Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism
Abstract
1. Introduction
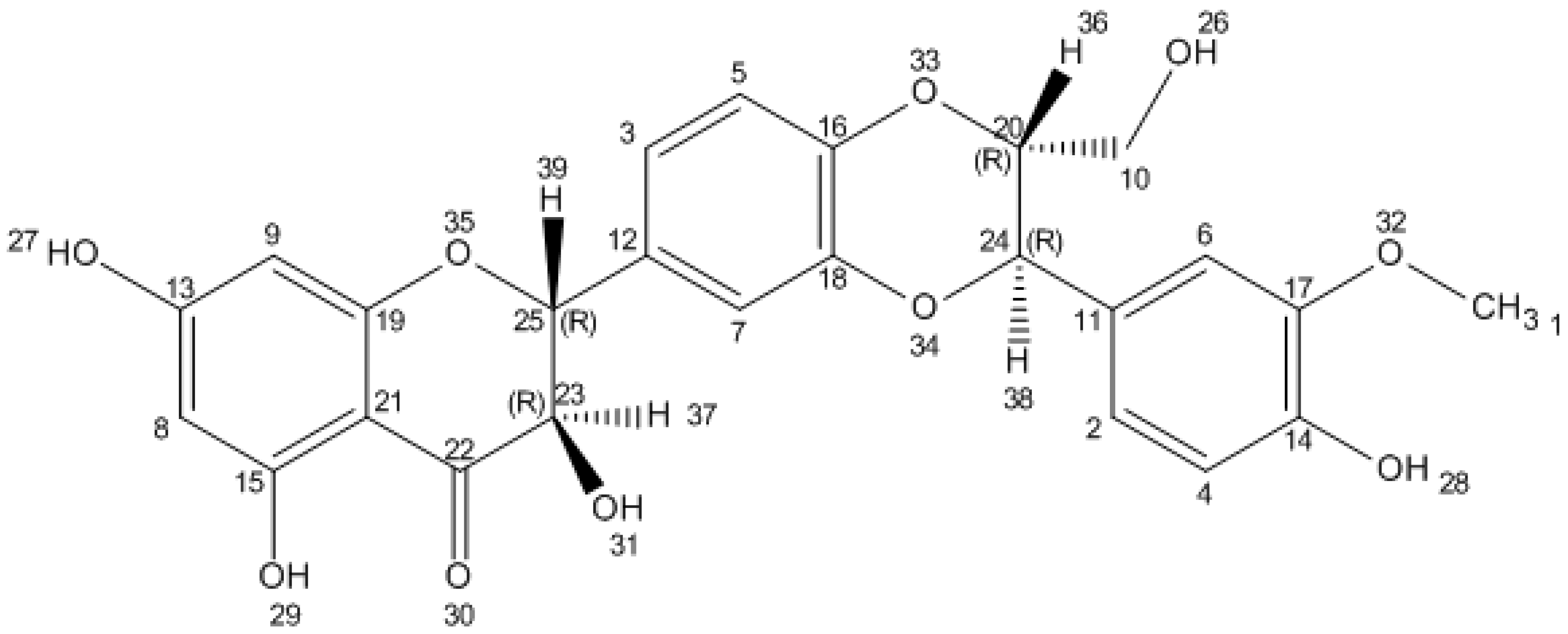
2. Silybin Structure and Chemistry
3. Silybin Metabolism
4. Bioavailability and Pharmacokinetics in Different Forms of Silybin Administration
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rainone, F. Milk thistle. Am. Fam. Physician 2005, 72, 1285–1288. [Google Scholar] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Salmi, H.A.; Sarna, S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand. J. Gastroenterol. 1982, 17, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Szilard, S.; Szentgyorgyi, D.; Demeter, I. Protective effect of Legalon in workers exposed to organic solvents. Acta Med. Hung. 1988, 45, 249–256. [Google Scholar] [PubMed]
- Feher, J.; Deak, G.; Muzes, G.; Lang, I.; Niederland, V.; Nekam, K.; Karteszi, M. Liver-protective action of silymarin therapy in chronic alcoholic liver diseases. Orv. Hetil. 1989, 130, 2723–2727. [Google Scholar] [PubMed]
- Wesolowska, O.; Lania-Pietrzak, B.; Kuzdzal, M.; Stanczak, K.; Mosiadz, D.; Dobryszycki, P.; Ozyhar, A.; Komorowska, M.; Hendrich, A.B.; Michalak, K. Influence of silybin on biophysical properties of phospholipid bilayers. Acta Pharmacol. Sin. 2007, 28, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Walterova, D. Silybin and silymarin—New effects and applications. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. Repub. 2005, 149, 29–41. [Google Scholar] [CrossRef]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin—New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Narayan, M.; Barrett, J.S. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 845, 9–103. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Flavonolignans—Compounds not only for liver treatment. Pol. Merkur. Lekarski 2017, 42, 34–37. [Google Scholar] [PubMed]
- Bijak, M.; Ponczek, M.B.; Nowak, P. Polyphenol compounds belonging to flavonoids inhibit activity of coagulation factor X. Int. J. Biol. Macromol. 2014, 65, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Ziewiecki, R.; Saluk, J.; Ponczek, M.; Pawlaczyk, I.; Krotkiewski, H.; Wachowicz, B.; Nowak, P. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014, 23, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Szelenberger, R.; Saluk, J.; Nowak, P. Flavonolignans inhibit ADP induced blood platelets activation and aggregation in whole blood. Int. J. Biol. Macromol. 2017, 95, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Saluk-Bijak, J. Flavonolignans reduce the response of blood platelet to collagen. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Saluk-Bijak, J. Flavonolignans inhibit the arachidonic acid pathway in blood platelets. BMC Complement. Altern. Med. 2017, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Dziedzic, A.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Flavonolignans inhibit IL1-beta-induced cross-talk between blood platelets and leukocytes. Nutrients 2017, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Pelter, A.; Hansel, R. The structure of silybin (silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968, 9, 2911–2916. [Google Scholar] [CrossRef]
- Pelter, A.; Hansel, R. Structure of silybin. 1. Degradative experiments. Chem. Ber.-Recl. 1975, 108, 790–802. [Google Scholar] [CrossRef]
- Althagafy, H.S.; Meza-Avina, M.E.; Oberlies, N.H.; Croatt, M.P. Mechanistic study of the biomimetic synthesis of flavonolignan diastereoisomers in milk thistle. J. Org. Chem. 2013, 78, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, V.A. Phenylpropanoids from medicinal plants: Distribution, classification, structural analysis, and biological activity. Chem. Nat. Compd. 2003, 39, 123–153. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavrikova, E.; Cvak, L.; Kren, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Zhu, J.; Hu, J.; Zhang, H.; Huang, C. Solubility of silybin in aqueous hydrochloric acid solution. Fluid Phase Equilib. 2007, 254, 204–210. [Google Scholar] [CrossRef]
- Van Wenum, E.; Jurczakowski, R.; Litwinienko, G. Media effects on the mechanism of antioxidant action of silybin and 2,3-dehydrosilybin: Role of the enol group. J. Org. Chem. 2013, 78, 9102–9112. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, J.G.; Lankin, D.C.; Graf, T.N.; Friesen, J.B.; Chen, S.N.; McAlpine, J.B.; Oberlies, N.H.; Pauli, G.F. HiFSA fingerprinting applied to isomers with near-identical NMR spectra: The silybin/isosilybin case. J. Org. Chem. 2013, 78, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Liu, Y. Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle). J. Nat. Prod. 2003, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Weyhenmeyer, R.; Mascher, H.; Birkmayer, J. Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992, 30, 134–138. [Google Scholar] [PubMed]
- Rickling, B.; Hans, B.; Kramarczyk, R.; Krumbiegel, G.; Weyhenmeyer, R. Two high-performance liquid chromatographic assays for the determination of free and total silibinin diastereomers in plasma using column switching with electrochemical detection and reversed-phase chromatography with ultraviolet detection. J. Chromatogr. B Biomed. Appl. 1995, 670, 267–277. [Google Scholar] [CrossRef]
- Han, Y.H.; Lou, H.X.; Ren, D.M.; Sun, L.R.; Ma, B.; Ji, M. Stereoselective metabolism of silybin diastereoisomers in the glucuronidation process. J. Pharm. Biomed. Anal. 2004, 34, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Gazak, R.; Marhol, P.; Biedermann, D.; Purchartova, K.; Fedrigo, M.; Riva, S.; Kren, V. Enzymatic kinetic resolution of silybin diastereoisomers. J. Nat. Prod. 2010, 73, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Freundenberg, K.; Neish, A. Constitution and Biosynthesis of Lignins; Molecular Biology, Biochemistry and Biophysics; Springer-Verlag: Berlin/Heidelberg, Germany, 1968; Volume 2, p. 132. [Google Scholar]
- Hansel, R.; Rimpler, H. Structure of silybin: Synthetic studies. Dtsch. Apoth. Ztg. 1968, 108, 1985. [Google Scholar] [CrossRef]
- Hansel, R.; Schulz, J.; Pelter, A. Structure of silybin: Synthetic studies. J. Chem. Soc. Chem. Commun. 1972, 1, 195–196. [Google Scholar] [CrossRef]
- Nyiredy, S.; Samu, Z.; Szucs, Z.; Gulacsi, K.; Kurtan, T.; Antus, S. New insight into the biosynthesis of flavanolignans in the white-flowered variant of Silybum marianum. J. Chromatogr. Sci. 2008, 46, 93–96. [Google Scholar] [CrossRef] [PubMed]
- AbouZid, S. Silymarin, Natural Flavonolignans from Milk Thistle. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; InTech: London, UK, 2012; pp. 255–272. [Google Scholar]
- Saller, R.; Brignoli, R.; Melzer, J.; Meier, R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch. Komplement. 2008, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.; Lucker, P.W.; Mennicke, W.H.; Wetzelsberger, N. Pharmacokinetic studies with silymarin in human serum and bile. Methods Find. Exp. Clin. Pharmacol. 1984, 6, 655–661. [Google Scholar] [PubMed]
- Morazzoni, P.; Montalbetti, A.; Malandrino, S.; Pifferi, G. Comparative pharmacokinetics of silipide and silymarin in rats. Eur. J. Drug Metab. Pharmacokinet. 1993, 18, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar] [PubMed]
- Sridar, C.; Goosen, T.C.; Kent, U.M.; Williams, J.A.; Hollenberg, P.F. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab. Dispos. 2004, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Beckmann-Knopp, S.; Rietbrock, S.; Weyhenmeyer, R.; Bocker, R.H.; Beckurts, K.T.; Lang, W.; Hunz, M.; Fuhr, U. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol. Toxicol. 2000, 86, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Ramachandran, V.; Komoroski, B.J.; Zhang, S.; Schiff, P.L.; Strom, S.C. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab. Dispos. 2000, 28, 1270–1273. [Google Scholar] [PubMed]
- Zuber, R.; Modriansky, M.; Dvorak, Z.; Rohovsky, P.; Ulrichova, J.; Simanek, V.; Anzenbacher, P. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother. Res. 2002, 16, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi-Suzuki, M.; Frye, R.F.; Zhu, H.J.; Brinda, B.J.; Chavin, K.D.; Bernstein, H.J.; Markowitz, J.S. The effects of milk thistle (Silybum marianum) on human cytochrome P450 activity. Drug Metab. Dispos. 2014, 42, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Jancova, P.; Anzenbacherova, E.; Papouskova, B.; Lemr, K.; Luzna, P.; Veinlichova, A.; Anzenbacher, P.; Simanek, V. Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab. Dispos. 2007, 35, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Gunaratna, C.; Zhang, T. Application of liquid chromatography-electrospray ionization-ion trap mass spectrometry to investigate the metabolism of silibinin in human liver microsomes. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 794, 303–310. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Ulrichova, J.; Kosina, P.; Stevenson, D.; Sedmera, P.; Prikrylova, V.; Halada, P.; Simanek, V. Chemoenzymatic preparation of silybin beta-glucuronides and their biological evaluation. Drug Metab. Dispos. 2000, 28, 1513–1517. [Google Scholar] [PubMed]
- Hawke, R.L.; Schrieber, S.J.; Soule, T.A.; Wen, Z.; Smith, P.C.; Reddy, K.R.; Wahed, A.S.; Belle, S.H.; Afdhal, N.H.; Navarro, V.J.; et al. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J. Clin. Pharmacol. 2010, 50, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.N.; Zhu, Y.; Wang, L.; Peng, M.; Tong, S.S.; Cao, X.; Qiu, H.; Xu, X.M. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol. Sin. 2010, 31, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Pepping, J. Milk thistle: Silybum marianum. Am. J. Health Syst. Pharm. 1999, 56, 1195–1197. [Google Scholar] [PubMed]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Voinovich, D.; Perissutti, B.; Grassi, M.; Passerini, N.; Bigotto, A. Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems. J. Pharm. Sci. 2009, 98, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Ahmad, M.; Madni, A.; Akhtar, N.; Asghar, W.; Aghtar, M.; Atif, M.; Qamar-uz-zaman, M. In-vivo Kinetics of Silymarin (Milk Thistle) on healthy male volunteers. Trop. J. Pharm. Res. 2009, 8, 311–316. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, E.J.; Lee, E.D.; Kim, J.H.; Jang, S.W.; Kim, Y.G.; Kwon, J.W.; Kim, W.B.; Lee, M.G. Comparative bioavailability of silibinin in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 2003, 41, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Arcari, M.; Brambilla, A.; Brandt, A.; Caponi, R.; Corsi, G.; Di, R.M.; Solinas, F.; Wachter, W.P. A new inclusion complex of silibinin and beta-cyclodextrins: In vitro dissolution kinetics and in vivo absorption in comparison with traditional formulations. Boll. Chim. Farm. 1992, 131, 205–209. [Google Scholar] [PubMed]
- Awasthi, R.; Kulkarni, G.; Pawar, V. Phytosomes: An approach to increase the bioavailability of plant extracts. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–3. [Google Scholar]
- Gandhi, A.; Dutta, A.; Pal, A.; Bakshi, P. Recent trends of phytosomes for delivering herbal extract with improved bioavailability. J. Pharmacogn. Phytochem. 2012, 1, 6–14. [Google Scholar]
- Barzaghi, N.; Crema, F.; Gatti, G.; Pifferi, G.; Perucca, E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Morazzoni, P.; Magistretti, M.J.; Giachetti, C.; Zanolo, G. Comparative bioavailability of Silipide, a new flavanolignan complex, in rats. Eur. J. Drug Metab. Pharmacokinet. 1992, 17, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, J.; Zhao, H.Z.; Liu, C.X. Development of a HPLC-UV assay for silybin-phosphatidylcholine complex (silybinin capsules) and its pharmacokinetic study in healthy male Chinese volunteers. Eur. J. Drug Metab. Pharmacokinet. 2006, 31, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Filburn, C.R.; Kettenacker, R.; Griffin, D.W. Bioavailability of a silybin-phosphatidylcholine complex in dogs. J. Vet. Pharmacol. Ther. 2007, 30, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Di, S.A.; Bendia, E.; Taffetani, S.; Omenetti, A.; Candelaresi, C.; Marzioni, M.; De, M.S.; Benedetti, A. Hepatoprotective and antifibrotic effect of a new silybin-phosphatidylcholine-Vitamin E complex in rats. Dig. Liver Dis. 2005, 37, 869–876. [Google Scholar]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Sallee, V.L.; Dietschy, J.M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J. Lipid Res. 1973, 14, 475–484. [Google Scholar] [PubMed]
- Westergaard, H.; Dietschy, J.M. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J. Clin. Investig. 1976, 58, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.A. Intestinal transport of bile acids. Am. J. Physiol. 1981, 241, G83–G92. [Google Scholar] [PubMed]
- O’Reilly, J.; Corrigan, O.; O’Driscoll, C. The effect of mixed micellar systems, bile acid/fatty acids, on the solubility and intestinal absorption of clofazimine (B663) in the anesthetized rat. Int. J. Phram. 1994, 109, 147–194. [Google Scholar] [CrossRef]
- Magee, G.A.; French, J.; Gibbon, B.; Luscombe, C. Bile salt/lecithin mixed micelles optimized for the solubilization of a poorly soluble steroid molecule using statistical experimental design. Drug Dev. Ind. Pharm. 2003, 29, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, X.; Shen, B.; Dai, L.; Xu, H.; Shen, C.; Yuan, H.; Ha, J. A solid phospholipid-bile salts-mixed micelles based on the fast dissolving oral films to improve the oral bioavailability of poorly water-soluble drugs. J. Nanopart. Res. 2014, 16, 2455. [Google Scholar] [CrossRef]
- Garidel, P.; Hildebrand, A.; Knauf, K.; Blume, A. Membranolytic activity of bile salts: Influence of biological membrane properties and composition. Molecules 2007, 12, 2292–2326. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: Preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6633–6644. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Vavia, P.R. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007, 9, E344–E352. [Google Scholar] [CrossRef] [PubMed]
- Charman, S.A.; Charman, W.N.; Rogge, M.C.; Wilson, T.D.; Dutko, F.J.; Pouton, C.W. Self-emulsifying drug delivery systems: Formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm. Res. 1992, 9, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S93–S98. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Q.; Huang, Y.; Zhou, Y.; Liu, Y. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech 2010, 11, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Kim, T.S.; Park, J.H.; Chi, S.C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharm. Res. 2007, 30, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Ahmad, S. Stability studies of silymarin nanoemulsion containing Tween 80 as a surfactant. J. Pharm. Bioallied Sci. 2015, 7, 321–324. [Google Scholar] [PubMed]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Oil based nanocarrier for improved oral delivery of silymarin: In vitro and in vivo studies. Int. J. Pharm. 2011, 413, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Liu, Z.; Liu, G.; Duan, C.; Jia, L.; Feng, F.; Zhang, X.; Shi, Y.; Zhang, Q. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology 2010, 21, 155104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Kim, Y.I.; Cho, K.H.; Poudel, B.K.; Choi, J.Y.; Kim, D.W.; Shin, Y.J.; Bae, O.N.; Yousaf, A.M.; Yong, C.S.; et al. A novel solid dispersion system for natural product-loaded medicine: Silymarin-Loaded solid dispersion with enhanced oral bioavailability and hepatoprotective activity. J. Microencapsul. 2014, 31, 619–626. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. https://doi.org/10.3390/molecules22111942
Bijak M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules. 2017; 22(11):1942. https://doi.org/10.3390/molecules22111942
Chicago/Turabian StyleBijak, Michal. 2017. "Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism" Molecules 22, no. 11: 1942. https://doi.org/10.3390/molecules22111942
APA StyleBijak, M. (2017). Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules, 22(11), 1942. https://doi.org/10.3390/molecules22111942




