Accelerated Hypofractionated Magnetic Resonance Guided Adaptive Radiation Therapy for Ultracentral Lung Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Accelerated Hypofractionated MRgART
2.2.1. Radiation Therapy Simulation
2.2.2. Targets and Organ-at-Risk Contouring
2.2.3. Treatment Planning and Delivery
2.3. Analysis of Dosimetric and Clinical Outcomes
3. Results
3.1. Patient Characteristics
3.2. MR Linac Treatment
3.3. Online Plan Adaptation
3.4. Locoregional Control
3.5. Acute Toxicity
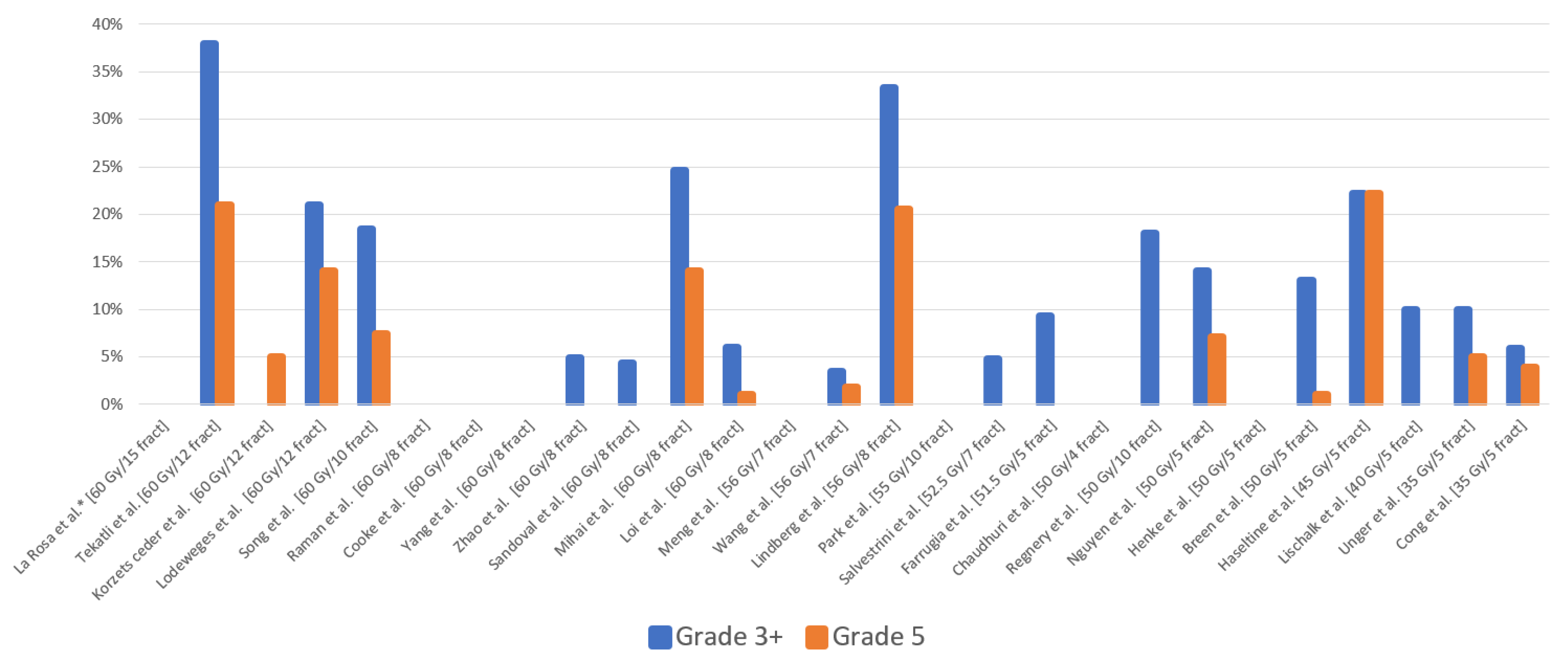
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, A.; Mittauer, K.E.; Chuong, M.D.; Hall, M.D.; Kutuk, T.; Bassiri, N.; McCulloch, J.; Alvarez, D.; Herrera, R.; Gutierrez, A.N.; et al. Accelerated hypofractionated magnetic resonance-guided adaptive radiotherapy for oligoprogressive non-small cell lung cancer. Med. Dosim. 2023, 48, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Lin, S.H.; Wei, C.; Allen, P.; Cox, J.D.; Komaki, R. Accelerated hypofractionated radiation therapy compared to conventionally fractionated radiation therapy for the treatment of inoperable non-small cell lung cancer. Radiat. Oncol. 2012, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Zhang-Velten, E.; Court, L.; Westover, K.; Yan, Y.; Lin, M.H.; Xiong, Z.; Patel, M.; Rivera, D.; Chang, J.; et al. Accelerated Hypofractionated Image-Guided vs. Conventional Radiotherapy for Patients with Stage II/III Non-Small Cell Lung Cancer and Poor Performance Status: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1497–1505. [Google Scholar] [CrossRef]
- Noel, C.E.; Parikh, P.J.; Spencer, C.R.; Green, O.L.; Hu, Y.; Mutic, S.; Olsen, J.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015, 54, 1474–1482. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Tang, C.; Binkley, M.S.; Jin, M.; Wynne, J.F.; von Eyben, R.; Hara, W.Y.; Trakul, N.; Loo, B.W., Jr.; Diehn, M. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Mittauer, K.E.; Hill, P.M.; Bassetti, M.F.; Bayouth, J.E. Validation of an MR-guided online adaptive radiotherapy (MRgoART) program: Deformation accuracy in a heterogeneous, deformable, anthropomorphic phantom. Radiother. Oncol. 2020, 146, 97–109. [Google Scholar] [CrossRef]
- Tandberg, D.J.; Tong, B.C.; Ackerson, B.G.; Kelsey, C.R. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer 2018, 124, 667–678. [Google Scholar] [CrossRef]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Grozman, V.; Karlsson, K.; Lindberg, S.; Lax, I.; Wersäll, P.; Persson, G.F.; Josipovic, M.; Khalil, A.A.; Moeller, D.S.; et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated with Stereotactic Body Radiotherapy. J. Thorac. Oncol. 2021, 16, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Haseltine, J.M.; Rimner, A.; Gelblum, D.Y.; Modh, A.; Rosenzweig, K.E.; Jackson, A.; Yorke, E.D.; Wu, A.J. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract. Radiat. Oncol. 2016, 6, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Choi, W.; Shin, S.; Lee, S.; Ahn, S.; Kim, J.; Je, H.U.; Park, C.I.; Lee, J.S.; Choi, E.K. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009, 66, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Unger, K.; Ju, A.; Oermann, E.; Suy, S.; Yu, X.; Vahdat, S.; Subramaniam, D.; William Harter, K.; Collins, S.P.; Dritschilo, A.; et al. CyberKnife for hilar lung tumors: Report of clinical response and toxicity. J. Hematol. Oncol. 2010, 3, 39. [Google Scholar] [CrossRef]
- Lischalk, J.W.; Malik, R.M.; Collins, S.P.; Collins, B.T.; Matus, I.A.; Anderson, E.D. Stereotactic body radiotherapy (SBRT) for high-risk central pulmonary metastases. Radiat. Oncol. 2016, 11, 28. [Google Scholar] [CrossRef][Green Version]
- Tekatli, H.; Haasbeek, N.; Dahele, M.; De Haan, P.; Verbakel, W.; Bongers, E.; Hashemi, S.; Nossent, E.; Spoelstra, F.; de Langen, A.J.; et al. Outcomes of Hypofractionated High-Dose Radiotherapy in Poor-Risk Patients with “Ultracentral” Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 1081–1089. [Google Scholar] [CrossRef]
- Chang, J.H.; Poon, I.; Erler, D.; Zhang, L.; Cheung, P. The safety and effectiveness of stereotactic body radiotherapy for central versus ultracentral lung tumors. Radiother Oncol. 2018, 129, 277–283. [Google Scholar] [CrossRef]
- Raman, S.; Yau, V.; Pineda, S.; Le, L.W.; Lau, A.; Bezjak, A.; Cho, B.C.J.; Sun, A.; Hope, A.J.; Giuliani, M. Ultracentral Tumors Treated with Stereotactic Body Radiotherapy: Single-Institution Experience. Clin. Lung Cancer 2018, 19, e803–e810. [Google Scholar] [CrossRef]
- Korzets Ceder, Y.; Fenig, E.; Popvtzer, A.; Peled, N.; Kramer, M.R.; Saute, M.; Bragilovsky, D.; Schochat, T.; Allen, A.M. Stereotactic body radiotherapy for central lung tumors, yes we can! Radiat. Oncol. 2018, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, A.; Campeau, M.P.; Mathieu, D.; Bahig, H.; Lambert, L.; Vu, T.; Roberge, D.; Bilodeau, L.; Filion, E. Risk-adapted stereotactic ablative radiotherapy for central and ultra-central lung tumours. Radiother. Oncol. 2019, 134, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Sun, B.; Wang, J.; Meng, X.; Xuan, L.; Zhang, J.; Liu, J.; Shen, G.; Wu, S. Outcomes and toxicity of stereotactic body radiation therapy for advanced stage ultra-central non-small cell lung cancer. Thorac. Cancer 2019, 10, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, Y.; Yoon, W.S.; Rim, C.H. A preliminary experience of moderate-intensity stereotactic body radiation therapy for ultra-central lung tumor. Int. J. Radiat. Biol. 2019, 95, 1287–1294. [Google Scholar] [CrossRef]
- Meng, M.-B.; Wang, H.-H.; Zaorsky, N.G.; Sun, B.-S.; Zhu, L.; Song, Y.-C.; Li, F.-T.; Dong, Y.; Wang, J.-S.; Chen, H.-M.; et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci. 2019, 110, 3553–3564. [Google Scholar] [CrossRef]
- Henke, L.E.; Olsen, J.R.; Contreras, J.A.; Curcuru, A.; DeWees, T.A.; Green, O.L.; Michalski, J.; Mutic, S.; Roach, M.C.; Bradley, J.D.; et al. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. Adv. Radiat. Oncol. 2019, 4, 201–209. [Google Scholar] [CrossRef]
- Cooke, R.; Camilleri, P.; Chu, K.-Y.; O’Cathail, S.M.; Robinson, M.; Van Den Heuvel, F.; Hawkins, M.A. Stereotactic body radiotherapy for moderately central and ultra-central oligometastatic disease: Initial outcomes. Tech. Innov. Patient Support Radiat. Oncol. 2020, 13, 24–30. [Google Scholar] [CrossRef]
- Wang, C.; Rimner, A.; Gelblum, D.Y.; Dick-Godfrey, R.; McKnight, D.; Torres, D.; Flynn, J.; Zhang, Z.; Sidiqi, B.; Jackson, A.; et al. Analysis of pneumonitis and esophageal injury after stereotactic body radiation therapy for ultra-central lung tumors. Lung Cancer 2020, 147, 45–48. [Google Scholar] [CrossRef]
- Yang, D.; Cui, J.; Zhao, J.; You, J.; Yu, R.; Yu, H.; Jiang, L.; Li, D.; Xu, B.; Shi, A. Stereotactic ablative radiotherapy of 60 Gy in eight fractions is safe for ultracentral non-small cell lung cancer. Thorac. Cancer 2020, 11, 754–761. [Google Scholar] [CrossRef]
- Zhao, Y.; Khawandanh, E.; Thomas, S.; Zhang, S.; Dunne, E.M.; Liu, M.; Schellenberg, D. Outcomes of stereotactic body radiotherapy 60 Gy in 8 fractions when prioritizing organs at risk for central and ultracentral lung tumors. Radiat. Oncol. 2020, 15, 61. [Google Scholar] [CrossRef]
- Breen, W.G.; Jeans, E.B.; Gergelis, K.R.; Garces, Y.I.; Park, S.S.; Merrell, K.W.; Peikert, T.D.; Mansfield, A.S.; Wigle, D.A.; Harmsen, W.S.; et al. Ablative radiotherapy for ultracentral lung cancers: Dosimetric, geometric, and volumetric predictors of outcomes and toxicity. Radiother. Oncol. 2021, 158, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.; Ma, S.J.; Hennon, M.; Nwogu, C.; Dexter, E.; Picone, A.; Demmy, T.; Yendamuri, S.; Yu, H.; Fung-Kee-Fung, S.; et al. Exceeding Radiation Dose to Volume Parameters for the Proximal Airways with Stereotactic Body Radiation Therapy Is More Likely for Ultracentral Lung Tumors and Associated with Worse Outcome. Cancers 2021, 13, 3463. [Google Scholar] [CrossRef]
- Lodeweges, J.; van Rossum, P.; Bartels, M.; van Lindert, A.; Pomp, J.; Peters, M.; Verhoeff, J.J.C. Ultra-central lung tumors: Safety and efficacy of protracted stereotactic body radiotherapy. Acta Oncol. 2021, 60, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Franceschini, D.; Dominici, L.; Chiola, I.; Franzese, C.; D’Agostino, G.R.; Navarria, P.; Marzo, M.; Paganini, L.; Comito, T.; et al. Dose coverage impacts local control in ultra-central lung oligometastases treated with stereotactic radiotherapy. Strahlenther. Onkol. 2021, 197, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sidiqi, B.U.; Eckstein, J.; Nosrati, J.D.; Baker, J.; Antone, J.; Malesevic, V.; Seetharamu, N.; Sharma, R.; Ghaly, M. Analysis of Toxicity and Local Control for Ultra-Central Lung Tumors Undergoing Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, e424. [Google Scholar] [CrossRef]
- Mihai, A.M.; Armstrong, P.J.; Hickey, D.; Milano, M.T.; Dunne, M.; Healy, K.; Thirion, P.; Heron, D.E.; ElBeltagi, N.; Armstrong, J.G. Late Toxicity and Long-Term Local Control in Patients with Ultra-Central Lung Tumours Treated by Intensity-Modulated Radiotherapy-Based Stereotactic Ablative Body Radiotherapy with Homogenous Dose Prescription. Clin. Oncol. 2021, 33, 627–637. [Google Scholar] [CrossRef]
- Guillaume, E.; Tanguy, R.; Ayadi, M.; Claude, L.; Sotton, S.; Moncharmont, C.; Magné, N.; Martel-Lafay, I. Toxicity and efficacy of stereotactic body radiotherapy for ultra-central lung tumours: A single institution real life experience. Br. J. Radiol. 2022, 95, 20210533. [Google Scholar] [CrossRef]
- Salvestrini, V.; Duijm, M.; Loi, M.; Nuyttens, J.J. Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy. Cancers 2022, 14, 5908. [Google Scholar] [CrossRef]
- Wang, B.; Dong, Y.; Yu, X.; Li, F.; Wang, J.; Chen, H.; Niu, Z.; Song, Y.; Yuan, Z.; Tao, Z. Safety and Efficacy of Stereotactic Ablative Radiotherapy for Ultra-Central Lung Cancer. Front. Oncol. 2022, 12, 868844. [Google Scholar] [CrossRef]
- Sandoval, M.L.; Sim, A.J.; Bryant, J.M.; Bhandari, M.; Wuthrick, E.J.; Perez, B.A.; Dilling, T.J.; Redler, G.; Andreozzi, J.; Nardella, L.; et al. Magnetic Resonance-Guided Stereotactic Body Radiation Therapy/Hypofractionated Radiation therapy for Metastatic and Primary Central and Ultracentral Lung Lesions. JTO Clin. Res. Rep. 2023, 4, 100488. [Google Scholar] [CrossRef]
- Song, X.; Zhao, L.; Jiang, N.; Ding, N.; Zong, D.; Zhang, N.; Wang, D.; Wen, J.; He, X.; Kong, C.; et al. Long-term outcomes in patients with central and ultracentral non-small cell lung cancer treated with stereotactic body radiotherapy: Single-institution experience. Curr. Probl. Cancer 2023, 47, 100956. [Google Scholar] [CrossRef] [PubMed]
- Regnery, S.; Katsigiannopulos, E.; Hoegen, P.; Weykamp, F.; Sandrini, E.; Held, T.; Deng, M.; Eichkorn, T.; Buchele, C.; Rippke, C.; et al. To fly or not to fly: Stereotactic MR-guided adaptive radiotherapy effectively treats ultracentral lung tumors with favorable long-term outcomes. Lung Cancer 2023, 179, 107175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.N.B.; Hause, D.J.; Novak, J.; Monjazeb, A.M.; Daly, M.E. Tumor Control and Toxicity after SBRT for Ultracentral, Central, and Paramediastinal Lung Tumors. Pract. Radiat. Oncol. 2019, 9, e196–e202. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Louie, A.V.; Kotecha, R.; Ashfaq Ahmed, M.; Zhang, Z.; Guckenberger, M.; Kim, M.S.; Lo, S.S.; Scorsetti, M.; Tree, A.C.; et al. Stereotactic body radiotherapy for Ultra-Central lung Tumors: A systematic review and Meta-Analysis and International Stereotactic Radiosurgery Society practice guidelines. Lung Cancer 2023, 182, 107281. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, A.; Wierzbicki, M.; Parpia, S.; Wright, J.R.; Tsakiridis, T.K.; Okawara, G.S.; Kundapur, V.; Bujold, A.; Ahmed, N.; Hirmiz, K.; et al. Canadian Phase III Randomized Trial of Stereotactic Body Radiotherapy Versus Conventionally Hypofractionated Radiotherapy for Stage I, Medically Inoperable Non-Small-Cell Lung Cancer—Rationale and Protocol Design for the Ontario Clinical Oncology Group (OCOG)-LUSTRE Trial. Clin. Lung Cancer 2017, 18, 250–254. [Google Scholar] [CrossRef]
- Westover, K.D.; Loo, B.W., Jr.; Gerber, D.E.; Iyengar, P.; Choy, H.; Diehn, M.; Hughes, R.; Schiller, J.; Dowell, J.; Wardak, Z.; et al. Precision Hypofractionated Radiation Therapy in Poor Performing Patients with Non-Small Cell Lung Cancer: Phase 1 Dose Escalation Trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 72–81. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef]
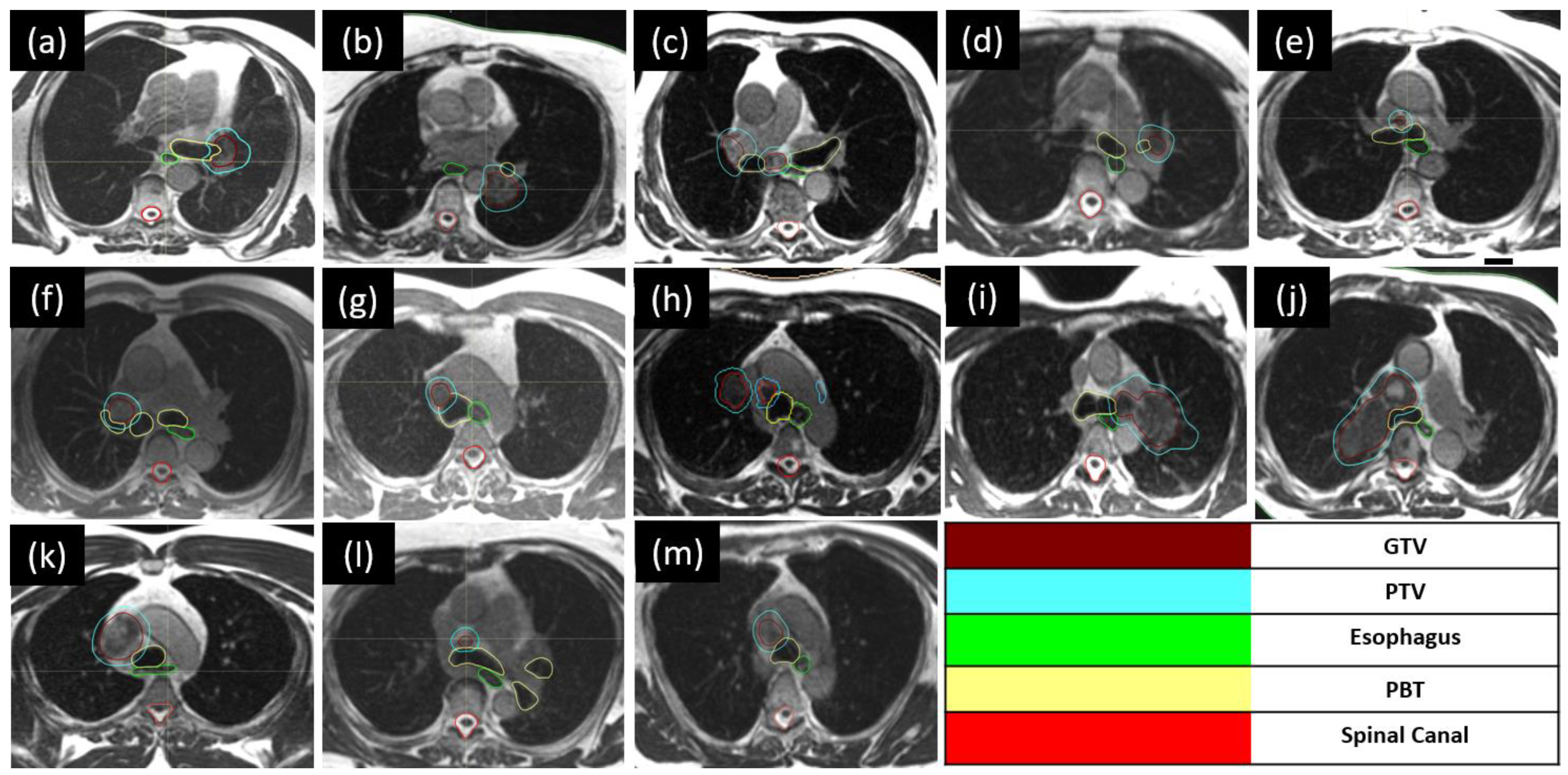

| Age | Gender | Smoking Status | Anti-Platelets Anticoagulant | COPD, Asthma, or Other Lung Disease | Primary | Disease Status | Site | reRT | GTV (cc) | PTV (cc) | Concurrent Systemic Therapy | G2+ Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | M | Ex-smoker | Yes | No | ADC | OP | 11L | No | 18.71 | 54.05 | Pembrolizumab | - |
| 84 | M | Ex-smoker | Yes | No | ADC | OP | LLL | Yes | 31.59 | 64.81 | Durvalumab | - |
| 85 | M | Ex-smoker | Yes | Yes | SCC | OR | 7 | No | 20.63 | 67.15 | Carboplatin and Paclitaxel | Cough (G2) Fatigue (G2) |
| 75 | F | Never | No | No | SCC | OP | LUL + 10L | No | 31.11 | 68.82 | Osimertinib | Pneumonitis (G2) Fatigue (G2) |
| 81 | F | Ex-smoker | Yes | No | SqCC | OP | 7 + RUL | No | 26.5 | 68.56 | Paclitaxel | - |
| 78 | M | Smoker | No | No | ADC | OP | 11L | No | 0.54 | 3.87 | Capmatinib | - |
| 50 | M | Ex-smoker | No | No | ADC | OP | 4R | No | 4.58 | 15.83 | None | - |
| 56 | M | No | No | No | Melanoma | OP | 2R, 11 + RUL | No | 20.81 | 58.25 | Nivolumab | - |
| 46 | F | Smoker | No | No | ADC | OP | LUL | No | 46.95 | 99.71 | Osimertinib | Fatigue (G2) |
| 71 | M | Smoker | No | No | ADC | OP | RUL | No | 121.65 | 211.81 | None | - |
| 59 | M | Smoker | No | Yes | ADC | OP | RUL | No | 100.76 | 173.36 | Atezolizumab | - |
| 68 | F | Never | No | No | Carcinosarcoma | OR | 4R + RUL | No | 1.6 | 7.94 | None | Esophagitis (G2) |
| 70 | M | Smoker | Yes | No | ADC | OP | 4R | No | 11.32 | 31.36 | None | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Rosa, A.; Mittauer, K.E.; Bassiri, N.; Rzepczynski, A.E.; Chuong, M.D.; Yarlagadda, S.; Kutuk, T.; McAllister, N.C.; Hall, M.D.; Gutierrez, A.N.; et al. Accelerated Hypofractionated Magnetic Resonance Guided Adaptive Radiation Therapy for Ultracentral Lung Tumors. Tomography 2024, 10, 169-180. https://doi.org/10.3390/tomography10010013
La Rosa A, Mittauer KE, Bassiri N, Rzepczynski AE, Chuong MD, Yarlagadda S, Kutuk T, McAllister NC, Hall MD, Gutierrez AN, et al. Accelerated Hypofractionated Magnetic Resonance Guided Adaptive Radiation Therapy for Ultracentral Lung Tumors. Tomography. 2024; 10(1):169-180. https://doi.org/10.3390/tomography10010013
Chicago/Turabian StyleLa Rosa, Alonso, Kathryn E. Mittauer, Nema Bassiri, Amy E. Rzepczynski, Michael D. Chuong, Sreenija Yarlagadda, Tugce Kutuk, Nicole C. McAllister, Matthew D. Hall, Alonso N. Gutierrez, and et al. 2024. "Accelerated Hypofractionated Magnetic Resonance Guided Adaptive Radiation Therapy for Ultracentral Lung Tumors" Tomography 10, no. 1: 169-180. https://doi.org/10.3390/tomography10010013
APA StyleLa Rosa, A., Mittauer, K. E., Bassiri, N., Rzepczynski, A. E., Chuong, M. D., Yarlagadda, S., Kutuk, T., McAllister, N. C., Hall, M. D., Gutierrez, A. N., Tolakanahalli, R., Mehta, M. P., & Kotecha, R. (2024). Accelerated Hypofractionated Magnetic Resonance Guided Adaptive Radiation Therapy for Ultracentral Lung Tumors. Tomography, 10(1), 169-180. https://doi.org/10.3390/tomography10010013









