Left Ventricular Diastolic Dysfunction with Elevated Filling Pressures Is Associated with Embolic Stroke of Undetermined Source and Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
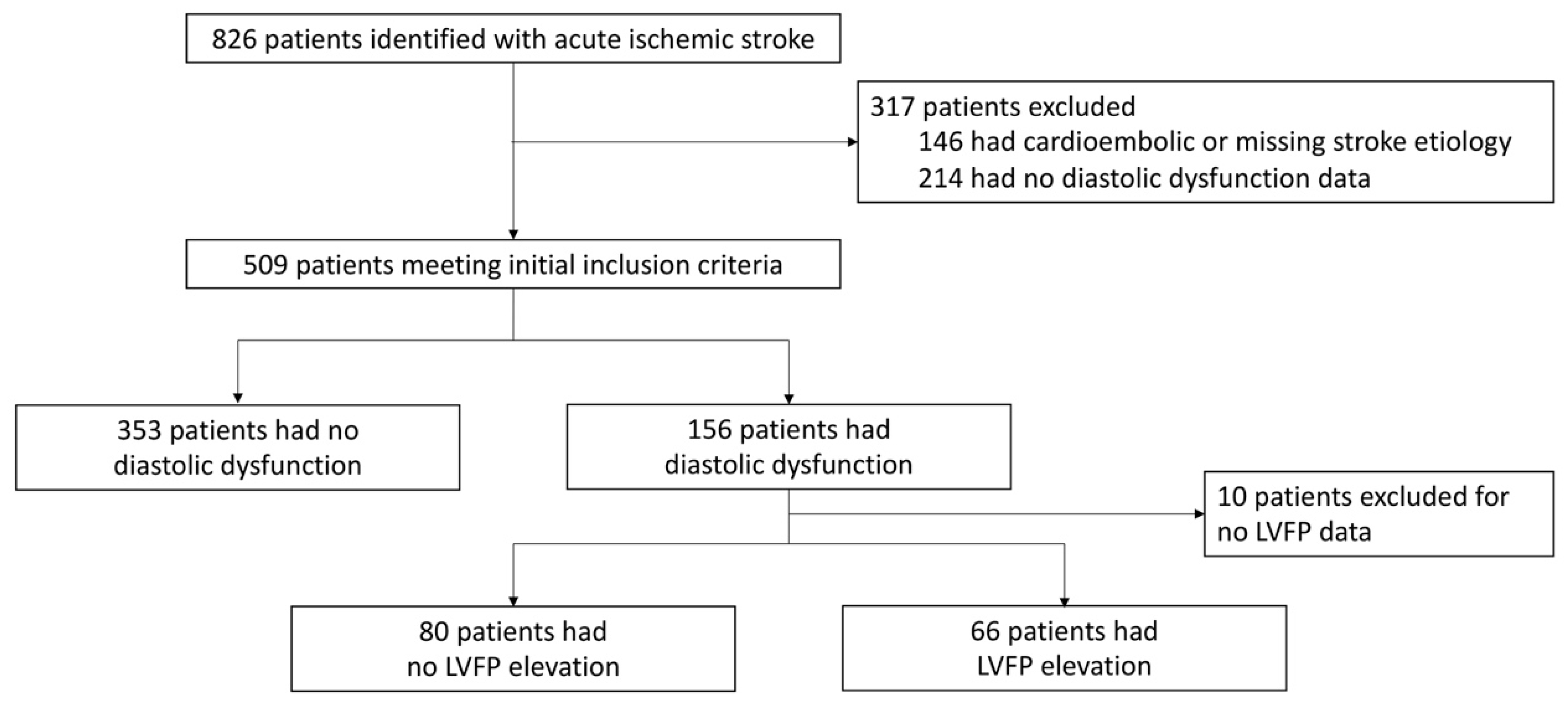
2.2. Patient Population
2.3. Patient Characteristics
2.4. Definition of LVDD and Elevated LVFP
2.5. Outcome
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. ESUS Association with LVDD and LVFP
3.3. AF Detection Association with LVDD and LVFP
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamel, H.; Merkler, A.E.; Iadecola, C.; Gupta, A.; Navi, B.B. Tailoring the Approach to Embolic Stroke of Undetermined Source: A Review. JAMA Neurol. 2019, 76, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Catanese, L.; Perera, K.S.; Ntaios, G.; Connolly, S.J. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke 2017, 48, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Bernstein, R.; Brachmann, J.; Camm, A.J.; Fromm, P.; Goto, S.; Granger, C.B.; Hohnloser, S.H.; Hylek, E.; Krieger, D.; et al. Reexamination of the Embolic Stroke of Undetermined Source Concept. Stroke 2021, 52, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Apostolakis, E.E.; Baikoussis, N.G.; Parissis, H.; Siminelakis, S.N.; Papadopoulos, G.S. Left ventricular diastolic dysfunction of the cardiac surgery patient; a point of view for the cardiac surgeon and cardio-anesthesiologist. J. Cardiothorac. Surg. 2009, 4, 67. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Gasiorek, P.; Wittczak, A.; Sakowicz, A.; Bytyçi, I.; Banach, M. Left Ventricular Diastolic Dysfunction as Predictor of Unfavorable Prognosis After ESUS. J. Multidiscip. Healthc. 2021, 14, 617–627. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Perera, K.S.; Vanassche, T.; Bosch, J.; Giruparajah, M.; Swaminathan, B.; Mattina, K.R.; Berkowitz, S.D.; Arauz, A.; O’Donnell, M.J.; Ameriso, S.F.; et al. Embolic strokes of undetermined source: Prevalence and patient features in the ESUS Global Registry. Int. J. Stroke 2016, 11, 526–533. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Fuster, V.; Ryden, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Heuzey, J.Y.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006, 114, e257–e354. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Ntaios, G.; Papavasileiou, V.; Milionis, H.; Makaritsis, K.; Manios, E.; Spengos, K.; Michel, P.; Vemmos, K. Embolic strokes of undetermined source in the Athens stroke registry: A descriptive analysis. Stroke 2015, 46, 176–181. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef]
- Wachter, R.; Gröschel, K.; Gelbrich, G.; Hamann, G.F.; Kermer, P.; Liman, J.; Seegers, J.; Wasser, K.; Schulte, A.; Jürries, F.; et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF(RANDOMISED)): An open-label randomised controlled trial. Lancet Neurol. 2017, 16, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Kosiuk, J.; Van Belle, Y.; Bode, K.; Kornej, J.; Arya, A.; Rolf, S.; Husser, D.; Hindricks, G.; Bollmann, A. Left ventricular diastolic dysfunction in atrial fibrillation: Predictors and relation with symptom severity. J. Cardiovasc. Electrophysiol. 2012, 23, 1073–1077. [Google Scholar] [CrossRef]
- Nagarakanti, R.; Ezekowitz, M. Diastolic dysfunction and atrial fibrillation. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2008, 22, 111–118. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythmia Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.J.; Prasad, S.B.; Marwick, T.H. Prognostic implications of left ventricular filling pressure with exercise. Circ. Cardiovasc. Imaging 2010, 3, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W., Jr.; Creswell, L.L.; Gutterman, D.D.; Fleisher, L.A. Epidemiology, mechanisms, and risks: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005, 128 (Suppl. S2), 9s–16s. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Achkar, M.; Laveau, F.; Boubrit, L.; Djebbar, M.; Allali, Y.; Komajda, M.; Isnard, R. Left atrial volume predicts abnormal exercise left ventricular filling pressure. Eur. J. Heart Fail. 2014, 16, 1089–1095. [Google Scholar] [CrossRef]
- Hammoudi, N.; Charbonnier, M.; Levy, P.; Djebbar, M.; Stankovic Stojanovic, K.; Ederhy, S.; Girot, R.; Cohen, A.; Isnard, R.; Lionnet, F. Left atrial volume is not an index of left ventricular diastolic dysfunction in patients with sickle cell anaemia. Arch. Cardiovasc. Dis. 2015, 108, 156–162. [Google Scholar] [CrossRef]
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shim, C.Y.; Park, J.H.; Nam, C.M.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J. Cardiol. 2016, 68, 104–109. [Google Scholar] [CrossRef]
- Jin, X.; Hummel, Y.M.; Tay, W.T.; Nauta, J.F.; Bamadhaj, N.S.S.; van Melle, J.P.; Lam, C.S.P.; Voors, A.A.; Hoendermis, E.S. Short- and long-term haemodynamic consequences of transcatheter closure of atrial septal defect and patent foramen ovale. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2021, 29, 402–408. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Haeusler, K.G.; Healey, J.S.; Freedman, B.; Boriani, G.; Brachmann, J.; Brandes, A.; Bustamante, A.; Casadei, B.; Crijns, H.; et al. Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation 2019, 140, 1834–1850. [Google Scholar] [CrossRef] [PubMed]
- Douen, A.G.; Pageau, N.; Medic, S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke 2008, 39, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.E.; Martin, P.J.; Ring, L.; Warburton, E.A.; Belham, M.; Pugh, P.J. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013, 80, 1546–1550. [Google Scholar] [CrossRef]
- Etgen, T.; Hochreiter, M.; Mundel, M.; Freudenberger, T. Insertable cardiac event recorder in detection of atrial fibrillation after cryptogenic stroke: An audit report. Stroke 2013, 44, 2007–2009. [Google Scholar] [CrossRef]
- Ritter, M.A.; Kochhäuser, S.; Duning, T.; Reinke, F.; Pott, C.; Dechering, D.G.; Eckardt, L.; Ringelstein, E.B. Occult atrial fibrillation in cryptogenic stroke: Detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013, 44, 1449–1452. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Hart, R.G.; Sharma, M.; Mundl, H.; Kasner, S.E.; Bangdiwala, S.I.; Berkowitz, S.D.; Swaminathan, B.; Lavados, P.; Wang, Y.; Wang, Y.; et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2018, 378, 2191–2201. [Google Scholar] [CrossRef]
- Diener, H.C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Healey, J.S.; Gladstone, D.J.; Swaminathan, B.; Eckstein, J.; Mundl, H.; Epstein, A.E.; Haeusler, K.G.; Mikulik, R.; Kasner, S.E.; Toni, D.; et al. Recurrent Stroke With Rivaroxaban Compared With Aspirin According to Predictors of Atrial Fibrillation: Secondary Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurol. 2019, 76, 764–773. [Google Scholar] [CrossRef]
- Kamel, H.; Longstreth, W.T., Jr.; Tirschwell, D.L.; Kronmal, R.A.; Marshall, R.S.; Broderick, J.P.; Aragon Garcia, R.; Plummer, P.; Sabagha, N.; Pauls, Q.; et al. Apixaban to Prevent Recurrence After Cryptogenic Stroke in Patients With Atrial Cardiopathy: The ARCADIA Randomized Clinical Trial. JAMA 2024, 331, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Sieweke, J.T.; Biber, S.; Weissenborn, K.; Heuschmann, P.U.; Akin, M.; Zauner, F.; Gabriel, M.M.; Schuppner, R.; Berliner, D.; Bauersachs, J.; et al. Septal total atrial conduction time for prediction of atrial fibrillation in embolic stroke of unknown source: A pilot study. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2020, 109, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kwong, C.; Ling, A.Y.; Crawford, M.H.; Zhao, S.X.; Shah, N.H. A Clinical Score for Predicting Atrial Fibrillation in Patients with Cryptogenic Stroke or Transient Ischemic Attack. Cardiology 2017, 138, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.X.; Ziegler, P.D.; Crawford, M.H.; Kwong, C.; Koehler, J.L.; Passman, R.S. Evaluation of a clinical score for predicting atrial fibrillation in cryptogenic stroke patients with insertable cardiac monitors: Results from the CRYSTAL AF study. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419842698. [Google Scholar] [CrossRef]
- Lee, J.S.; Shim, C.Y.; Wi, J.; Joung, B.; Ha, J.W.; Lee, M.H.; Pak, H.N. Left ventricular diastolic function is closely associated with mechanical function of the left atrium in patients with paroxysmal atrial fibrillation. Circ. J. 2013, 77, 697–704. [Google Scholar] [CrossRef]
| Variable | No LVDD (n = 353) | LVDD (n = 156) | p Value |
|---|---|---|---|
| Age (mean ± SD) | 60.88 ± 14.17 | 71.69 ± 12.31 | <0.001 |
| Sex (% female) | 157/353 (44.5%) | 73/156 (46.8%) | 0.628 |
| Hypertension (%) | 207/352 (58.8%) | 127/155 (81.9%) | <0.001 |
| Diabetes (%) | 75/352 (21.3%) | 51/155 (32.9%) | 0.005 |
| Hyperlipidemia (%) | 129/351 (36.8%) | 88/155 (56.8%) | <0.001 |
| Coronary Artery Disease (%) | 37/353 (10.5%) | 37/155 (23.9%) | <0.001 |
| Congestive Heart Failure (%) * | 2/352 (0.6%) | 10/155 (6.5%) | <0.001 |
| Renal Disease (%) | 15/352 (4.3%) | 13/155 (8.4%) | 0.061 |
| Active Tobacco Use (%) | 99/277 (35.7%) | 23/96 (24.0%) | 0.034 |
| Left Atrial Dilation (%) | 18/344 (5.2%) | 39/128 (30.5%) | <0.001 |
| Systolic Blood Pressure | 146.03 ± 27.04 | 154.29 ± 28.69 | 0.003 |
| NIHSS (median, IQR) | 7 (3–16) | 9 (4–17) | 0.121 |
| ESUS Stroke (%) | 141/353 (39.9%) | 88/156 (56.4%) | 0.001 |
| NCE Stroke (%) | 212/353 (60.1%) | 68/156 (43.6%) | 0.001 |
| Atrial Fibrillation Detection (%) | 17/228 (7.5%) | 17/103 (16.5%) | 0.012 |
| Wall Motion Abnormalities (%) | 29/338 (8.6%) | 39/139 (28.1%) | <0.001 |
| Tricuspid Regurgitation Velocity Max (median, IQR) | 2.38 (2.17–2.57) | 2.51 (2.27–2.86) | <0.001 |
| Left Ventricular Ejection Fraction (median, IQR) | 65 (60–67) | 65 (55–69) | 0.042 |
| Left Atrial Volume (median, IQR) | 36 (25–44) | 38 (29.5–55) | 0.287 |
| Left Atrial Volume Index (median, IQR) | 17 (13–22.5) | 19.5 (15–27) | 0.205 |
| Mitral Valve E/A Ratio (median, IQR) | 0.97 (0.76–1.2) | 0.84 (0.72–1.09) | 0.003 |
| Mitral Valve E/E′ Ratio Septal (median, IQR) | 10.38 (8.5–12.62) | 14.62 (10.78–18.38) | <0.001 |
| Mitral Valve E/E′ Ratio Lateral (median, IQR) | 7.9 (6.23–9.75) | 11.85 (8.91–15.32) | <0.001 |
| Mitral Valve E/E′ Ratio Mean (median, IQR) | 8.95 (7.31–10.92) | 12.89 (9.86–16.17) | <0.001 |
| Variable | No LVDD (n = 353) | LVDD with Normal LVFP (n = 80) | LVDD with Elevated LVFP (n = 66) | p Value |
|---|---|---|---|---|
| Age (mean ± SD) | 60.88 ± 14.17 | 69.81 ± 11.65 | 74.58 ± 12.45 | 0.062 |
| Sex (% female) | 157/353 (44.5%) | 30/80 (37.5%) | 39/66 (59.1%) | 0.028 |
| Hypertension (%) | 207/352 (58.8%) | 59/79 (74.7%) | 59/66 (89.4%) | <0.001 |
| Diabetes (%) | 75/352 (21.3%) | 23/79 (29.1%) | 23/66 (34.8%) | 0.035 |
| Hyperlipidemia (%) | 129/351 (36.8%) | 39/79 (49.4%) | 42/66 (63.6%) | <0.001 |
| Coronary Artery Disease (%) | 37/353 (10.5%) | 15/79 (19.0%) | 19/66 (28.8%) | <0.001 |
| Congestive Heart Failure (%) * | 2/352 (0.6%) | 3/79 (3.8%) | 7/66 (10.6%) | <0.001 |
| Renal Disease (%) | 15/352 (4.3%) | 5/79 (6.3%) | 7/66 (10.6%) | 0.105 |
| Active Tobacco Use (%) | 99/277 (35.7%) | 15/48 (31.3%) | 8/44 (18.2%) | 0.068 |
| Left Atrial Dilation (%) | 18/344 (5.2%) | 7/76 (9.2%) | 29/45 (64.4%) | <0.001 |
| Systolic Blood Pressure | 146.03 ± 27.04 | 151.34 ± 27.21 | 158.03 ± 30.26 | 0.491 |
| NIHSS (median, IQR) | 7 (3–16) | 7 (4–17) | 11 (4.5–17) | 0.019 |
| ESUS Stroke (%) | 141/353 (39.9%) | 34/80 (42.5%) | 45/66 (68.2%) | <0.001 |
| NCE Stroke (%) | 212/353 (60.1%) | 46/80 (57.5%) | 21/66 (31.8%) | <0.001 |
| Atrial Fibrillation Detection (%) | 17/228 (7.5%) | 5/43 (11.6%) | 12/51 (23.5%) | 0.003 |
| Wall Motion Abnormalities (%) | 29/338 (8.6%) | 20/75 (26.7%) | 16/54 (29.6%) | <0.001 |
| Tricuspid Regurgitation Velocity Max (median, IQR) | 2.38 (2.17–2.57) | 2.4 (2.21–2.63) | 2.77 (2.4–2.96) | <0.001 |
| Left Ventricular Ejection Fraction (median, IQR) | 65 (60–67) | 65 (55–69) | 62 (55–70) | 0.149 |
| Left Atrial Volume (median, IQR) | 36 (25–44) | 34 (20–43) | 43 (36–57) | 0.018 |
| Left Atrial Volume Index (median, IQR) | 17 (13–22.5) | 16 (12–21) | 21 (19–33) | 0.006 |
| Mitral Valve E/A Ratio (median, IQR) | 0.97 (0.76–1.2) | 0.75 (0.67–0.84) | 1.04 (0.85–1.27) | <0.001 |
| Mitral Valve E/E′ Ratio Septal (median, IQR) | 10.38 (8.5–12.62) | 12 (9.47–14.62) | 17.8 (15.4–19.5) | <0.001 |
| Mitral Valve E/E′ Ratio Lateral (median, IQR) | 7.9 (6.23–9.75) | 9.47 (8–11.9) | 14.7 (12.74–17.7) | <0.001 |
| Mitral Valve E/E′ Ratio Mean (median, IQR) | 8.95 (7.31–10.92) | 10.38 (8.53–12.89) | 15.68 (14–18.29) | <0.001 |
| No LVDD | LVDD | |
|---|---|---|
| ESUS | ||
| Unadjusted OR (95% CI) | Reference | 1.95 (1.33–2.85) p < 0.001 |
| Adjusted † OR (95% CI) | Reference | 1.43 (0.90–2.27) p = 0.130 |
| AF Detection | ||
| Unadjusted OR (95% CI) | Reference | 2.72 (1.14–6.48) p = 0.024 |
| Adjusted † OR (95% CI) | Reference | 1.88 (0.75–4.72) p = 0.179 |
| No LVDD | LVDD with Normal LVFP | LVDD with Elevated LVFP | |
|---|---|---|---|
| ESUS | |||
| Unadjusted OR (95% CI) | Reference | 1.11 (0.68–1.82) p = 0.674 | 3.22 (1.84–5.64) p < 0.001 |
| Adjusted § OR (95% CI) | Reference | 1.03 (0.61–1.77) p = 0.903 | 2.17 (0.99–4.77) p = 0.054 |
| AF Detection | |||
| Unadjusted OR (95% CI) | Reference | 1.63 (0.52–5.10) p = 0.403 | 5.86 (1.89–18.17) p = 0.002 |
| Adjusted § OR (95% CI) | Reference | 1.30 (0.40–4.20) p = 0.658 | 3.95 (1.07–12.06) p = 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, Z.; Shu, L.; Guo, Y.; Chen, E.W.; Wang, S.; Goldstein, E.D.; Rana, M.; Kala, N.; Dai, X.; Mandel, D.; et al. Left Ventricular Diastolic Dysfunction with Elevated Filling Pressures Is Associated with Embolic Stroke of Undetermined Source and Atrial Fibrillation. Tomography 2024, 10, 1694-1705. https://doi.org/10.3390/tomography10100124
Bashir Z, Shu L, Guo Y, Chen EW, Wang S, Goldstein ED, Rana M, Kala N, Dai X, Mandel D, et al. Left Ventricular Diastolic Dysfunction with Elevated Filling Pressures Is Associated with Embolic Stroke of Undetermined Source and Atrial Fibrillation. Tomography. 2024; 10(10):1694-1705. https://doi.org/10.3390/tomography10100124
Chicago/Turabian StyleBashir, Zubair, Liqi Shu, Yuqian Guo, Edward W. Chen, Shuyuan Wang, Eric D. Goldstein, Maheen Rana, Narendra Kala, Xing Dai, Daniel Mandel, and et al. 2024. "Left Ventricular Diastolic Dysfunction with Elevated Filling Pressures Is Associated with Embolic Stroke of Undetermined Source and Atrial Fibrillation" Tomography 10, no. 10: 1694-1705. https://doi.org/10.3390/tomography10100124
APA StyleBashir, Z., Shu, L., Guo, Y., Chen, E. W., Wang, S., Goldstein, E. D., Rana, M., Kala, N., Dai, X., Mandel, D., Yaghi, S., Has, P., Xie, M., Wang, T., Simmons, J., Song, C., & Haines, P. (2024). Left Ventricular Diastolic Dysfunction with Elevated Filling Pressures Is Associated with Embolic Stroke of Undetermined Source and Atrial Fibrillation. Tomography, 10(10), 1694-1705. https://doi.org/10.3390/tomography10100124





