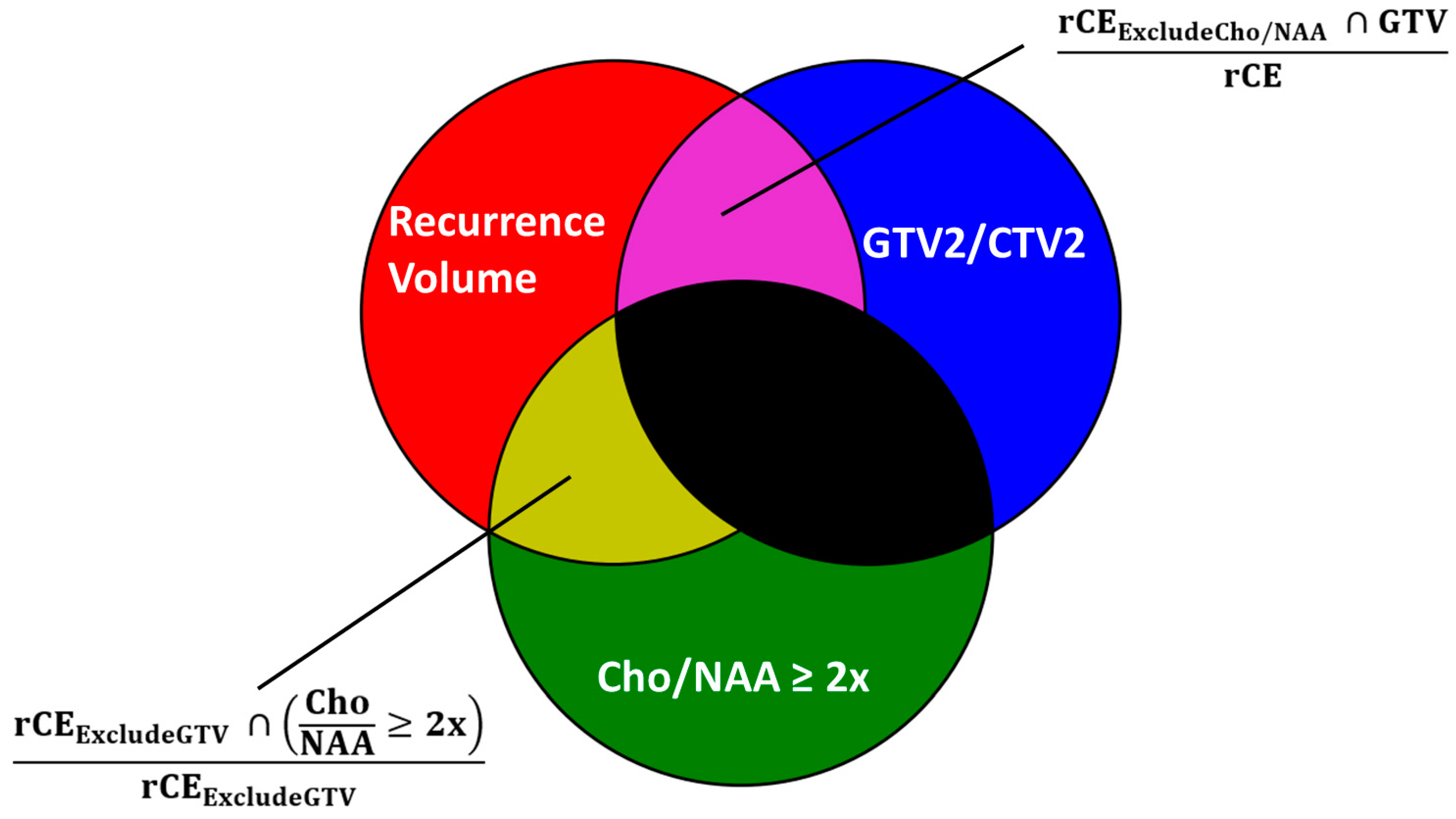
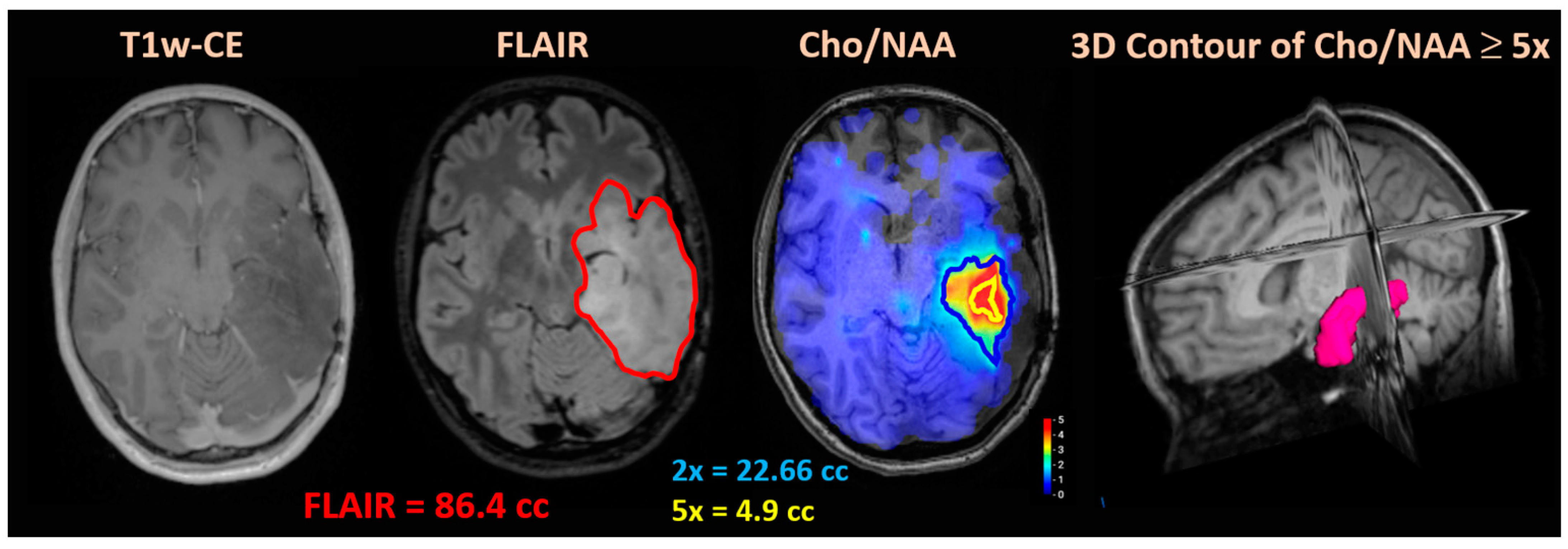

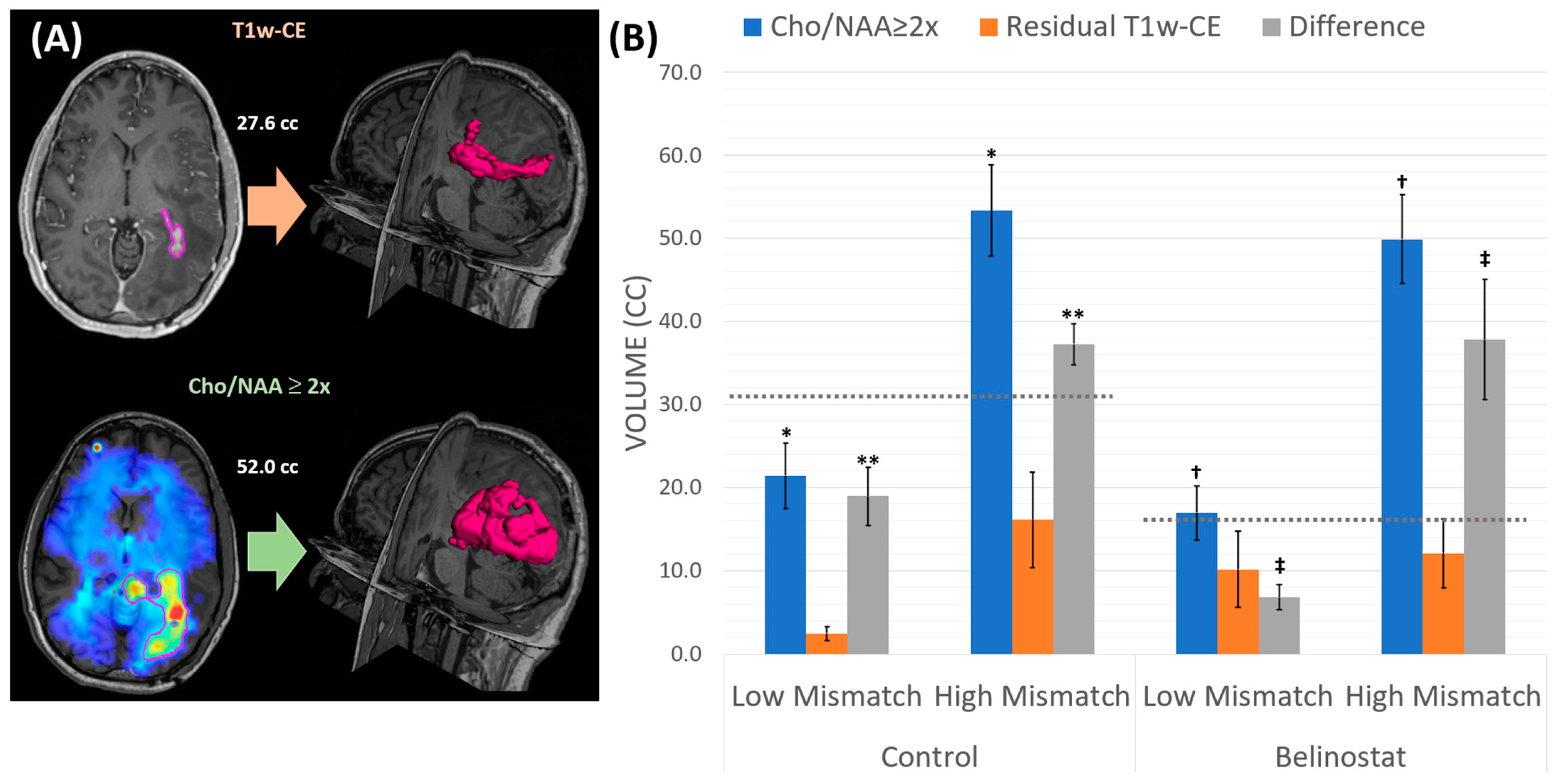

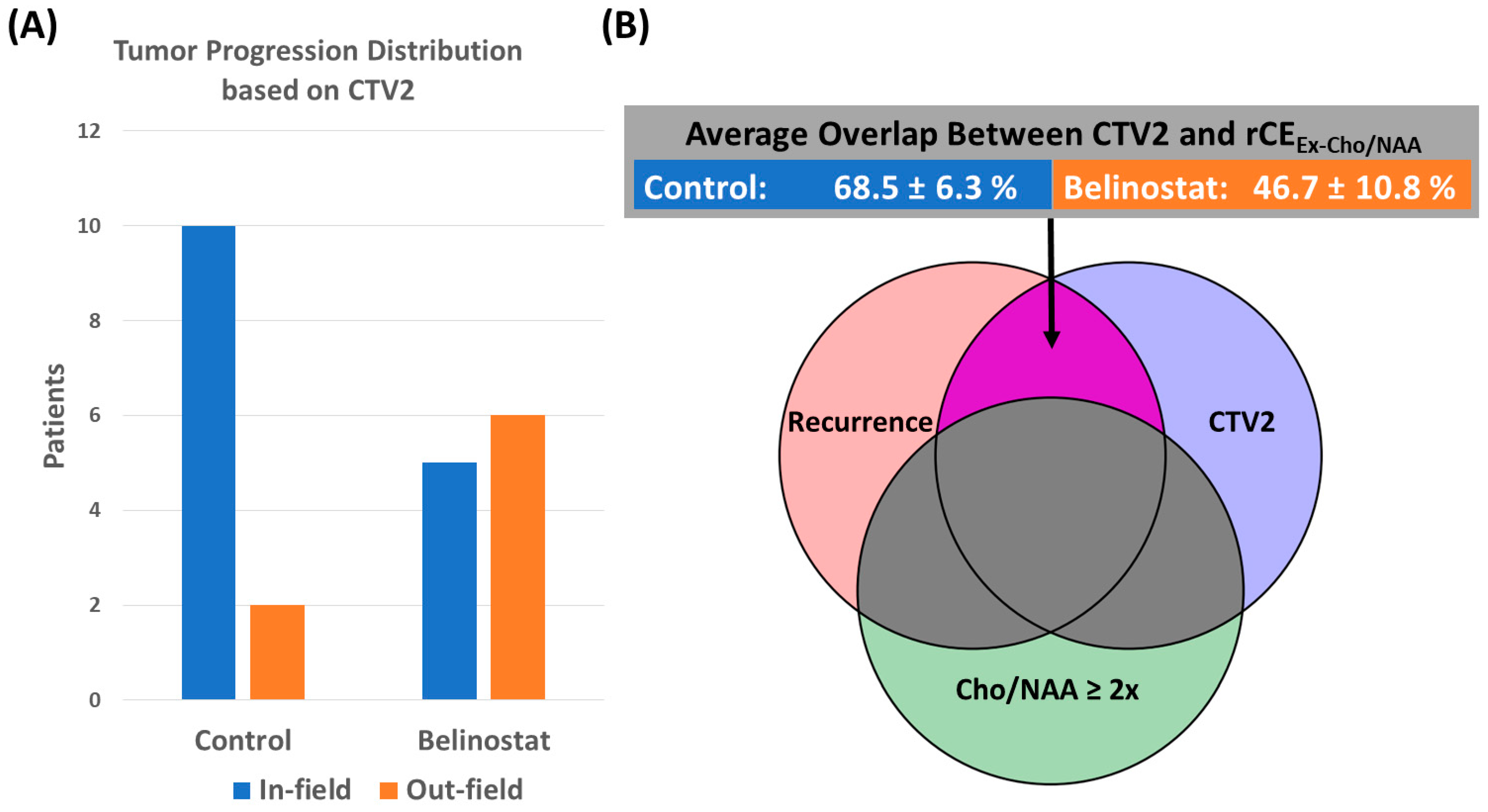

| Cohort | Mismatch | rCE (cc) | Cho/NAA ≥ 2x and rCEEx-GTV2 (%) | GTV2 and rCEEx-Cho/NAA (%) | Cho/NAA ≥ 2x and rCEEx-CTV2 (%) | CTV2 and rCEEx-Cho/NAA (%) |
|---|---|---|---|---|---|---|
| Control | Low | 14.7 ± 5.5 | 26.2 ± 13.6 | 58.6 ± 8.0 | 1.6 ± 1.5 | 71.8 ± 9.2 |
| High | 14.1 ± 7.1 | 25.3 ± 7.1 | 46.4 ± 6.7 | 4.9 ± 2.9 | 65.3 ± 8.3 | |
| Belinostat | Low | 9.8 ± 4.7 | 15.7 ± 5.2 | 22.5 ± 8.5 | 6.6 ± 2.7 | 40.6 ± 15.6 |
| High | 21.1 ± 9.5 | 18.8 ± 8.4 | 42.6 ± 15.2 | 7.7 ± 4.1 | 51.8 ± 14.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejimon, A.C.; Ramesh, K.K.; Trivedi, A.G.; Huang, V.; Schreibmann, E.; Weinberg, B.D.; Kleinberg, L.R.; Shu, H.-K.G.; Shim, H.; Olson, J.J. The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma. Tomography 2024, 10, 428-443. https://doi.org/10.3390/tomography10030033
Rejimon AC, Ramesh KK, Trivedi AG, Huang V, Schreibmann E, Weinberg BD, Kleinberg LR, Shu H-KG, Shim H, Olson JJ. The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma. Tomography. 2024; 10(3):428-443. https://doi.org/10.3390/tomography10030033
Chicago/Turabian StyleRejimon, Abinand C., Karthik K. Ramesh, Anuradha G. Trivedi, Vicki Huang, Eduard Schreibmann, Brent D. Weinberg, Lawrence R. Kleinberg, Hui-Kuo G. Shu, Hyunsuk Shim, and Jeffrey J. Olson. 2024. "The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma" Tomography 10, no. 3: 428-443. https://doi.org/10.3390/tomography10030033
APA StyleRejimon, A. C., Ramesh, K. K., Trivedi, A. G., Huang, V., Schreibmann, E., Weinberg, B. D., Kleinberg, L. R., Shu, H.-K. G., Shim, H., & Olson, J. J. (2024). The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma. Tomography, 10(3), 428-443. https://doi.org/10.3390/tomography10030033







