How Multifunctioning Joints Produce Highly Agile Limbs in Animals with Lessons for Robotics
Abstract
1. Introduction
2. The Human Wrist Joint
2.1. Wrist Anatomy
2.2. Requirements and Objectives
2.3. Wrist Functions and Common Parts
2.4. Actuation and Fine-Tuning
2.5. Integration, Reconfiguration and Miniaturisation
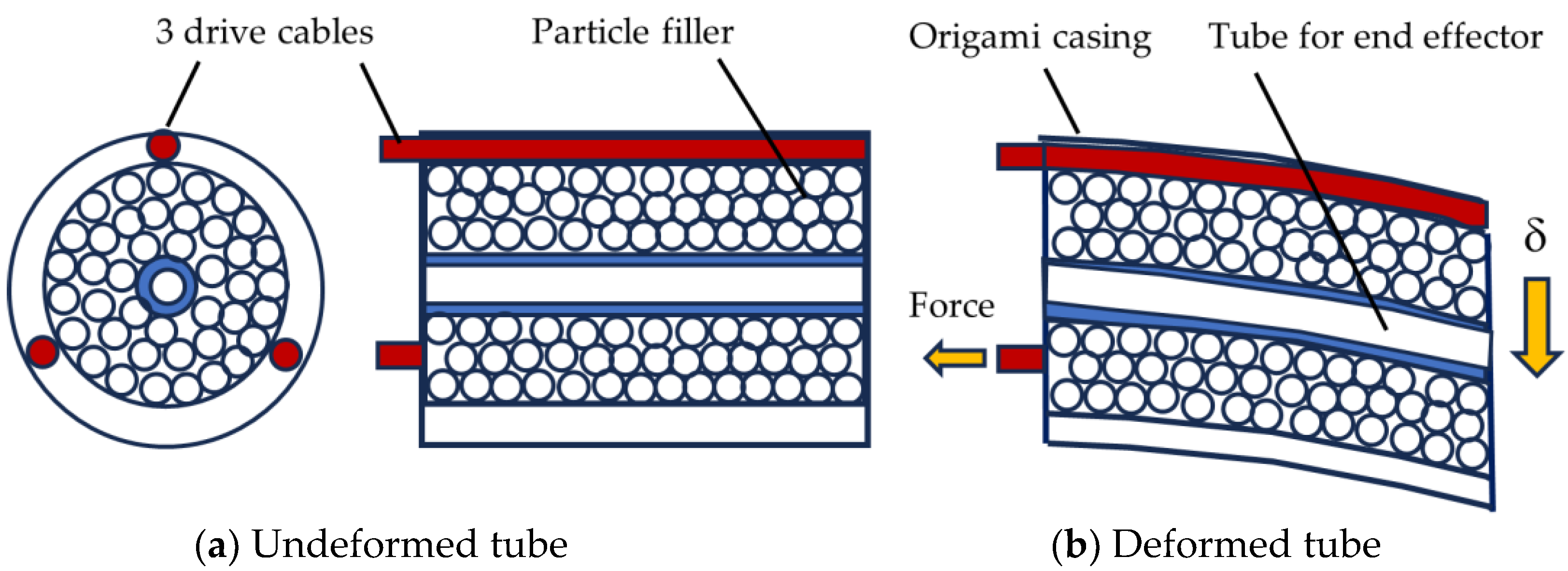
2.6. A Multifunctioning Robotic Wrist
3. The Human Knee Joint
3.1. Knee Joint Anatomy
3.2. Requirements and Objectives
3.3. Knee Functions and Common Parts
| Main Functions | Flexion–Extension | Rotation | Locking at Full Extension | Shock Absorber |
|---|---|---|---|---|
| Schematic | Femur rolls against tibia | Geometry of joint allows rotation | Geometry of joint allows locking | Meniscus absorbs shock |
| Common parts | Condyles Ligaments Meniscus | Condyles Ligaments Meniscus | Condyles Ligaments Meniscus | Condyles Meniscus |
| Actuation [51] | Seven muscles over-actuated | Five muscles over-actuated | One muscle for unlocking | |
| Fine tuning | Geometry of 4-bar linkage | Geometry of tibia plateau. Meniscus geometry | Joint geometry. Popliteus muscle alignment | Meniscus geometry |
| Integration | Ligaments, muscles and meniscus | Ligaments, muscles and meniscus | Ligaments, muscles and meniscus | Meniscus surrounding condyles |
| Reconfiguration | Unlocking with popliteus muscle | |||
| Miniaturisation | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication |
3.4. Actuation and Fine Tuning
3.5. Integration, Reconfiguration and Miniaturisation
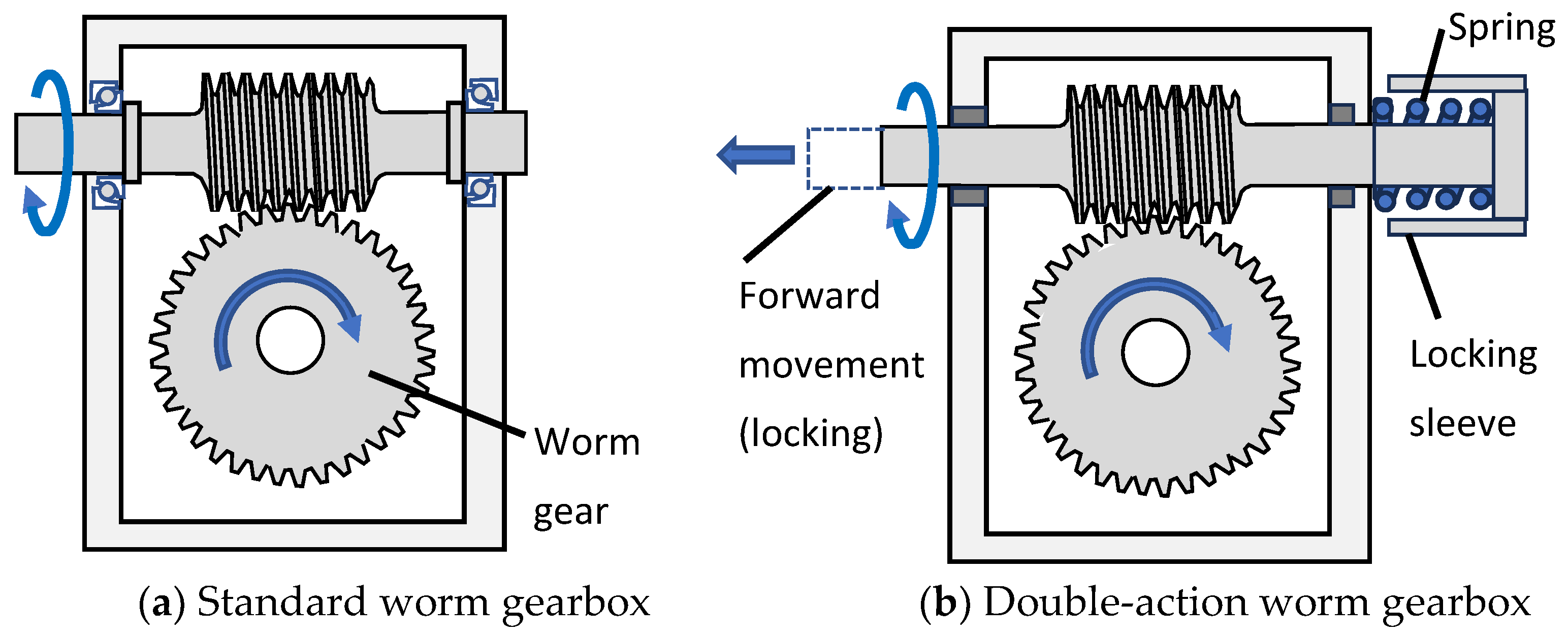
3.6. A Multifunctioning Lockable Robotic Joint
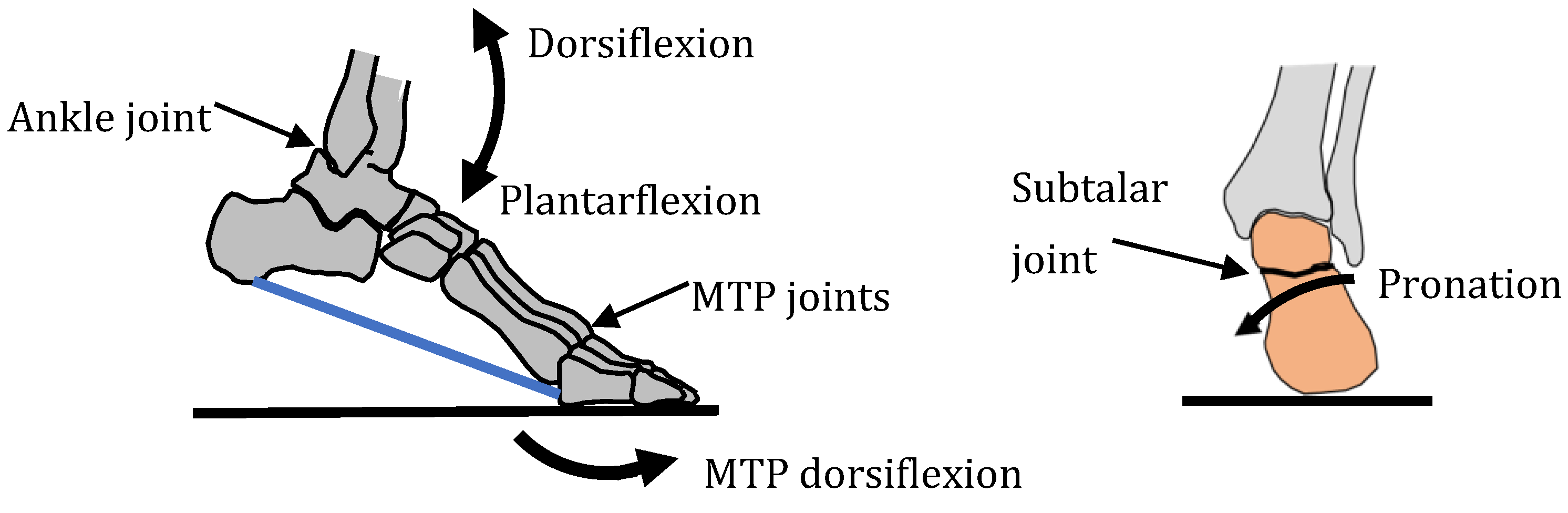
4. Human Foot Joints
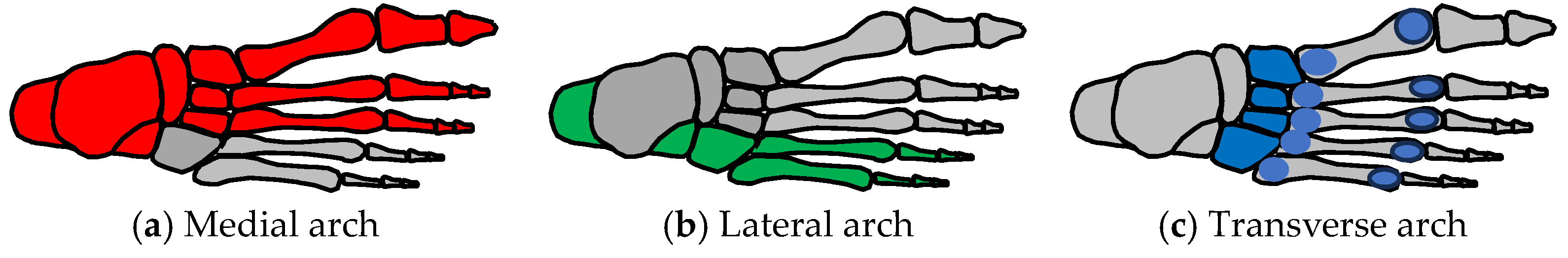
4.1. Foot Anatomy
4.2. Requirements and Objectives
4.3. Foot Functions and Common Parts
4.4. Actuation and Fine-Tuning
4.5. Integration, Reconfiguration and Miniaturisation
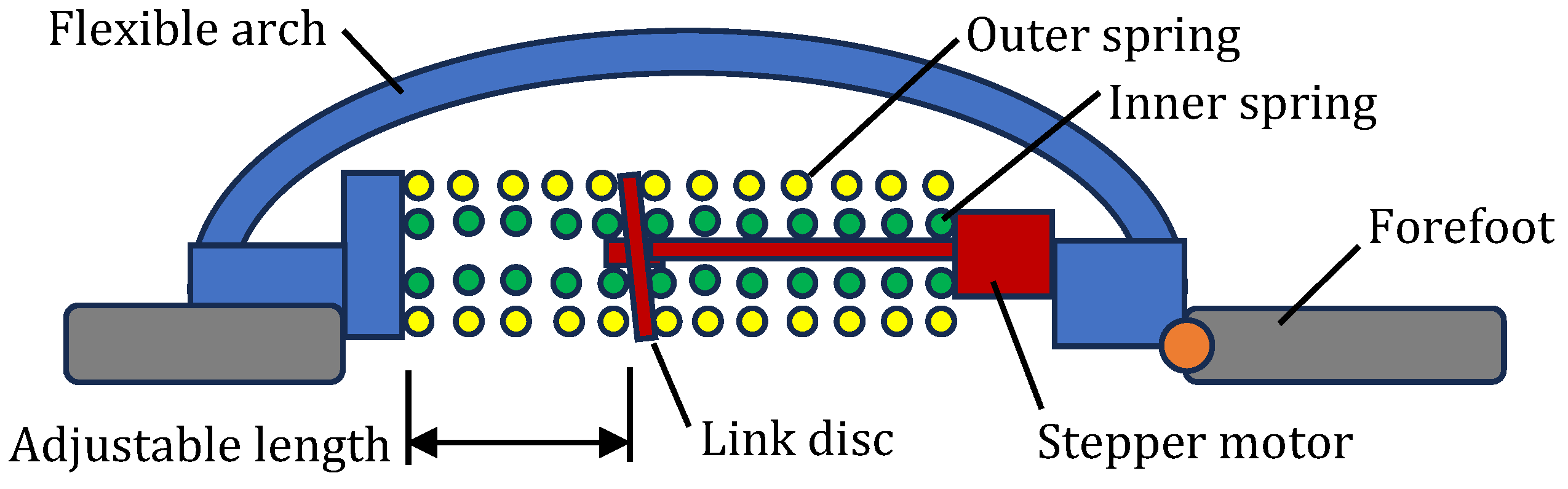
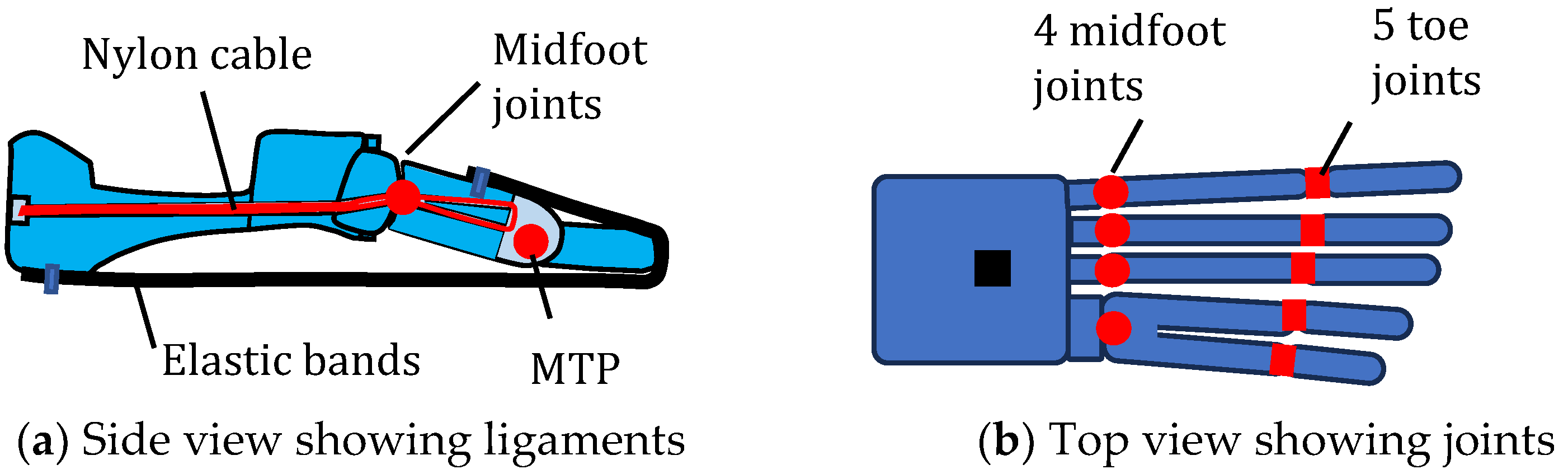
4.6. Multifunctioning Robotic Feet
5. Skeletal Muscle
5.1. Muscle Anatomy
5.2. Requirements and Objectives
5.3. Actuator Functions and Common Parts
5.4. Fine-Tuning
5.5. Integration, Reconfiguration and Miniaturisation
5.6. Bioinspired Muscle
6. Discussion
6.1. The Importance of Multifunctioning in Engineering Systems
6.2. Energy Benefits of Multifunctioning Limb Joints
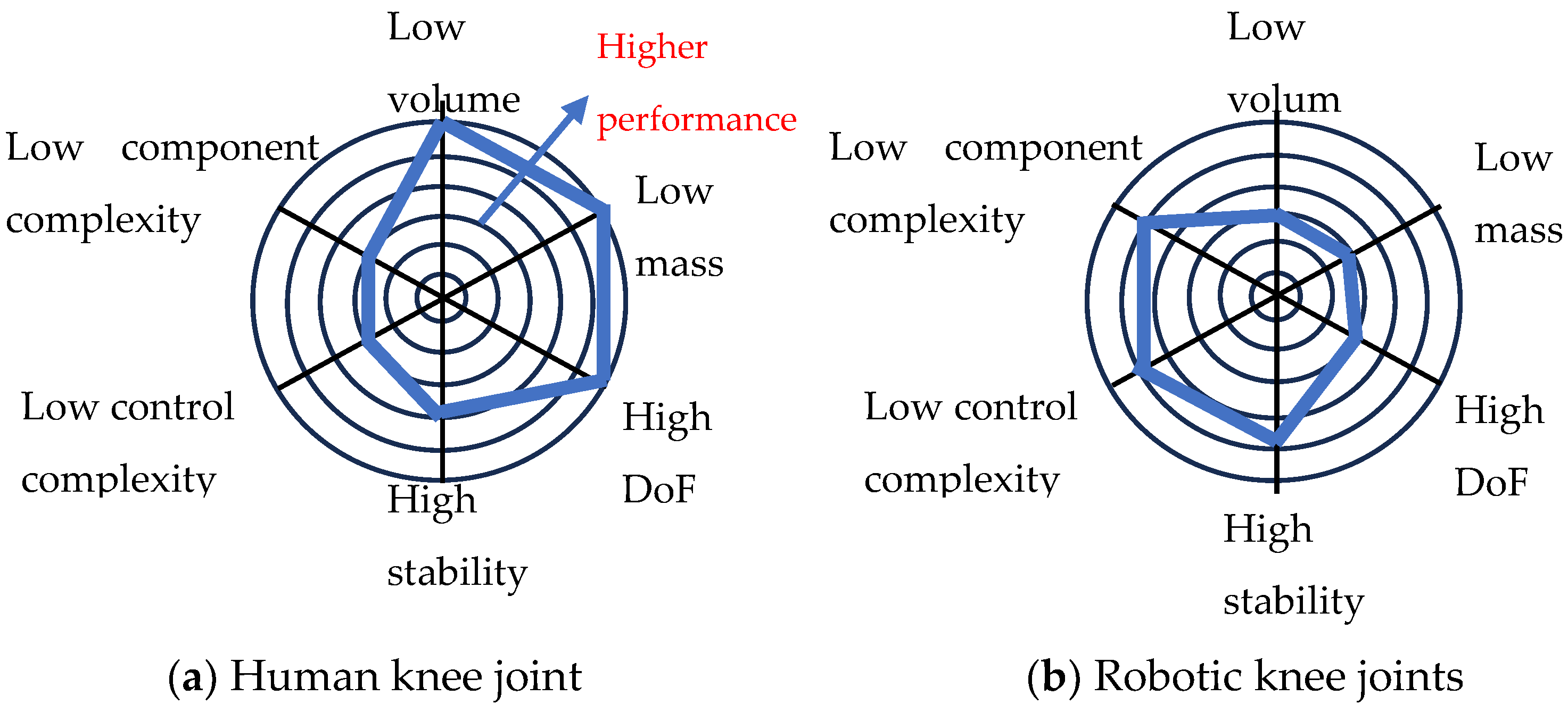
6.3. Comparison between Animal and Robot Joints
6.4. Strategies for Producing Multifunctioning
6.5. Trade-Offs for Multifunctioning
6.6. Design Process for Multifunctioning
6.7. The Emergence of Multifunctioning in Biology
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burden, S.A.; Libby, T.; Jayaram, K.; Sponberg, S.; Donelan, J.M. Why animals can outrun robots. Sci. Robot. 2024, 9, eadi9754. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Liu, H.; Zhang, Z. Advancements in humanoid robots: A comprehensive review and future prospects. IEEE/CAA J. Autom. Sin. 2024, 11, 301–328. [Google Scholar] [CrossRef]
- Egan, P.; Sinko, R.; LeDuc, P.; Keten, S. The role of mechanics in biological and bio-inspired systems. Nat Commun. 2015, 6, 7418. [Google Scholar] [CrossRef] [PubMed]
- Meglio, P.D.; Perera, G.K.; Nestle, F.O. The Multitasking Organ: Recent Insights into Skin Immune Function. Immunity 2011, 35, 857–869. [Google Scholar]
- Székely, G. A perfect design: The multifunctional muscle. Behav. Brain Sci. 1989, 12, 668–669. [Google Scholar] [CrossRef]
- Mellis, D.J.; Itzstein, C.; Helfrich, M.H.; Crockett, J.C. The skeleton: A multi-functional complex organ. The role of key signalling pathways in osteoclast differentiation and in bone resorption. J. Endocrinol. 2011, 211, 131–143. [Google Scholar] [CrossRef]
- Al-Maskari, N.S.; McAdams, D.A.; Reddy, J.N. Modelling of a biological material nacre: Multi-objective optimization model. Mech. Adv. Mater. Struct. 2019, 28, 430–439. [Google Scholar] [CrossRef]
- Burgess, S.C. Multi-optimisation and multi-functioning in feathers. J. Des. Nat. 2007, 1, 1–10. [Google Scholar]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional Roles of Plant Cuticle During Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Guilak, F. The Slippery Slope of Arthritis. Arthritis Rheum. 2005, 52, 1632–1633. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Tubbs, R.S. Anatomy of the Oral Cavity. In Atlas of Oral and Maxillofacial Anatomy; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Jung, J.; Ahn, H.K.; Huh, Y. Clinical and Functional Anatomy of the Urethral Sphincter. Int. Neurourol. J. 2012, 16, 102–106. [Google Scholar] [CrossRef]
- Garrett, J.N.; Fish, F.E. Kinematics of terrestrial locomotion in harbor seals and gray seals: Importance of spinal flexion by amphibious phocids. Mar. Mammal Sci. 2015, 31, 459–478. [Google Scholar] [CrossRef]
- Lock, R.J.; Burgess, S.C.; Vaidyanathan, R. Multi-modal locomotion: From animal to application. Bioinspir. Biomim. 2014, 9, 011001. [Google Scholar] [CrossRef]
- Cherian, V.T. Physiological Functions of Blood. In Blood Substitutes and Oxygen Biotherapeutics; Liu, H., Kaye, A.D., Jahr, J.S., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Chapple, C.E.; Robisson, B.; Spinelli, L.; Guien, C.; Becker, E.; Brun, C. Extreme multifunctional proteins identified from a human protein interaction network. Nat. Commun. 2015, 6, 7412. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Kottarappat, M.; Dileepan, N.; Wood, G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, F.M.; van Koningsbruggen, S.; Navascués, J.; Lamond, A.I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007, 8, 574–585. [Google Scholar] [CrossRef]
- Mahadevan, V. Anatomy of the vertebral column. Surgery 2018, 36, 327–332. [Google Scholar] [CrossRef]
- Ning, M.; Ma, Z.; Chen, H.; Cao, J.; Zhu, C.; Liu, Y.; Wang, Y. Design and analysis for a multifunctional rescue robot with four-bar wheel-legged structure. Adv. Mech. Eng. 2018, 10, 168781401774739. [Google Scholar] [CrossRef]
- Gobinath, A.; Manjula Devi, C.; Ezhilraj, B.; Sabarinathan, T.; Anandan, M.; Rajeswari, P. Design and Development of Multifunction Robotic Arm. In Proceedings of the 2024 IEEE Third International Conference on Intelligent Techniques in Control, Optimization and Signal Processing (INCOS), Krishnankoil, Virudhunagar District, Tamil Nadu, India, 14–16 March 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Ma, J.; Liu, Z.; Luo, Y.; Li, J.; Li, Y. Multifunctional Robotic Glove with Active-Passive Training Modes for Hand Rehabilitation and Assistance. In Proceedings of the 2021 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Prague, Czech Republic, 27 September–1 October 2021; pp. 4969–4974. [Google Scholar] [CrossRef]
- Narayana, K.J.; Burela, R.G. A review of recent research on multifunctional composite materials and structures with their applications. Mater. Today Proc. Energy Harvest. 2018, 5, 5580–5590. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Singh, A.K.; Cao, L.; Seo, J.; Rahn, C.D.; Bakis, C.E.; Hickner, M.A. Multifunctional structural lithium-ion battery for electric vehicles. J. Intell. Mater. Syst. Struct. 2017, 28, 1603–1613. [Google Scholar] [CrossRef]
- Eaton, J.; Naraghi, M.; Boyd, J.G. Evaluating multifunctional efficiency of a structural battery composite via thermo-electro-chemical modelling. Multifunct. Mater. 2022, 5, 015001. [Google Scholar] [CrossRef]
- Ranzani, T.; Russo, S.; Bartlett, N.W.; Wehner, M.; Wood, R.J. Increasing the Dimensionality of Soft Microstructures through Injection-Induced Self-Folding. Adv. Mater. 2018, 30, e1802739. [Google Scholar] [CrossRef]
- Jiang, M.; Gravish, N. Reconfigurable laminates enable multifunctional robotic building blocks. Smart Mater. Struct. 2021, 30, 035005. [Google Scholar] [CrossRef]
- Bajaj, N.M.; Spiers, A.J.; Dollar, A.M. State of the Art in Prosthetic Wrists: Commercial and Research Devices. In Proceedings of the IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015. [Google Scholar]
- Versluys, R.; Beyl, P.; Van Damme, M.; Desomer, A.; Van Ham, R.; Lefeber, D. Prosthetic feet: State-of-the-art review and the importance of mimicking human ankle–foot biomechanics. Disabil. Rehabil. Assist. Technol. 2009, 4, 65–75. [Google Scholar] [CrossRef]
- Shigemi, S. ASIMO and Humanoid Robot Research at Honda. In Humanoid Robotics: A Reference; Goswami, A., Vadakkepat, P., Eds.; Springer: Dordrecht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Koolen, T. Design of a Momentum-Based Control Framework and Application to the Humanoid Robot Atlas. Int. J. Humanoid Robot. 2016, 13, 1650007-1. [Google Scholar] [CrossRef]
- Gray, H. Anatomy of the Human Body, 20th ed.; Lewis, W.H., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1918. [Google Scholar]
- Berger, R.A. The Anatomy of the Ligaments of the Wrist and Distal Radioulnar Joints. Clin. Orthop. Relat. Res. 2001, 383, 32–40. [Google Scholar] [CrossRef]
- Mansfield, P.J.; Neumann, D.A. Essentials of Kinesiology for the Physical Therapist Assistant, 3rd ed.; Chapter 6—Structure and Function of the Wrist; Elsevier: Amsterdam, The Netherlands, 2019; pp. 120–140. [Google Scholar]
- Palmer, A.K.; Werner, F.W. The triangular fibrocartilage complex of the wrist—Anatomy and function. J. Hand Surg. Am. 1981, 6, 153–162. [Google Scholar] [CrossRef]
- Hagert, E.; Rein, S. Wrist proprioception-An update on scientific insights and clinical implications in rehabilitation of the wrist. J. Hand Ther. 2024, 37, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, J.; Li, J.; Quack, V.; Rath, B.; Baroncini, A.; Hildebrand, F.; Migliorini, F. Anatomy, Biomechanics, and Loads of the Wrist Joint. Life 2022, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Wrist. Available online: https://med.libretexts.org/Bookshelves/Anatomy_and_Physiology/Anatomy_and_Physiology_(Boundless)/9%3A_Muscular_System/9.9%3A_Muscles_of_the_Upper_Limb/9.9B%3A_Muscles_of_the_Wrist_and_Hand (accessed on 1 August 2024).
- Xiu, K.-H.; Kim, J.-H.; Li, Z.-M. Biomechanics of the Transverse Carpal Arch under Carpal Bone Loading. Clin. Biomech. (Bristol. Avon.) 2010, 25, 776–780. [Google Scholar] [CrossRef]
- Ayhan, C.; Ayhan, E. Chapter 13—inesiology of the Wrist and the Hand. In Comparative Kinesiology of the Human Body: Normal and Pathological Conditions; Angin, S., Şimşek, I.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 211–282. [Google Scholar]
- Rainbow, M.J.; Wolff, A.L.; Crisco, J.J.; Wolfe, S.W. Functional kinematics of the wrist. J. Hand Surg. Eur. Vol. 2015, 41, 7–21. [Google Scholar] [CrossRef]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, H.; Chen, W.; Cheng, S.S.; Liu, Y.-H. Bioinspired Soft Wrist Based on Multicable Jamming with Hybrid Motion and Stiffness Control for Dexterous Manipulation. IEEE/ASME Trans. Mechatron. 2023, 28, 1256–1267. [Google Scholar] [CrossRef]
- Abulhasan, J.F.; Grey, M.J. Anatomy and Physiology of Knee Stability. J. Funct. Morphol. Kinesiol. 2017, 2, 34. [Google Scholar] [CrossRef]
- Hallén, L.G.; Lindahl, O. The “Screw-Home” Movement in the Knee-Joint. Acta Orthop. Scand. 2009, 37, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H. Role of knee ligaments in proprioception and regulation of muscle stiffness. J. Electromyogr. Kinesiol. 1991, 1, 158–179. [Google Scholar] [CrossRef]
- Zarins, B.; Rowe, C.R.; Harris, B.A.; Watkins, M.P. Rotational motion of the knee. Am. J. Sports Med. 1983, 11, 152–156. [Google Scholar] [CrossRef]
- Knee. Available online: https://med.libretexts.org/Bookshelves/Anatomy_and_Physiology/Human_Anatomy_Laboratory_Manual_2021/07%3A_Joints/7.02%3A_Knee_Joint (accessed on 1 August 2024).
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. A novel classification of planar four-bar linkages and its application to the mechanical analysis of animal systems. Phil. Trans. R. Soc. Lond. B 1996, 351, 689–720. [Google Scholar]
- Etoundi, A.C.; Burgess, S.C.; Vaidyanathan, R. A Bio-Inspired Condylar Hinge for Robotic Limbs. ASME J. Mech. Rob. 2013, 5, 031011. [Google Scholar] [CrossRef]
- Burgess, S.C. A backwards design process for Mechanical Design. J. Mech. Des. ASME 2012, 134, 031002. [Google Scholar] [CrossRef]
- Righetti, P.; De Juana Gamo, J.; Sancho, F. Metop-C deployment and start of three-satellite operations. Aeronaut. J. 2020, 124, 902–916. [Google Scholar] [CrossRef]
- Angin, S.; Demirbüken, I. Chapter 23—Ankle and Foot Complex. In Comparative Kinesiology of the Human Body: Normal and Pathological Conditions; Angin, S., Şimşek, I.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 411–439. [Google Scholar]
- Lundberg, A.; Svensson, O.K.; Bylund, C.; Goldie, I.; Selvik, G. Kinematics of the ankle/foot complex—Part 2: Pronation and supination. Foot Ankle 1989, 9, 248–253. [Google Scholar] [CrossRef]
- Foot. Available online: https://med.libretexts.org/Bookshelves/Anatomy_and_Physiology/Anatomy_and_Physiology_(Boundless)/9%3A_Muscular_System/9.10%3A_Muscles_of_the_Lower_Limb/9.10C%3A_Muscles_that_Cause_Movement_at_the_Foot (accessed on 1 August 2024).
- Burdett, R. Forces predicted at the ankle during running. Med. Sci. Sports Exerc. 1981, 14, 308–316. [Google Scholar] [CrossRef]
- Salathe, E.P., Jr.; Arangio, G.A.; Salathe, E.P. The foot as a shock absorber. J. Biomech. 1990, 23, 655–659. [Google Scholar] [CrossRef]
- Ker, R.F.; Bennett, M.B.; Bibby, S.R.; Kester, R.C.; Alexander, R.M. The spring in the arch of the human foot. Nature 1987, 325, 147–149. [Google Scholar] [CrossRef]
- Alfuth, M.; Rosenbaum, D. Long distance running and acute effects on plantar foot sensitivity and plantar foot loading. Neurosci. Lett. 2011, 503, 58–62. [Google Scholar] [CrossRef]
- Venkadesan, M.; Yawar, A.; Eng, C.M.; Dias, M.A.; Singh, D.K.; Tommasini, S.M.; Haims, A.H.; Bandi, M.M.; Mandre, S. Stiffness of the human foot and evolution of the transverse arch. Nature 2020, 579, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Boonpratatong, A.; Ren, L. The human ankle-foot complex as a multi-configurable mechanism during the stance phase of walking. J. Bionic Eng. 2010, 7, 211–218. [Google Scholar] [CrossRef]
- Shah, N.S.; Umeda, Y.; Suriel Peguero, E.; Erwin, J.T.; Laughlin, R. Outcome reporting in total ankle arthroplasty: A systematic review. J. Foot Ankle Surg. 2021, 60, 770–776. [Google Scholar] [CrossRef]
- Grimmer, M.; Seyfarth, A. Mimicking Human-Like Leg Function in Prosthetic Limbs. In Neuro-Robotics; Springer: Dordrecht, The Netherlands, 2014; pp. 105–155. [Google Scholar]
- Russo, M.; Dayana, B.; Chaparro-Rico, M.; Pavone, L.; Pasqua, G.; Cafolla, D. A bioinspired humanoid foot mechanism. Appl. Sci. 2021, 11, 1686. [Google Scholar] [CrossRef]
- Qaisera, Z.; Kanga, L.; Johnson, S. Design of a bioinspired tunable stiffness robotic foot. Mech. Mach. Theory 2017, 110, 1–15. [Google Scholar] [CrossRef]
- Burgess, S.; Beeston, A.; Carr, J.; Siempou, K.; Simmonds, M.; Zanker, Y. A Bio-Inspired Arched Foot with Individual Toe Joints and Plantar Fascia. Biomimetics 2023, 8, 455. [Google Scholar] [CrossRef]
- Sun, J.; Song, G.; Chu, J.; Ren, L. An adaptive bioinspired foot mechanism based on tensegrity structures. Soft Robot. 2019, 6, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Buehler, M.J.; Redaelli, A. Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J. Mech. Behav. Biomed. Mater. 2009, 2, 130–137. [Google Scholar] [CrossRef]
- Roach, C.; Love, C.; Allen, T.; Proske, U. The contribution of muscle spindles to position sense measured with three different methods. Exp. Brain Res. 2023, 241, 2433–2450. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Jiang, Y.; Zang, W.; Cao, P.; Tian, M.; Ning, N.; Zhang, L. Dielectric elastomer actuators for artificial muscles: A comprehensive review of soft robot explorations. Resour. Chem. Mater. 2022, 1, 308–324. [Google Scholar] [CrossRef]
- Ibarz, A.; Felip, O.; Fernández-Borràs, J.; Martín-Pérez, M.; Blasco, J.; Torrella, J.R. Sustained swimming improves muscle growth and cellularity in gilthead sea bream. J. Comp. Physiol. B 2011, 181, 209–217. [Google Scholar] [CrossRef] [PubMed]
- West, T.G.; Toepfer, C.N.; Woledge, R.C.; Curtin, N.A.; Rowlerson, A.; Kalakoutis, M.; Hudson, P.; Wilson, A.M. Power output of skinned skeletal muscle fibres from the cheetah (Acinonyx jubatus). J. Exp. Biol. 2013, 216, 2974–2982. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. (1985) 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Roberts, T.J.; Konow, N. How tendons buffer energy dissipation by muscle. Exerc. Sport Sci. Rev. 2013, 41, 186–193. [Google Scholar] [CrossRef]
- Marshall, N.J.; Glaser, J.I.; Trautmann, E.M.; Amematsro, E.A.; Perkins, S.M.; Shadlen, M.N.; Abbott, L.F.; Cunningham, J.P.; Churchland, M.M. Flexible neural control of motor units. Nat. Neurosci. 2022, 25, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Haman, F.; Blondin, D.P. Shivering thermogenesis in humans: Origin, contribution and metabolic requirement. Temperature 2017, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Qian, Z.; Wang, K.; Zhao, F.; Ding, P.; Xu, D.; Wei, G.; Ren, L.; Ren, L. Bioinspired actuators with intrinsic muscle-like mechanical properties. iScience 2021, 24, 103023, ISSN 2589-0042. [Google Scholar] [CrossRef]
- Pei, Q.; Rosenthal, M.; Stanford, S.; Prahlad, H.; Pelrine, R. Multiple-degrees-of-freedom electroelastomer roll actuators. Smart Mater. Struct. 2004, 13, N86. [Google Scholar] [CrossRef]
- Hwang, T.; Frank, Z.; Neubauer, J.; Kim, K.J. High-performance polyvinyl chloride gel artificial muscle actuator with graphene oxide and plasticizer. Sci. Rep. 2019, 9, 9658. [Google Scholar] [CrossRef]
- Miriyev, A.; Stack, K.; Lipson, H. Soft material for soft actuators. Nat. Commun. 2017, 8, 596. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Sun, Y.; Wu, M.; Su, J.; Li, Y.; Yu, X.; Li, L.; Yu, J. Muscle-inspired soft robots based on bilateral dielectric elastomer actuators. Microsyst. Nanoeng. 2023, 9, 124. [Google Scholar] [CrossRef]
- Sairajana, K.K.; Agliettib, G.S.; Mania, K.M. A review of multifunctional structure technology for aerospace applications. Acta Astronaut. 2016, 120, 30–42. [Google Scholar] [CrossRef]
- Kilian, L.; Shahid, F.; Zhao, J.S.; Nayeri, C.N. Bioinspired morphing wings: Mechanical design and wind tunnel experiments. Bioinspir. Biomim. 2022, 17, 046019. [Google Scholar] [CrossRef] [PubMed]
- Khandalavala, K.; Shimon, T.; Flores, L.; Armijo, P.R.; Oleynikov, D. Emerging surgical robotic technology: A progression toward microbots. Ann. Laparosc. Endosc. Surg. 2020, 5, 3. [Google Scholar] [CrossRef]
- Kim, C. Evolution of Advanced Miniaturization for Active Implantable Medical Devices; Nano-Bio-Electronic, Photonic and MEMS Packaging; Wong, C.P.P., Moon, K.S., Li, Y., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- South, A. Miniaturization of medical products: The development challenge. Med. Device Technol. 1998, 9, 30–34. [Google Scholar]
- Wu, Y. Multifunctional devices from asymmetry. Nat. Electron. 2018, 1, 331–332. [Google Scholar] [CrossRef]
- Jiro Doke, J.; Donelan, M.; Kuo, A.D. Mechanics and energetics of swinging the human leg. J. Exp. Biol. 2005, 208, 439–445. [Google Scholar] [CrossRef]
- Rodrigo-Carranza, V.; González-Mohíno, F.; Santos-Concejero, J.; González-Ravé, J.M. Influence of Shoe Mass on Performance and Running Economy in Trained Runners. Front. Physiol. 2020, 11, 573660. [Google Scholar] [CrossRef]
- Taboga, P.; Lazzer, S.; Fessehatsion, R.; Agosti, F.; Sartorio, A.; di Prampero, P.E. Energetics and mechanics of running men: The influence of body mass. Eur. J. Appl. Physiol. 2012, 112, 4027–4033. [Google Scholar] [CrossRef]
- Burgess, S.C. Universal optimal design in the vertebrate limb pattern and lessons for bioinspired design. Bioinspir. Biomim. 2024, 19, 051004. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.; Cichella, V.; Lamuta, C. Bioinspired Soft Robotics: State of the Art, Challenges, and Future Directions. Curr. Robot. Rep. 2023, 4, 65–80. [Google Scholar] [CrossRef]
- Zhu, J.; Dexheimer, M.; Cheng, H. Reconfigurable systems for multifunctional electronics. NPJ Flex. Electron. 2017, 1, 8. [Google Scholar] [CrossRef]
- Yu, M.; Evangelou, S.A.; Dini, D. Advances in Active Suspension Systems for Road Vehicles. Engineering 2024, 33, 160–177. [Google Scholar] [CrossRef]
- Jordan Nickel, P.; Duimering, R.; Hurst, A. Manipulating the design space to resolve trade-offs: Theory and evidence. Des. Stud. 2022, 79, 101095. [Google Scholar] [CrossRef]
- Liu-Ambrose, T. The anterior cruciate ligament and functional stability of the knee joint. BCMJ 2003, 45, 495–499. [Google Scholar]
- Al-Ghuwairi, A.R.; Al-Fraihat, D.; Sharrab, Y.; Alrashidi, H.; Almujally, N.; Kittaneh, A.; Ali, A. Visualizing software refactoring using radar charts. Sci. Rep. 2023, 13, 19530. [Google Scholar] [CrossRef]
- Cross, N. Engineering Design Methods, Strategies for Product Design, 5th ed.; Wiley: Chichester, UK, 2021. [Google Scholar]
- Hülagü, R.; Timur, Ş. Using Morphological Chart for Analysing Existing Designs. Arch. Des. Res. 2024, 37, 27–41. [Google Scholar] [CrossRef]
- Smith, G.; Troy, T.J.; Summers, J.D. Concept Exploration Through Morphological Charts: An Experimental Study. In Proceedings of the ASME 2006 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, Philadelphia, PA, USA, 10–13 September 2006; Volume 4a, pp. 495–504. [Google Scholar]
- Fröhlich, T.; Klaiber, D.; Türck, D.E.; Vietor, T. Function in a box: An approach for multi-functional design by function integration and separation. In Proceedings of the 29th CIRP Design, Póvoa de Varzim, Portgal, 8–10 May 2019. [Google Scholar]
- Fröhlich, T.; Vietor, T. Design Principles and Multi-Level Structures for Multi-Functional and Multi-Material Design. Proc. Des. Soc. 2022, 2, 395–404. [Google Scholar] [CrossRef]
- Svendsen, N.W.; Lenau, T.A. Approaches to analyzing multi-functional problems. In Proceedings of the DS 101: Proceedings of NordDesign 2020, Lyngby, Denmark, 12–14 August 2020. [Google Scholar]
- Hébert-Dufresne, L.; Allard, A.; Garland, J.; Hobson, E.A.; Zaman, L. The path of complexity. NPJ Complex. 2024, 1, 4. [Google Scholar] [CrossRef]
- Jantzen, B.C. An Introduction to Design Arguments. Cambridge Introductions to Philosophy; Cambridge University Press: Cambridge, UK, 2014; pp. 189–206. [Google Scholar]
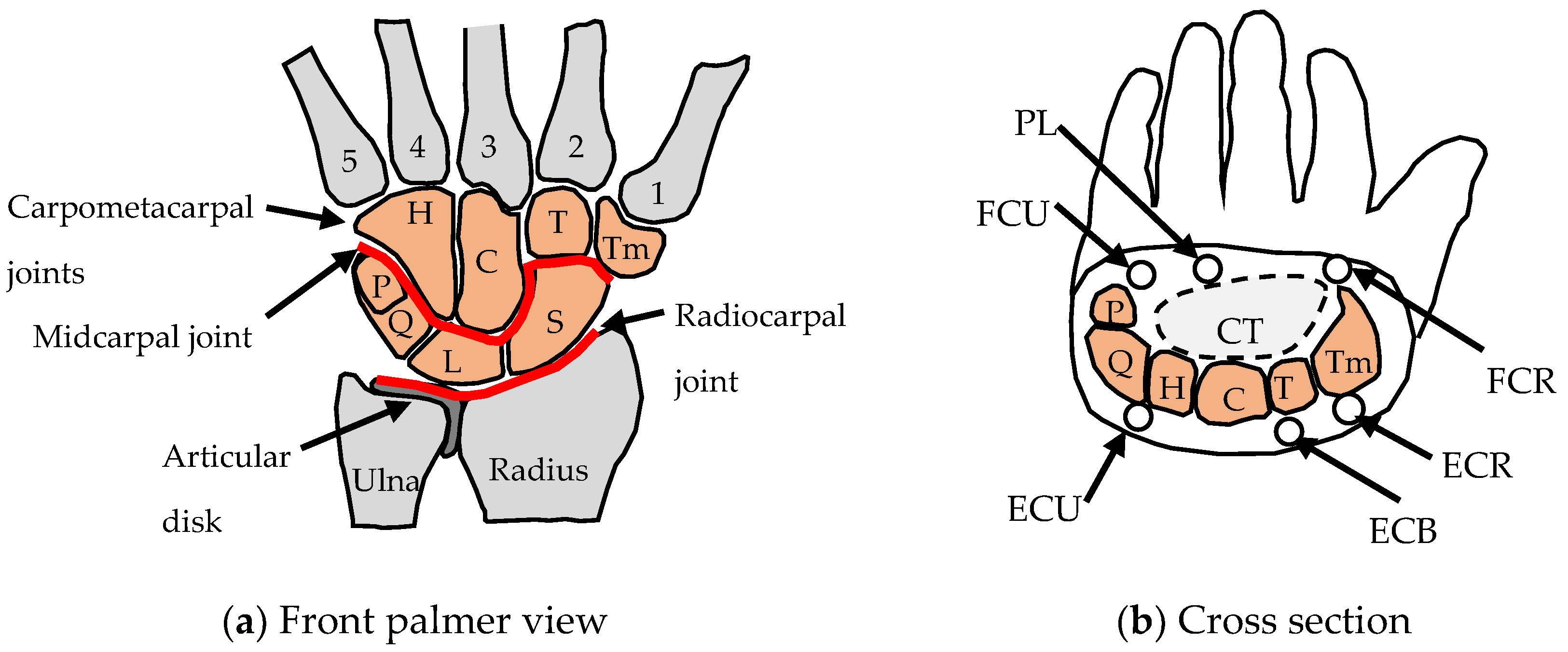


| Main Functions | Flexion–Extension | Abduction–Adduction | Longitudinal Load Paths | Carpal Arch |
|---|---|---|---|---|
| Schematic | Midcarpal joint Radiocarpal joint  | Midcarpal joint Radiocarpal joint  | Two load columns Two arches  | Arch in cross-section of hand |
| Common parts (wrist bones) | All of top row Three of bottom row  | Three of top row Three of bottom row  | All of top row Two of bottom row  | All of top row Two of bottom row  |
| Actuation [41] | Multifunctioning muscles | Multifunctioning muscles | ||
| Fine tuning | Common centre of rotation | Common centre of rotation | Alignment of metacarpals with top row of wrist bones. | Arch segments aligning simultaneously in two different planes |
| Integration | Network of 8 bones and >25 ligaments | Multiple joint interfaces (Capitate = 5) | Capitate styloid process | Carpal arch integrated with tendons/nerves |
| Reconfiguration | Muscle tension to stiffen wrist during loading | Muscle tension to stiffen wrist during loading | ||
| Miniaturisation | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication |
| Main Functions | Stiff Lever (for Push-Off) | Flexible Lever (for Landing) | Stand on Ball of Feet | Three-Point Contact (for Standing) |
|---|---|---|---|---|
| Schematic | Medial arch | All arches Pronation  | Five MTP joints | All arches |
| Common parts | Medial arch | Medial arch Lateral arch Transverse arch | Medial arch Lateral arch Transverse arch | Medial arch Lateral arch Transverse arch |
| Actuation [59] | Seven muscles over-actuated | Four muscles over-actuated | Twenty-two muscles over-actuated | n/a |
| Fine tuning | Alignment of talus bone with medial arch | Spring ligament alignment | Alignment of five MTP joints | Maximised spacing of contact points |
| Integration | Bones of medial arch | Integration of three arches | Integration of three arches | Integration pf three arches |
| Reconfiguration | Tightening of muscles and ligaments | |||
| Miniaturisation | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication |
| Foot Example | Schematic | Flexible Arch | Tuneable Stiffness | MTP Joint | Individual Toes |
|---|---|---|---|---|---|
| [68] | 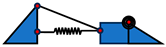 | Y | N | Y | N |
| [69] |  | Y | Y | Y | N |
| [70] | 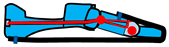 | Y | N | Y | Y |
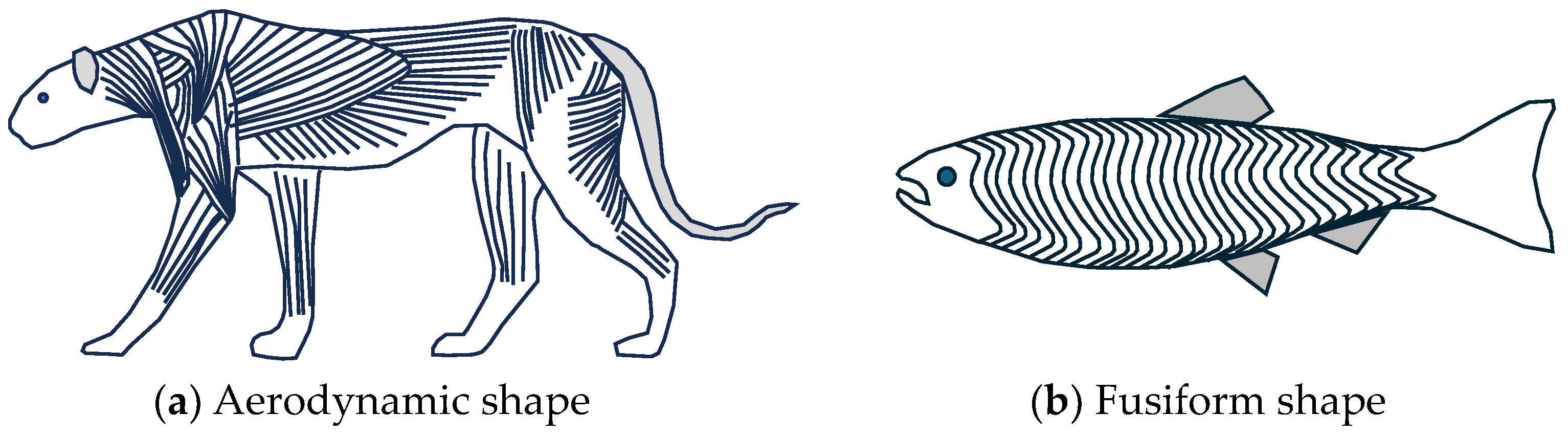
| Main Functions | Actuator (e.g., Move Joints) | Shape (e.g., Optimise Hydrodynamics) | Cushioning (e.g., Protect Organs & Bones) | Heat Source (for Blood Vessels) |
|---|---|---|---|---|
| Schematic | Muscle contraction | Muscle defines animal shape | Protective barrier Thigh section 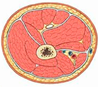 | Heat transfer 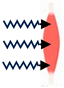 |
| Common parts | Muscle and tendon | Muscle and tendon | Muscle and tendon | Muscle |
| Fine-tuning | MUs recruited from smallest to largest three types of muscle | Animals: aerodynamic shape Fish: hydrodynamic shape | Distribution Self-healing | Maximal heat transfer |
| Integration | Nerves for each muscle unit | Muscles with bones and organs | Muscles with bones and organs | Blood vessels |
| Reconfiguration | Shivering for generating heat [9] | |||
| Miniaturisation | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication | Sensors, nerves, blood vessels, lubrication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgess, S.C. How Multifunctioning Joints Produce Highly Agile Limbs in Animals with Lessons for Robotics. Biomimetics 2024, 9, 529. https://doi.org/10.3390/biomimetics9090529
Burgess SC. How Multifunctioning Joints Produce Highly Agile Limbs in Animals with Lessons for Robotics. Biomimetics. 2024; 9(9):529. https://doi.org/10.3390/biomimetics9090529
Chicago/Turabian StyleBurgess, Stuart C. 2024. "How Multifunctioning Joints Produce Highly Agile Limbs in Animals with Lessons for Robotics" Biomimetics 9, no. 9: 529. https://doi.org/10.3390/biomimetics9090529
APA StyleBurgess, S. C. (2024). How Multifunctioning Joints Produce Highly Agile Limbs in Animals with Lessons for Robotics. Biomimetics, 9(9), 529. https://doi.org/10.3390/biomimetics9090529










