Residual Lung Abnormalities in Survivors of Severe or Critical COVID-19 at One-Year Follow-Up Computed Tomography: A Narrative Review Comparing the European and East Asian Experiences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
3. Results
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cerqua, A.; Di Stefano, R. When Did Coronavirus Arrive in Europe? Stat. Methods Appl. 2022, 31, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Maroldi, R. COVID-19 Outbreak in Italy: Experimental Chest X-Ray Scoring System for Quantifying and Monitoring Disease Progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Europe—Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 7 October 2023).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on Covid-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Hwang, M.; Kim, Y.-H.; Chung, M.J.; Sim, B.H.; Chae, K.J.; Yoo, J.Y.; Jeong, Y.J. Imaging and Clinical Features of COVID-19 Breakthrough Infections: A Multicenter Study. Radiology 2022, 303, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Maroldi, R. Vaccination and Reduced Severity of COVID-19 Pneumonia Viewed at Chest Radiography. Radiology 2022, 304, E47. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and Safety of COVID-19 Vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- So, M.; Kabata, H.; Fukunaga, K.; Takagi, H.; Kuno, T. Radiological and Functional Lung Sequelae of COVID-19: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2021, 21, 97. [Google Scholar] [CrossRef]
- Besutti, G.; Monelli, F.; Schirò, S.; Milone, F.; Ottone, M.; Spaggiari, L.; Facciolongo, N.; Salvarani, C.; Croci, S.; Pattacini, P.; et al. Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study. Tomography 2022, 8, 97. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Zhang, X.; Jia, X.; Zheng, Y.; Shi, H. Fibrotic Interstitial Lung Abnormalities at 1-Year Follow-up CT after Severe COVID-19. Radiology 2021, 301, E438–E440. [Google Scholar] [CrossRef]
- Pan, F.; Yang, L.; Liang, B.; Ye, T.; Li, L.; Li, L.; Liu, D.; Wang, J.; Hesketh, R.L.; Zheng, C. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19. Radiology 2022, 302, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Wi, Y.M. Residual Lung Lesions at 1-Year CT after COVID-19. Radiology 2022, 302, 720–721. [Google Scholar] [CrossRef] [PubMed]
- Luger, A.K.; Sonnweber, T.; Gruber, L.; Schwabl, C.; Cima, K.; Tymoszuk, P.; Gerstner, A.K.; Pizzini, A.; Sahanic, S.; Boehm, A.; et al. Chest CT of Lung Injury 1 Year after COVID-19 Pneumonia: The CovILD Study. Radiology 2022, 304, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Marando, M.; Fusi-Schmidhauser, T.; Tamburello, A.; Grazioli Gauthier, L.; Rigamonti, E.; Argentieri, G.; Puligheddu, C.; Pagnamenta, A.; Valenti, A.; Pons, M.; et al. 1-Year Radiological, Functional and Quality-of-Life Outcomes in Patients with SARS-CoV-2 Pneumonia—A Prospective Observational Study. NPJ Prim. Care Respir. Med. 2022, 32, 8. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Belletti, A.; Palumbo, D.; Calvi, M.R.; Guzzo, F.; Fominskiy, E.V.; Ortalda, A.; Nardelli, P.; Ripa, M.; Baiardo Redaelli, M.; et al. One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Carvalho, C.R.; Lamas, C.A.; Chate, R.C.; Salge, J.M.; Sawamura, M.V.Y.; De Albuquerque, A.L.P.; Toufen Junior, C.; Lima, D.M.; Garcia, M.L.; Scudeller, P.G.; et al. Long-Term Respiratory Follow-up of ICU Hospitalized COVID-19 Patients: Prospective Cohort Study. PLoS ONE 2023, 18, e0280567. [Google Scholar] [CrossRef]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-Term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology 2023, 306, e221806. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Xiao, M.; Guan, X.; Lei, Y.; Diao, T.; Long, P.; Zeng, R.; Lai, X.; Cai, H.; et al. Long-Term Outcomes of COVID-19 Convalescents: An 18.5-Month Longitudinal Study in Wuhan. Int. J. Infect. Dis. 2023, 127, 85–92. [Google Scholar] [CrossRef]
- Zhou, F.; Tao, M.; Shang, L.; Liu, Y.; Pan, G.; Jin, Y.; Wang, L.; Hu, S.; Li, J.; Zhang, M.; et al. Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis. Front. Med. 2021, 8, 717194. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health Outcomes in People 2 Years after Surviving Hospitalisation with COVID-19: A Longitudinal Cohort Study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Tonkin, J.; Devaraj, A.; Philip, K.E.J.; Orton, C.M.; Desai, S.R.; Shah, P.L. CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge. Radiology 2022, 303, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Lieto, R.; Romano, F.; Sica, G.; Bocchini, G.; Muto, E.; Capitelli, L.; Sequino, D.; Valente, T.; Fiorentino, G.; et al. Chest CT–Based Assessment of 1-Year Outcomes after Moderate COVID-19 Pneumonia. Radiology 2022, 305, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Van Raaij, B.F.M.; Stöger, J.L.; Hinnen, C.; Penfornis, K.M.; De Jong, C.M.M.; Klok, F.A.; Roukens, A.H.E.; Veldhuijzen, D.S.; Arbous, M.S.; Noordam, R.; et al. Fibrotic-like Abnormalities Notably Prevalent One Year after Hospitalization with COVID-19. Respir. Med. Res. 2022, 82, 100973. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year Follow-up CT Findings in COVID -19 Patients: A Systematic Review and Meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Baricich, A.; Patrucco, F.; Zeppegno, P.; Gramaglia, C.; Balbo, P.E.; Carriero, A.; Amico, C.S.; Avanzi, G.C.; Barini, M.; et al. Long-Term Sequelae Are Highly Prevalent One Year after Hospitalization for Severe COVID-19. Sci. Rep. 2021, 11, 22666. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Caroli, A.; Bonaffini, P.A.; Conti, C.; Arrigoni, A.; Mercanzin, E.; Imeri, G.; Anelli, M.; Balbi, M.; Pace, M.; et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022, 8, 216. [Google Scholar] [CrossRef]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-Year Pulmonary Impairment after Severe COVID-19: A Prospective, Multicenter Follow-up Study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, C.; Yu, L.; Guo, W.; Feng, X.; Yu, L.; Su, J.; Xu, T.; Ren, C.; Shi, D.; et al. One-Year Follow-up of Chest CT Findings in Patients after SARS-CoV-2 Infection. BMC Med. 2021, 19, 191. [Google Scholar] [CrossRef]
- Lee, J.H.; Yim, J.-J.; Park, J. Pulmonary Function and Chest Computed Tomography Abnormalities 6–12 Months after Recovery from COVID-19: A Systematic Review and Meta-Analysis. Respir. Res. 2022, 23, 233. [Google Scholar] [CrossRef]
- Lenoir, A.; Christe, A.; Ebner, L.; Beigelman-Aubry, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Erkosar, B.; Garzoni, C.; Geiser, T.; et al. Pulmonary Recovery 12 Months after Non-Severe and Severe COVID-19: The Prospective Swiss COVID-19 Lung Study. Respiration 2023, 102, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Tarraso, J.; Safont, B.; Carbonell-Asins, J.A.; Fernandez-Fabrellas, E.; Sancho-Chust, J.N.; Naval, E.; Amat, B.; Herrera, S.; Ros, J.A.; Soler-Cataluña, J.J.; et al. Lung Function and Radiological Findings 1 Year after COVID-19: A Prospective Follow-Up. Respir. Res. 2022, 23, 242. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Zuil, M.; Benítez, I.D.; De Gonzalo-Calvo, D.; Aguilar, M.; Santisteve, S.; Vaca, R.; Minguez, O.; Seck, F.; Torres, G.; et al. One Year Overview and Follow-Up in a Post-COVID Consultation of Critically Ill Patients. Front. Med. 2022, 9, 897990. [Google Scholar] [CrossRef] [PubMed]
- Lorent, N.; Vande Weygaerde, Y.; Claeys, E.; Guler Caamano Fajardo, I.; De Vos, N.; De Wever, W.; Salhi, B.; Gyselinck, I.; Bosteels, C.; Lambrecht, B.N.; et al. Prospective Longitudinal Evaluation of Hospitalised COVID-19 Survivors 3 and 12 Months after Discharge. ERJ Open Res. 2022, 8, 00004–02022. [Google Scholar] [CrossRef] [PubMed]
- Eberst, G.; Claudé, F.; Laurent, L.; Meurisse, A.; Roux-Claudé, P.; Barnig, C.; Vernerey, D.; Paget-Bailly, S.; Bouiller, K.; Chirouze, C.; et al. Result of One-Year, Prospective Follow-up of Intensive Care Unit Survivors after SARS-CoV-2 Pneumonia. Ann. Intensive Care 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, D.; Mei, N.; Yin, B.; Li, X.; Zheng, Y.; Xiao, A.; Yu, X.; Qiu, X.; Lu, Y.; et al. Longitudinal Radiological Findings in Patients With COVID-19 With Different Severities: From Onset to Long-Term Follow-Up After Discharge. Front. Med. 2021, 8, 711435. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhou, Y.; Yu, H.; Li, R.; Zhan, Q.; Ni, F.; Fang, S.; Lu, Y.; Ding, X.; et al. 3-Month, 6-Month, 9-Month, and 12-Month Respiratory Outcomes in Patients Following COVID-19-Related Hospitalisation: A Prospective Study. Lancet Respir. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Gamberini, L.; Mazzoli, C.A.; Prediletto, I.; Sintonen, H.; Scaramuzzo, G.; Allegri, D.; Colombo, D.; Tonetti, T.; Zani, G.; Capozzi, C.; et al. Health-Related Quality of Life Profiles, Trajectories, Persistent Symptoms and Pulmonary Function One Year after ICU Discharge in Invasively Ventilated COVID-19 Patients, a Prospective Follow-up Study. Respir. Med. 2021, 189, 106665. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Huang, J.; Alwalid, O.; Jia, X.; Yuan, M.; Cao, Y.; Shao, G.; Cui, Y.; Liu, J.; et al. Follow-up Study of Pulmonary Sequelae in Discharged COVID-19 Patients with Diabetes or Secondary Hyperglycemia. Eur. J. Radiol. 2021, 144, 109997. [Google Scholar] [CrossRef]
- Bernardinello, N.; Cocconcelli, E.; Giraudo, C.; Daverio, M.; Castelli, G.; Petrarulo, S.; Bovo, M.; Fichera, G.; Cavinato, S.; Cattelan, A.M.; et al. Predictors of Pulmonary Sequelae after COVID-19 Pneumonia: A 12-Month Follow-up Study. Front. Med. 2023, 10, 1084002. [Google Scholar] [CrossRef]
- Martino, G.P.; Benfaremo, D.; Bitti, G.; Valeri, G.; Postacchini, L.; Marchetti, A.; Angelici, S.; Moroncini, G. 6 and 12 Month Outcomes in Patients Following COVID-19-Related Hospitalization: A Prospective Monocentric Study. Intern. Emerg. Med. 2022, 17, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liao, X.; Ma, Z.; Zhang, L.; Dong, J.; Zheng, G.; Zi, M.; Peng, W.; Wei, L.; Li, Z.; et al. Clinical Status of Patients 1 Year after Hospital Discharge Following Recovery from COVID-19: A Prospective Cohort Study. Ann. Intensive Care 2022, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Meng, D.; Xiong, L.; Wu, S.; Yang, L.; Wang, S.; Zhou, M.; He, X.; Cao, X.; Xiong, H.; et al. Long-Term Effects of COVID-19 on Health Care Workers 1-Year Post-Discharge in Wuhan. Infect. Dis. Ther. 2022, 11, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, C.; An, X.; Xiong, Y.; Shang, Y.; He, J.; Qiu, Y.; Zhang, N.; Huang, L.; Jia, J.; et al. Follow-up Study on COVID-19 Survivors One Year after Discharge from Hospital. Int. J. Infect. Dis. 2021, 112, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, L.; Fan, Y.; Alwalid, O.; Jia, X.; Zheng, Y.; Liu, J.; Li, Y.; Cao, Y.; Gu, J.; et al. Longitudinal Assessment of Chest CT Findings and Pulmonary Function after COVID-19 Infection. Radiology 2023, 307, e222888. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, S.; Kwee, A.K.A.L.; Houweling, L.; De Wit-van Wijck, Y.; Mohamed Hoesein, F.A.A.; Downward, G.S.; Nossent, E.J.; Maitland-van Der Zee, A.H. A Systematic Review of Chest Imaging Findings in Long COVID Patients. JPM 2023, 13, 282. [Google Scholar] [CrossRef]
- Fabbri, L.; Moss, S.; Khan, F.A.; Chi, W.; Xia, J.; Robinson, K.; Smyth, A.R.; Jenkins, G.; Stewart, I. Parenchymal Lung Abnormalities Following Hospitalisation for COVID-19 and Viral Pneumonitis: A Systematic Review and Meta-analysis. Thorax 2023, 78, 191–201. [Google Scholar] [CrossRef]
- Bocchino, M.; Rea, G.; Capitelli, L.; Lieto, R.; Bruzzese, D. Chest CT Lung Abnormalities 1 Year after COVID-19: A Systematic Review and Meta-Analysis. Radiology 2023, 308, e230535. [Google Scholar] [CrossRef]
- WHO. Clinical Management of COVID-19: Living Guideline, 18 August 2023; World Health Organization: Geneva, Switzerland, 2023; (WHO/2019-nCoV/clinical/2023.2). [Google Scholar]
- Yamamoto, N.; Bauer, G. Apparent Difference in Fatalities between Central Europe and East Asia Due to SARS-COV-2 and COVID-19: Four Hypotheses for Possible Explanation. Med. Hypotheses 2020, 144, 110160. [Google Scholar] [CrossRef]
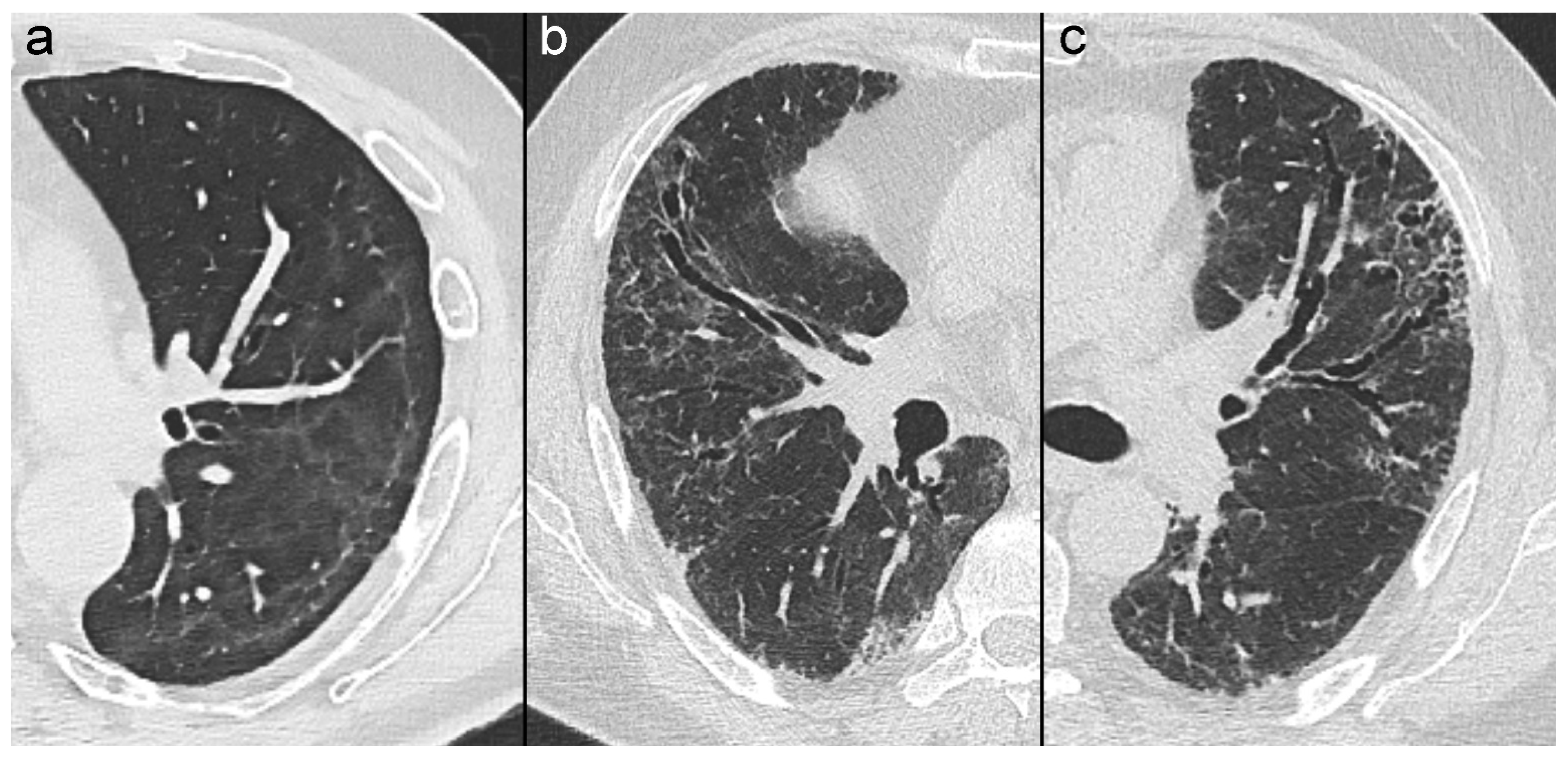

| First Author | Date † | Country | Design | Study Patient | Severe or Critical COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Age * (Years) | Men | No. | Age * (Years) | Men | ||||
| Gamberini [39] | September 2021 | Italy | Prospective/MC | 178 | 64 | 129 (72) | 178 (100) | 64 | 129 (72) |
| Eberst [36] | September 2021 | France | Prospective/SC | 85 | 68 | 67 (79) | 85 (100) | 68 | 67 (79) |
| Martino [42] | September 2021 | Italy | Prospective/SC | 64 | 68 | 41 (64) | 64 (100) | 68 | 41 (64) |
| Faverio [29] | October 2021 | Italy | Prospective/MC | 287 | 61 | 213 (74) | 90 (31) ^ | 60 | 74 (82) |
| Lorent [35] | January 2022 | Belgium | Prospective/MC | 299 | 59 | 205 (69) | 94 (31) | 60 | 78 (83) |
| Gonzalez [34] | March 2022 | Spain | Prospective/MC | 181 | 61 | 121 (67) | 181 (100) | 61 | 121 (67) |
| Tarraso [33] | May 2022 | Spain | Prospective/MC | 284 | 61 | 157 (55) | 52 (18) | 63 | 38 (73) |
| Van Raaij [25] | August 2022 | Netherlands | Prospective/SC | 66 | 61 | 46 (70) | 28 (42) | 60 | 19 (68) |
| Corsi [28] | September 2022 | Italy | Retrospective/SC | 71 | 66 | 45 (63) | 71 (100) | 66 | 45 (63) |
| First Author | Date † | Country | Design | Study Patient | Severe or Critical COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Age * (Years) | Men | No. | Age * (Years) | Men | ||||
| Han [11] | April 2021 | China | Prospective/MC | 62 | 57 | 34 (55) | 62 (100) | 57 | 34 (55) |
| Wu [38] | May 2021 | China | Prospective/SC | 83 | 60 | 47 (57) | 83 (100) | 60 | 47 (57) |
| Zhou [20] | May 2021 | China | Prospective/MC | 120 | 52 | 49 (41) | 16 (13) | 53 | 8 (50) |
| Zhao [45] | July 2021 | China | Prospective/SC | 94 | 48 | 54 (57) | 43 (46) | 51 | 29 (67) |
| Huang [21] | August 2021 | China | Prospective/SC | 1276 | 59 | 681 (53) | 94 (7) | 58 | 63 (67) |
| Liao [44] | September 2021 | China | Prospective/SC | 303 | 39 | 59 (19) | 190 (63) | 39 | 37 (19) |
| Li [43] | January 2022 | China | Prospective/SC | 230 | 46 | 116 (50) | 52 (23) | 55 | 33 (63) |
| First Author | Patients with 1-Year CT Follow-Up | CT Lung Abnormalities at 1-Year Follow-Up after Severe or Critical COVID-19 | ||||||
|---|---|---|---|---|---|---|---|---|
| Any | GGOs | Reticular Opacities | Linear Opacities | Consolidation | Bronchiectasis (+/− Traction) | Honeycomb | ||
| Gamberini [39] ° | 37 (21) | NA | 21 (57) | 13 (35) | 26 (70) | 3 (8) | 10 (27) | 3 (8) |
| Eberst [36] * | 64 (75) | 60 (94) | 32 (50) | 51 (80) | NA | NA | 44 (69) | 3 (5) |
| Martino [42] ^ | 47 (73) | 30 (64) | 7 (15) | 19 (40) | 5 (11) | 7 (15) | 4 (9) | 2 (4) |
| Faverio [29] * | 85 (94) | 68 (80) | 60 (71) | 42 (49) | NA | 2 (2) | 24 (28) | 1 (1) |
| Lorent [35] † | 57 (61) | 40 (65) | 21 (37) | 36 (63) | NA | 0 (0) | 14 (25) | NA |
| Gonzalez [34] ° | 41 (23) | 41 (100) | 27 (66) | 22 (54) | 41 (100) | 3 (7) | 37 (90) | NA |
| Tarraso [33] * | 57 (100) | 54 (95) | 31 (54) | 23 (40) | 32 (56) | 11 (19) | 28 (49) | NA |
| Van Raaij [25] * | 26 (93) | 21 (81) | 11 (42) | 10 (39) | 19 (73) | 2 (8) | 16 (62) | NA |
| Corsi [28] ^ | 63 (89) | 48 (76) | 2 (3) | 38 (60) | NA | 2 (3) | 42 (67) | NA |
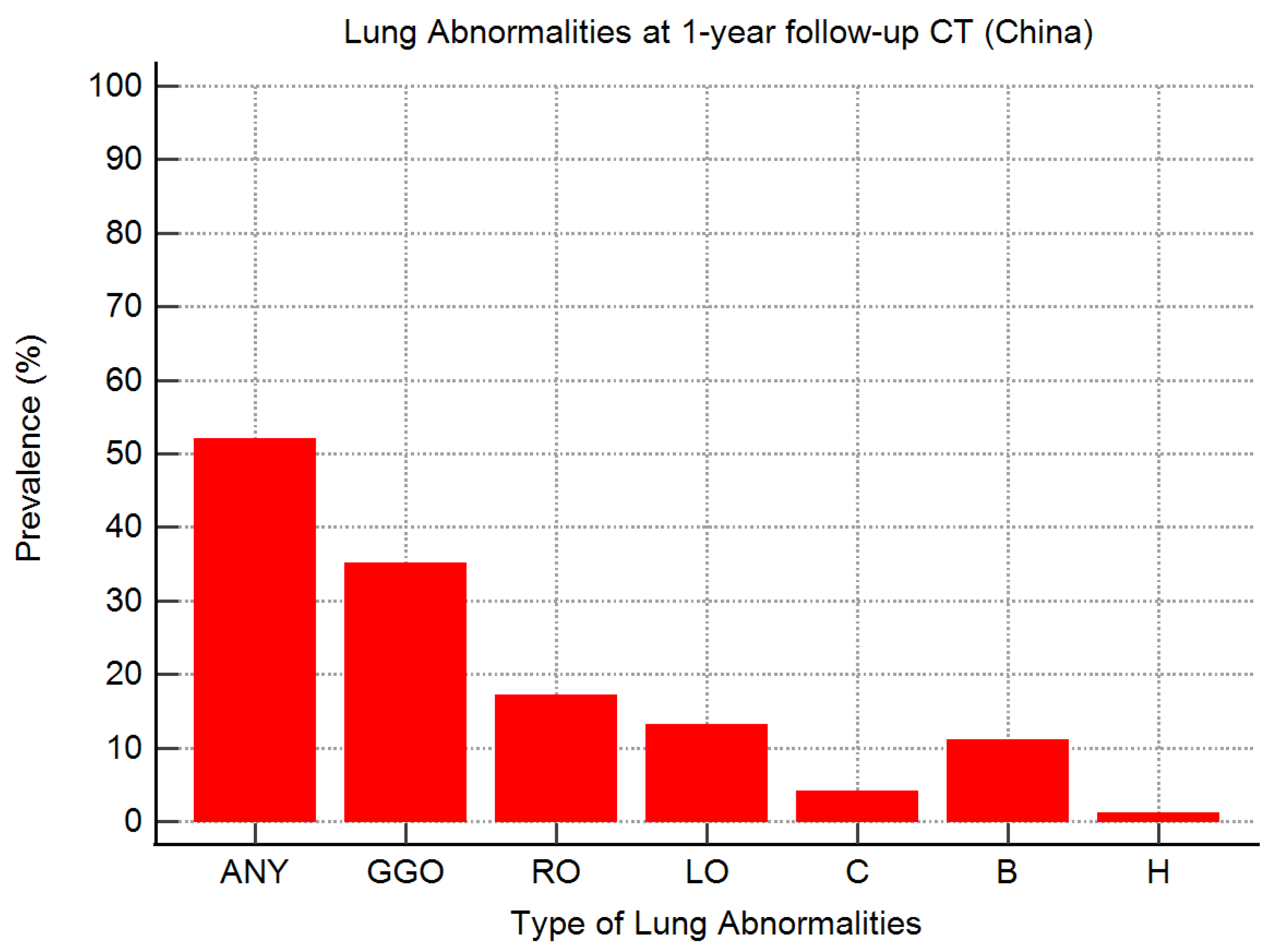
| First Author | Patients with 1-Year CT Follow-Up | CT Lung Abnormalities at 1-Year Follow-Up after Severe or Critical COVID-19 | ||||||
|---|---|---|---|---|---|---|---|---|
| Any | GGOs | Reticular Opacities | Linear Opacities | Consolidation | Bronchiectasis (+/− Traction) | Honeycomb | ||
| Han [11] * | 62 (100) | 45 (73) | 7 (11) | 32 (52) | NA | 6 (10) | 27 (44) | NA |
| Wu [38] * | 83 (100) | 20 (24) | 19 (23) | 3 (4) | 5 (6) | 0 (0) | 1 (1) | 0 (0) |
| Zhou [20] ^ | 14 (88) | 8 (57) | 5 (36) | NA | 5 (36) | NA | 1 (7) | NA |
| Zhao [45] † | 43 (100) | NA | 20 (47) | 3 (7) | 8 (19) | 2 (5) | NA | NA |
| Huang [21] ^ | 38 (40) | 33 (87) | 29 (76) | 3 (8) | 23 (61) | 1 (3) | NA | NA |
| Liao [44] ° | 158 (83) | 63 (40) | 43 (27) | 2 (1) | 5 (3) | 7 (4) | 2 (1) | 0 (0) |
| Li [43] ° | 48 (92) | 41 (85) | 35 (73) | 29 (60) | 3 (6) | 1 (2) | 8 (17) | 2 (4) |
| First Author | Patients with 1-Year CT Follow-Up | Extent of CT Lung Abnormalities at 1-Year Follow-Up | |
|---|---|---|---|
| Visual Assessment (Percentage) | Software-Based Analysis (Percentage) | ||
| Martino [42] | 47 | 5 (0–10) | - |
| Van Raaij [25] | 26 | 11 (4–26) | - |
| Corsi [28] | 63 | - | 12 (9–16) |
| Zhou [20] | 14 | - | 0 (0–0.02) |
| Liao [44] | 158 | - | 0 (0–0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghesi, A.; Ciolli, P.; Antonelli, E.; Monti, A.; Scrimieri, A.; Ravanelli, M.; Maroldi, R.; Farina, D. Residual Lung Abnormalities in Survivors of Severe or Critical COVID-19 at One-Year Follow-Up Computed Tomography: A Narrative Review Comparing the European and East Asian Experiences. Tomography 2024, 10, 25-36. https://doi.org/10.3390/tomography10010003
Borghesi A, Ciolli P, Antonelli E, Monti A, Scrimieri A, Ravanelli M, Maroldi R, Farina D. Residual Lung Abnormalities in Survivors of Severe or Critical COVID-19 at One-Year Follow-Up Computed Tomography: A Narrative Review Comparing the European and East Asian Experiences. Tomography. 2024; 10(1):25-36. https://doi.org/10.3390/tomography10010003
Chicago/Turabian StyleBorghesi, Andrea, Pietro Ciolli, Elisabetta Antonelli, Alessandro Monti, Alessandra Scrimieri, Marco Ravanelli, Roberto Maroldi, and Davide Farina. 2024. "Residual Lung Abnormalities in Survivors of Severe or Critical COVID-19 at One-Year Follow-Up Computed Tomography: A Narrative Review Comparing the European and East Asian Experiences" Tomography 10, no. 1: 25-36. https://doi.org/10.3390/tomography10010003
APA StyleBorghesi, A., Ciolli, P., Antonelli, E., Monti, A., Scrimieri, A., Ravanelli, M., Maroldi, R., & Farina, D. (2024). Residual Lung Abnormalities in Survivors of Severe or Critical COVID-19 at One-Year Follow-Up Computed Tomography: A Narrative Review Comparing the European and East Asian Experiences. Tomography, 10(1), 25-36. https://doi.org/10.3390/tomography10010003






