Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications
Abstract
1. Introduction
2. The Broken Homeostasis of Gut Microbiota Caused by SD
3. Functional Impairment by SD and the Role of Gut Microbiota in This Process
3.1. Weakened Immune Defenses or Colonization Resistance against Infections
3.1.1. SD-Induced Depletion of Immune Defenses
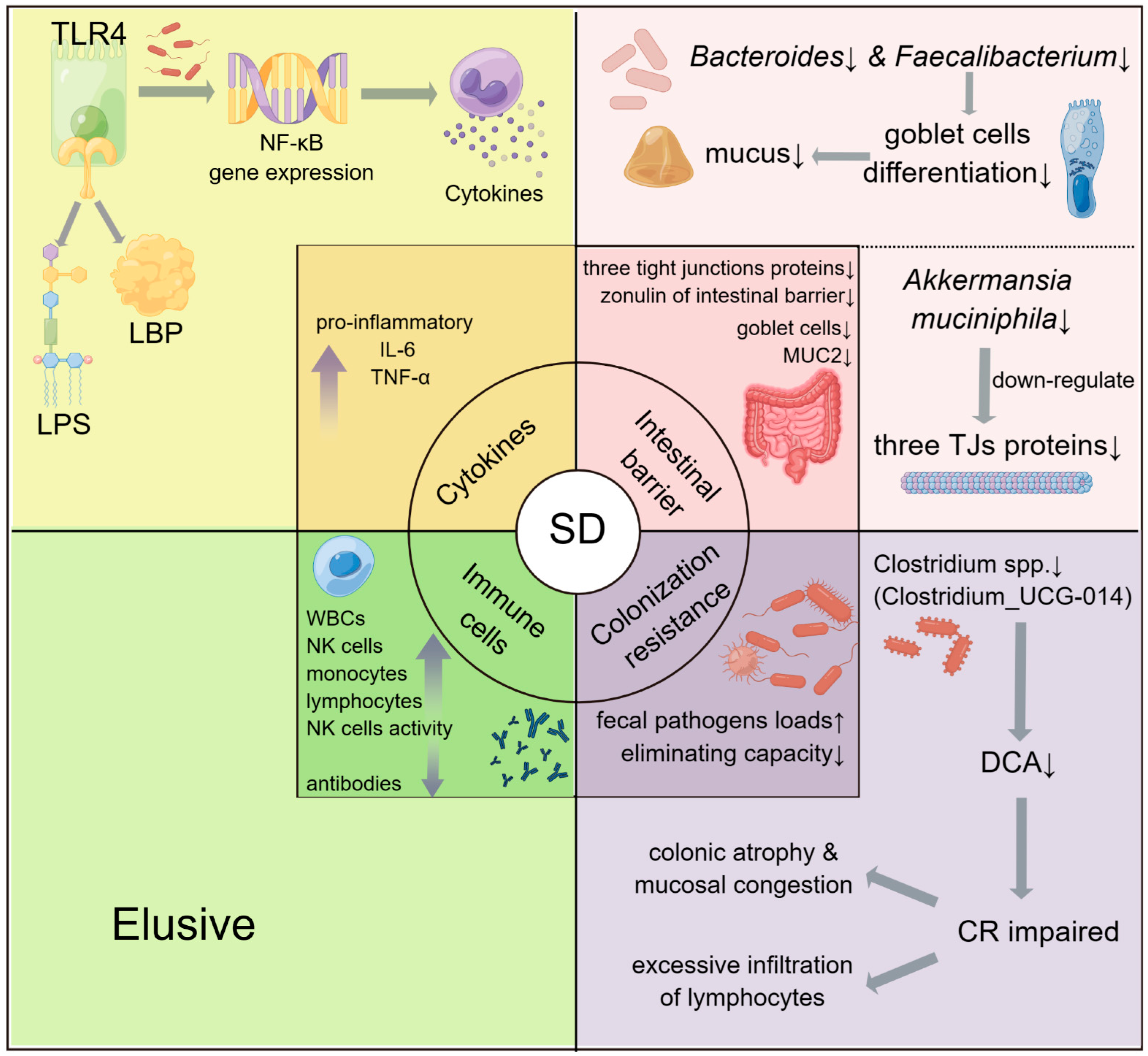
3.1.2. The Potential Roles of Gut Microbiota in SD-Induced Immune Diseases
- (1)
- Through the regulation of TLR4 and NF-κB gene expression.
- (2)
- Through the intestinal barrier.
- (3)
- Through colonization resistance.
3.2. Metabolic Diseases
3.3. Gut-Brain Axis
3.4. Other Sleep-Induced Diseases
4. Conclusions and Implications
- Most of the results so far have been obtained in animal models, and there is still a lack of human clinical data to support these findings. Moreover, the time periods for SD treatment have been too concentrated. Such experiment designs tend to be ideal and not in line with the actual situation.
- It is not known what the detailed mechanisms behind the disruption of gut microbiota (caused by SD) are. Whether altered intestinal environments also play an important role in this process is unknown. In addition, there is a lack of enough evidence to explain how gut microbiota affects the development of human diseases in the context of SD.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology from Chronic Sleep Restriction and Total Sleep Deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-S.; Hu, P.T.; Gujar, N.; Jolesz, F.A.; Walker, M.P. A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 2007, 10, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Bathalon, G.P.; Falco, C.M.; Kramer, F.M.; Morgan, C.A., III; Niro, P. Severe decrements in cognition function and mood induced by sleep loss, heat, dehydration, and undernutrition during simulated combat. Biol. Psychiatry 2005, 57, 422–429. [Google Scholar] [CrossRef]
- Owens, J.A.; Weiss, M.R. Insufficient sleep in adolescents: Causes and consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef]
- Triplett, J.; Braddock, A.; Roberts, E.; Ellis, D.; Chan, V. Identification of sleep fragmentation-induced gut microbiota alteration and prediction of functional impact in Sprague Dawley rats harboring microbiome derived from multiple human donors. Sleep Sci. 2022, 15, 7–19. [Google Scholar] [CrossRef]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef]
- Tavares, L.; Lador, A.; Valderrábano, M. Sleep Apnea and Atrial Fibrillation: Role of the Cardiac Autonomic Nervous System. Methodist DeBakey Cardiovasc. J. 2021, 17, 49–52. [Google Scholar] [CrossRef]
- Covassin, N.; Singh, P. Sleep Duration and Cardiovascular Disease Risk: Epidemiologic and Experimental Evidence. Sleep Med. Clin. 2016, 11, 81–89. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Sheridan, J.F.; Van Cauter, E. Effect of sleep deprivation on response to immunization. JAMA 2002, 288, 1471–1472. [Google Scholar] [CrossRef]
- Magee, L.; Hale, L. Longitudinal associations between sleep duration and subsequent weight gain: A systematic review. Sleep Med. Rev. 2012, 16, 231–241. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sigua, N.L. What Is Sleep Deprivation? Am. J. Respir. Crit. Care Med. 2019, 199, P11–P12. [Google Scholar] [CrossRef]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2016, 42, 129–155. [Google Scholar] [CrossRef]
- Abrams, R.M. Sleep Deprivation. Obstet. Gynecol. Clin. N. Am. 2015, 42, 493–506. [Google Scholar] [CrossRef]
- Gulia, K.K.; Kumar, V.M. Sleep disorders in the elderly: A growing challenge. Psychogeriatrics 2018, 18, 155–165. [Google Scholar] [CrossRef]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef]
- Sen, P.; Molinero-Perez, A.; O’riordan, K.J.; McCafferty, C.P.; O’halloran, K.D.; Cryan, J.F. Microbiota and sleep: Awakening the gut feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627.e17. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell. Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef]
- Yang, D.-F.; Huang, W.-C.; Wu, C.W.; Huang, C.-Y.; Yang, Y.-C.S.; Tung, Y.-T. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023, 268, 127292. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Patel, S. The epidemiology of sleep and obesity. Sleep Health 2017, 3, 383–388. [Google Scholar] [CrossRef]
- Lee, J.; Kang, J.; Kim, Y.; Lee, S.; Oh, C.-M.; Kim, T. Integrated analysis of the microbiota-gut-brain axis in response to sleep deprivation and diet-induced obesity. Front. Endocrinol. 2023, 14, 1117259. [Google Scholar] [CrossRef]
- Ogilvie, R.P.; Patel, S.R. The Epidemiology of Sleep and Diabetes. Curr. Diabetes Rep. 2018, 18, 82. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, Y.; Wu, Z.; Wang, R.; Zhang, X. New hints for improving sleep: Tea polyphenols mediate gut microbiota to regulate circadian disturbances. Food Front. 2023, 4, 47–59. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Jiao, L.; Duan, Z.; Sangihaghpeykar, H.; Hale, L.; White, D.; Elserag, H.B. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br. J. Cancer 2013, 108, 213–221. [Google Scholar] [CrossRef]
- Kakizaki, M.; Kuriyama, S.; Sone, T.; Ohmori-Matsuda, K.; Hozawa, A.; Nakaya, N.; Fukudo, S.; Tsuji, I. Sleep duration and the risk of breast cancer: The Ohsaki Cohort Study. Br. J. Cancer 2008, 99, 1502–1505. [Google Scholar] [CrossRef]
- Lewis, L.D. The interconnected causes and consequences of sleep in the brain. Science 2021, 374, 564–568. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Sumida, K.; Kovesdy, C.P. The gut-kidney-heart axis in chronic kidney disease. Physiol. Int. 2019, 106, 195–206. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha; Sharma, K.K. Gut-organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2016, 43, 81–95. [Google Scholar] [CrossRef]
- Jia, X.; Yang, R.; Li, J.; Zhao, L.; Zhou, X.; Xu, X. Gut-Bone Axis: A Non-Negligible Contributor to Periodontitis. Front. Cell. Infect. Microbiol. 2021, 11, 752708. [Google Scholar] [CrossRef]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nature Reviews. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.-H.; Li, S.-X.; He, Z.-M.; Zhu, W.-L.; Ji, Y.-B.; Wang, Z.; Zhu, X.-M.; Yuan, K.; Bao, Y.-P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Kumar, V.; Thukral, A.K.; Sharma, A.; Bhardwaj, R. Extending the concept of entropy-negentropy for the assessment of ecological dominance and diversity at alpha, beta and gamma levels. Geol. Ecol. Landsc. 2023, 7, 27–39. [Google Scholar] [CrossRef]
- Bowers, S.J.; Vargas, F.; Gonzalez, A.; He, S.; Jiang, P.; Dorrestein, P.C.; Knight, R.; Wright, K.P., Jr.; Lowry, C.A.; Fleshner, M.; et al. Repeated sleep disruption in mice leads to persistent shifts in the fecal microbiome and metabolome. PLoS ONE 2020, 15, e0229001. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.-M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef]
- Liu, G.-H.; Zhuo, X.-C.; Huang, Y.-H.; Liu, H.-M.; Wu, R.-C.; Kuo, C.-J.; Chen, N.-H.; Chuang, L.-P.; Lin, S.-W.; Chen, Y.-L.; et al. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology 2022, 11, 962. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Nohroudi, K.; Born, J. Number and Function of Circulating Human Antigen Presenting Cells Regulated by Sleep. Sleep 2007, 30, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Hansen, K.; Mölle, M.; Fehm, H.L. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997, 158, 4454–4464. [Google Scholar] [CrossRef]
- Fang, D.; Xu, T.; Sun, J.; Shi, J.; Li, F.; Yin, Y.; Wang, Z.; Liu, Y. Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections. Adv. Sci. 2023, 10, e2207170. [Google Scholar] [CrossRef]
- Dinges, D.F.; Douglas, S.D.; Zaugg, L.; Campbell, D.E.; McMann, J.M.; Whitehouse, W.G.; Orne, E.C.; Kapoor, S.C.; Icaza, E.; Orne, M.T. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J. Clin. Investig. 1994, 93, 1930–1939. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Mishima, K.; Satoh, K.; Tozawa, T.; Mishima, Y.; Shimizu, T.; Hishikawa, Y. Total sleep deprivation induces an acute and transient increase in NK cell activity in healthy young volunteers. Sleep 2001, 24, 806–811. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflügers Arch. Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, ecosalplus.ESP-0001-2018. [Google Scholar] [CrossRef]
- Ray, A.; Cot, M.; Puzo, G.; Gilleron, M.; Nigou, J. Bacterial cell wall macroamphiphiles: Pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie 2013, 95, 33–42. [Google Scholar] [CrossRef]
- Morris, M.C.; Gilliam, E.A.; Li, L. Innate Immune Programing by Endotoxin and Its Pathological Consequences. Front. Immunol. 2015, 5, 680. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflammation 2019, 16, 180. [Google Scholar] [CrossRef]
- Zweigner, J.; Gramm, H.-J.; Singer, O.C.; Wegscheider, K.; Schumann, R.R. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 2001, 98, 3800–3808. [Google Scholar] [CrossRef]
- Blairon, L.; Wittebole, X.; Laterre, P. Lipopolysaccharide-Binding Protein Serum Levels in Patients with Severe Sepsis Due to Gram-Positive and Fungal Infections. J. Infect. Dis. 2003, 187, 287–291. [Google Scholar] [CrossRef]
- Hernández-Chirlaque, C.; Aranda, C.J.; Ocón, B.; Capitán-Cañadas, F.; Ortega-González, M.; Carrero, J.J.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Germ-free and Antibiotic-treated Mice are Highly Susceptible to Epithelial Injury in DSS Colitis. J. Crohn’s Colitis 2016, 10, 1324–1335. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Löfmark, S.; Jernberg, C.; Jansson, J.K.; Edlund, C. Clindamycin-induced enrichment and long-term persistence of resistant Bacteroides spp. and resistance genes. J. Antimicrob. Chemother. 2006, 58, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; A Miller, M. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Hear. J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab. Clin. Exp. 2018, 84, 56–66. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Heiman, M.; Dimarchi, R. Leptin: Structure, function and biology. Vitam. Horm. 2005, 71, 345–372. [Google Scholar]
- Mosavat, M.; Mirsanjari, M.; Arabiat, D.; Smyth, A.; Whitehead, L. The Role of Sleep Curtailment on Leptin Levels in Obesity and Diabetes Mellitus. Obes. Facts 2021, 14, 214–221. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Asakawa, A.; Fujimiya, M.; Lee, S.-D.; Inui, A. Ghrelin Gene Products and the Regulation of Food Intake and Gut Motility. Pharmacol. Rev. 2009, 61, 430–481. [Google Scholar] [CrossRef]
- Li, Z.; Mulholland, M.; Zhang, W. Ghrelin O-acyltransferase (GOAT) and energy metabolism. Sci. China Life Sci. 2016, 59, 281–291. [Google Scholar] [CrossRef]
- Mullington, J.M.; Chan, J.L.; Van Dongen, H.P.A.; Szuba, M.P.; Samaras, J.; Price, N.J.; Meier-Ewert, H.K.; Dinges, D.F.; Mantzoros, C.S. Sleep Loss Reduces Diurnal Rhythm Amplitude of Leptin in Healthy Men. J. Neuroendocr. 2003, 15, 851–854. [Google Scholar] [CrossRef]
- Guilleminault, C.; Powell, N.B.; Martinez, S.; Kushida, C.; Raffray, T.; Palombini, L.; Philip, P. Preliminary observations on the effects of sleep time in a sleep restriction paradigm. Sleep Med. 2003, 4, 177–184. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; L’hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; O’Keeffe, M.; Roberts, A.L.; RoyChoudhury, A.; Laferrère, B. Short Sleep Duration, Glucose Dysregulation and Hormonal Regulation of Appetite in Men and Women. Sleep 2012, 35, 1503–1510. [Google Scholar] [CrossRef]
- de Oliveira, E.M.; Visniauskas, B.; Tufik, S.; Andersen, M.L.; Chagas, J.R.; Campa, A. Serum Amyloid A Production Is Triggered by Sleep Deprivation in Mice and Humans: Is That the Link between Sleep Loss and Associated Comorbidities? Nutrients 2017, 9, 311. [Google Scholar] [CrossRef]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 2259. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Muñoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2018, 72, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Majewski, C.; Xie, M.; Yan, P.; Guo, Y.; Bao, W.; Rong, Y.; Jackson, C.L.; Hu, F.B.; Liu, L. Sleep Duration and Risk of Type 2 Diabetes: A Meta-analysis of Prospective Studies. Diabetes Care 2015, 38, 529–537. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef]
- Stranges, S.; Dorn, J.M.; Cappuccio, F.P.; Donahue, R.P.; Rafalson, L.B.; Hovey, K.M.; Freudenheim, J.L.; Kandala, N.-B.; A Miller, M.; Trevisan, M. A population-based study of reduced sleep duration and hypertension: The strongest association may be in premenopausal women. J. Hypertens. 2010, 28, 896–902. [Google Scholar] [CrossRef]
- Meng, L.; Zheng, Y.; Hui, R. The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertens. Res. 2013, 36, 985–995. [Google Scholar] [CrossRef]
- Fung, M.M.; Peters, K.; Redline, S.; Ziegler, M.G.; Ancoli-Israel, S.; Barrett-Connor, E.; Stone, K.L.; Osteoporotic Fractures in Men Research Group. Decreased Slow Wave Sleep Increases Risk of Developing Hypertension in Elderly Men. Hypertension 2011, 58, 596–603. [Google Scholar] [CrossRef]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases- a review. Sleep Med. 2020, 77, 192–204. [Google Scholar] [CrossRef]
- Bishir, M.; Bhat, A.; Essa, M.M.; Ekpo, O.; Ihunwo, A.O.; Veeraraghavan, V.P.; Mohan, S.K.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2011, 13, 93–110. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef]
- Soulet, D.; Rivest, S. Microglia. Curr. Biol. 2008, 18, R506–R508. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Korostovtseva, L.; Bochkarev, M.; Sviryaev, Y. Sleep and Cardiovascular Risk. Sleep Med. Clin. 2021, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Humbert, M.; Chaouat, A. Sleep-related breathing disorders and pulmonary hypertension. Eur. Respir. J. 2020, 57, 2002258. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, E.A.; de Mutsert, R.; le Cessie, S.; Appelman-Dijkstra, N.M.; Rosendaal, F.R.; van Heemst, D.; den Heijer, M.; Biermasz, N.R.; group, N.E.O.s. Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: The NEO study. PLoS ONE 2017, 12, e0176685. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Gonzalez-Suarez, M.L.; Srivali, N.; Ungprasert, P.; Kittanamongkolchai, W.; Caples, S.M.; Erickson, S.B. The effects of short sleep duration on proteinuria and chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 991–996. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.d. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sport. Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Bodenmann, S.; Hohoff, C.; Freitag, C.; Deckert, J.; Rétey, J.V.; Bachmann, V.; Landolt, H.P. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br. J. Pharmacol. 2012, 165, 1904–1913. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, S.; Ma, W.; Wang, Q.; Li, Y.; Xia, C.; Xu, Y.; Zhang, T.; Yang, L.; Zhou, M. The Impact of Instant Coffee and Decaffeinated Coffee on the Gut Microbiota and Depression-Like Behaviors of Sleep-Deprived Rats. Front. Microbiol. 2022, 13, 32. [Google Scholar] [CrossRef]
- Devasagayam, T.; Kamat, J.; Mohan, H.; Kesavan, P. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1996, 1282, 63–70. [Google Scholar] [CrossRef]
- Mitani, T.; Nagano, T.; Harada, K.; Yamashita, Y.; Ashida, H. Caffeine-Stimulated Intestinal Epithelial Cells Suppress Lipid Accumulation in Adipocytes. J. Nutr. Sci. Vitaminol. 2017, 63, 331–338. [Google Scholar] [CrossRef]
- Urry, E.; Landolt, H.-P. Adenosine, caffeine, and performance: From cognitive neuroscience of sleep to sleep pharmacogenetics. Sleep Neuronal Plast. Brain Funct. 2015, 25, 331–366. [Google Scholar]
- Szentirmai, É.; Millican, N.S.; Massie, A.R.; Kapas, L. Butyrate, a metabolite of intestinal bacteria, enhances sleep. Sci. Rep. 2019, 9, 7035. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2012, 6, 39–51. [Google Scholar] [CrossRef]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Haarhuis, J.; Kardinaal, A.; Kortman, G. Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Benef. Microbes 2022, 13, 169–182. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Whittle, N.; Singewald, N. HDAC inhibitors as cognitive enhancers in fear, anxiety and trauma therapy: Where do we stand? Biochem. Soc. Trans. 2014, 42, 569–581. [Google Scholar] [CrossRef]


| Subjects | Experiment Protocols | Results | Ref. |
|---|---|---|---|
| C57BL/6 mice | repeated SD 20 h SD/day for 5 days | F:B ratio↑ g_Lactobacillus↓ g_Bifidobacterium↓ phylum Actinobacteria↓ | [57] |
| Nine normal-weight men | two nights of PSD; sleep opportunity 02:45–07:00 h | F:B ratio↑ families Coriobacteriaceae and Erysipelotrichaceae↑ phylum Tenericutes↓ | [58] |
| Twenty-five healthy participants (13 males) | 40 h of SD | α-diversity: 24 h SD↓, 40 h SD↓↓ β-diversity obvious different g_Prevotella↓ g_Sutterella↓ g_Parasutterella↓ g_Alloprevotella↓ g_Anaeroplasma↓ g_Elusimicrobium↓ | [54] |
| CD1 mice (male) | Continuous SD for three days | α-diversity↓ ACE, Chao and Shannon indexes↓ Simpson index↑ phylum Bacteroidetes↓ phylum Firmicutes↑ F:B ratio↑ | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Fang, D.; Wang, Z.; Liu, Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int. J. Mol. Sci. 2023, 24, 9603. https://doi.org/10.3390/ijms24119603
Sun J, Fang D, Wang Z, Liu Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. International Journal of Molecular Sciences. 2023; 24(11):9603. https://doi.org/10.3390/ijms24119603
Chicago/Turabian StyleSun, Jingyi, Dan Fang, Zhiqiang Wang, and Yuan Liu. 2023. "Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications" International Journal of Molecular Sciences 24, no. 11: 9603. https://doi.org/10.3390/ijms24119603
APA StyleSun, J., Fang, D., Wang, Z., & Liu, Y. (2023). Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. International Journal of Molecular Sciences, 24(11), 9603. https://doi.org/10.3390/ijms24119603






