Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration
Abstract
1. Introduction
2. Calcium-Based Materials
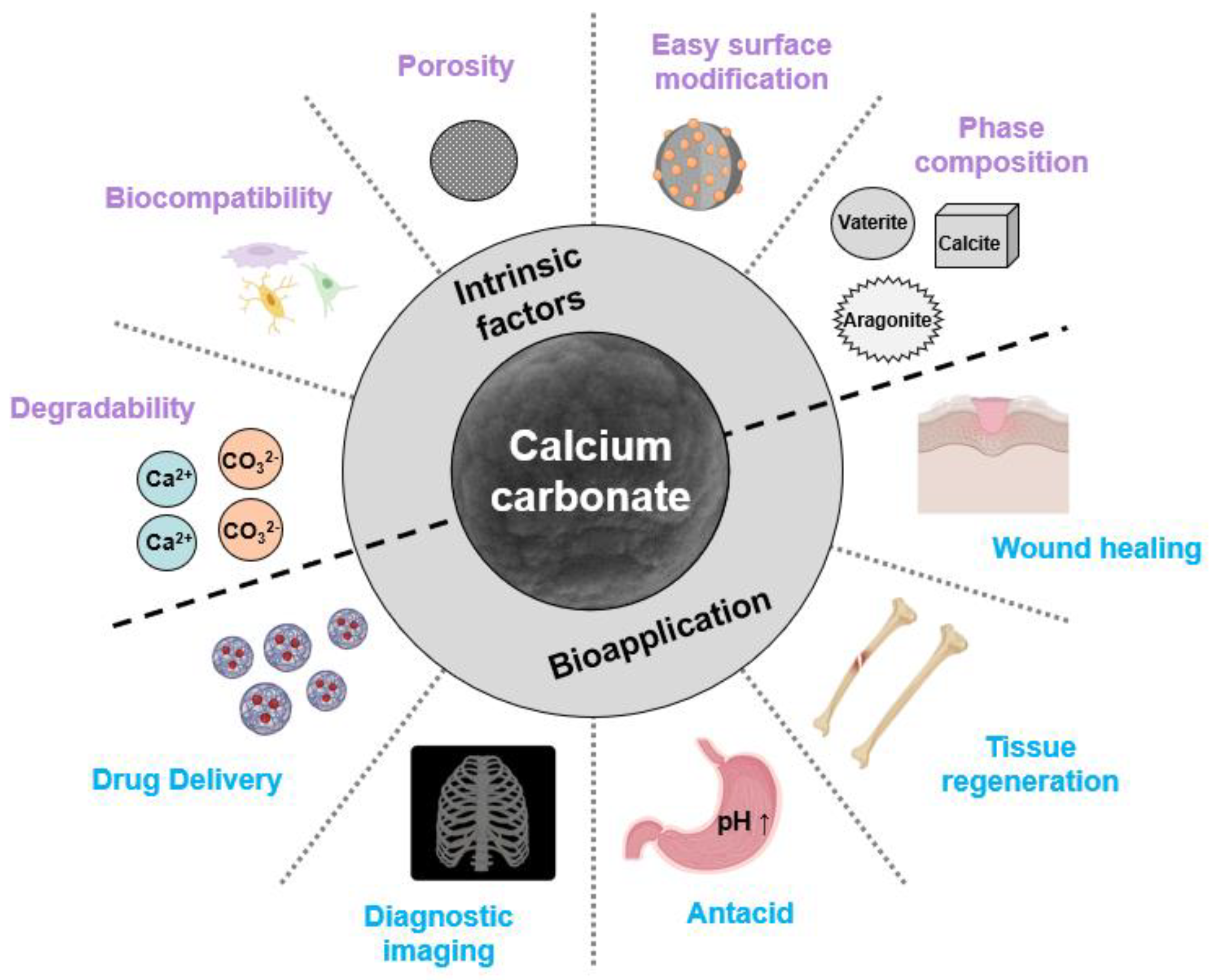
2.1. Calcium Carbonate
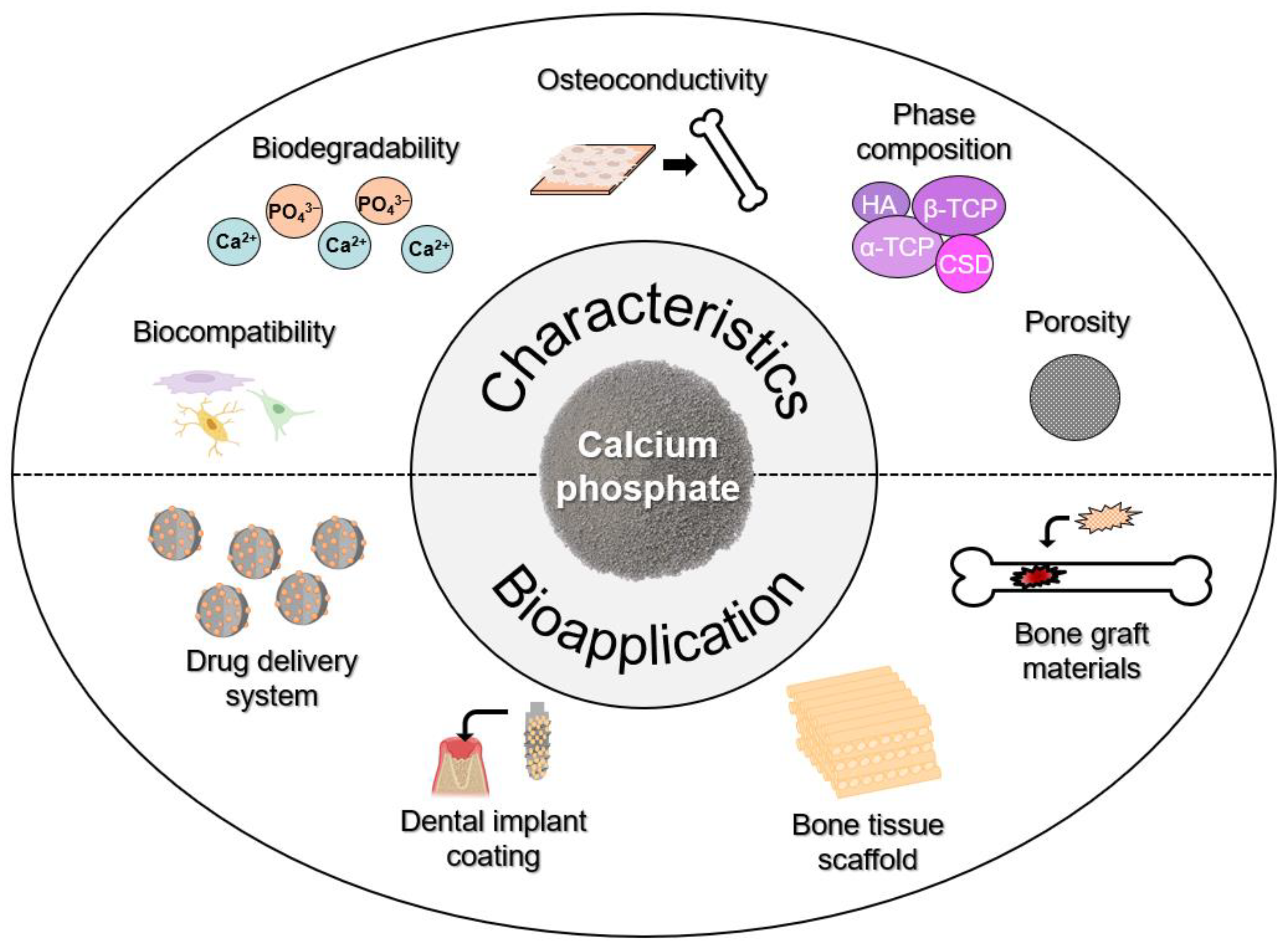
2.2. Calcium Phosphate
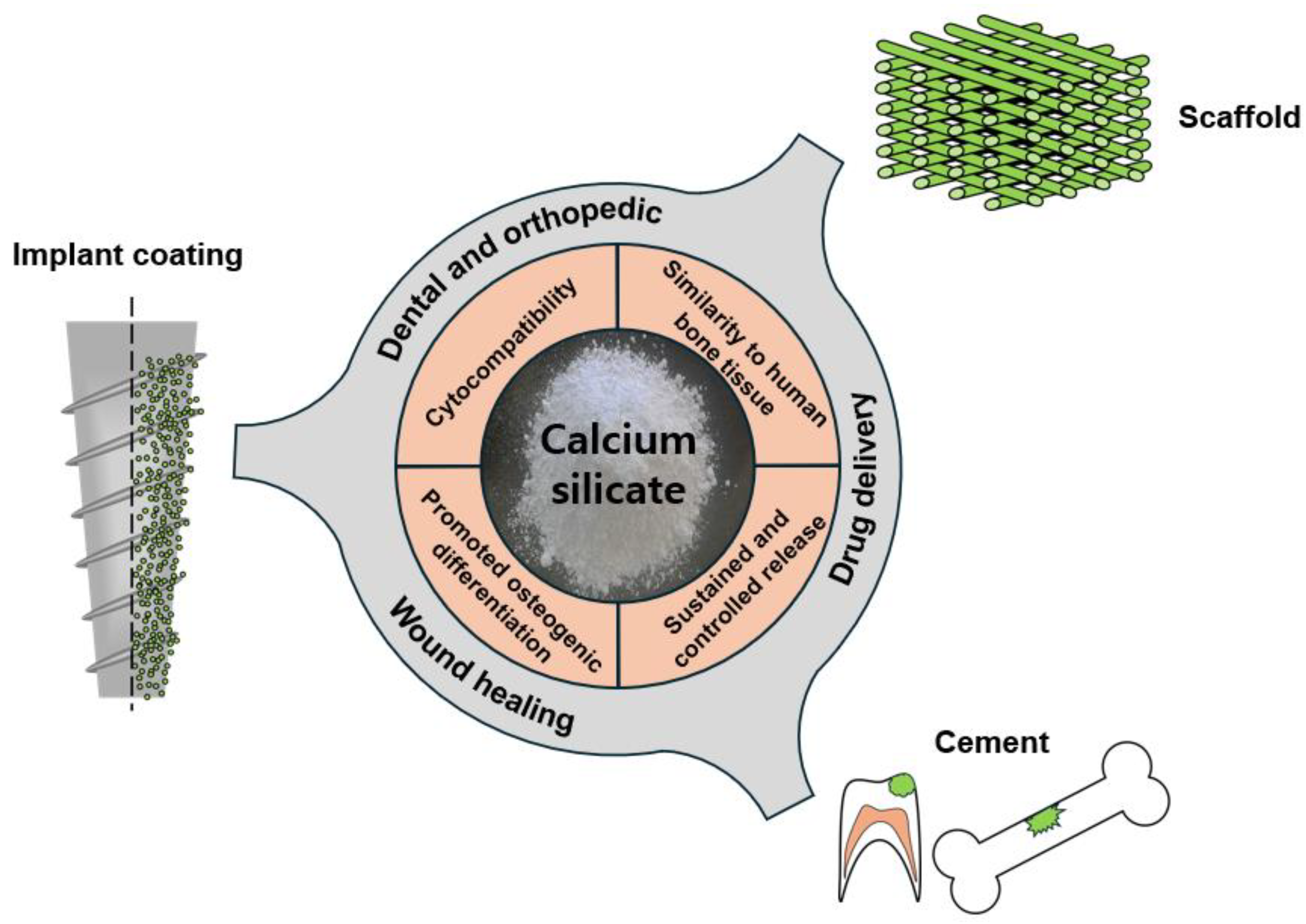
2.3. Calcium Silicate
3. Calcium-Based Materials for Biomedical Applications
3.1. Calcium-Carbonate-Based Applications
3.2. Calcium-Phosphate-Based Applications
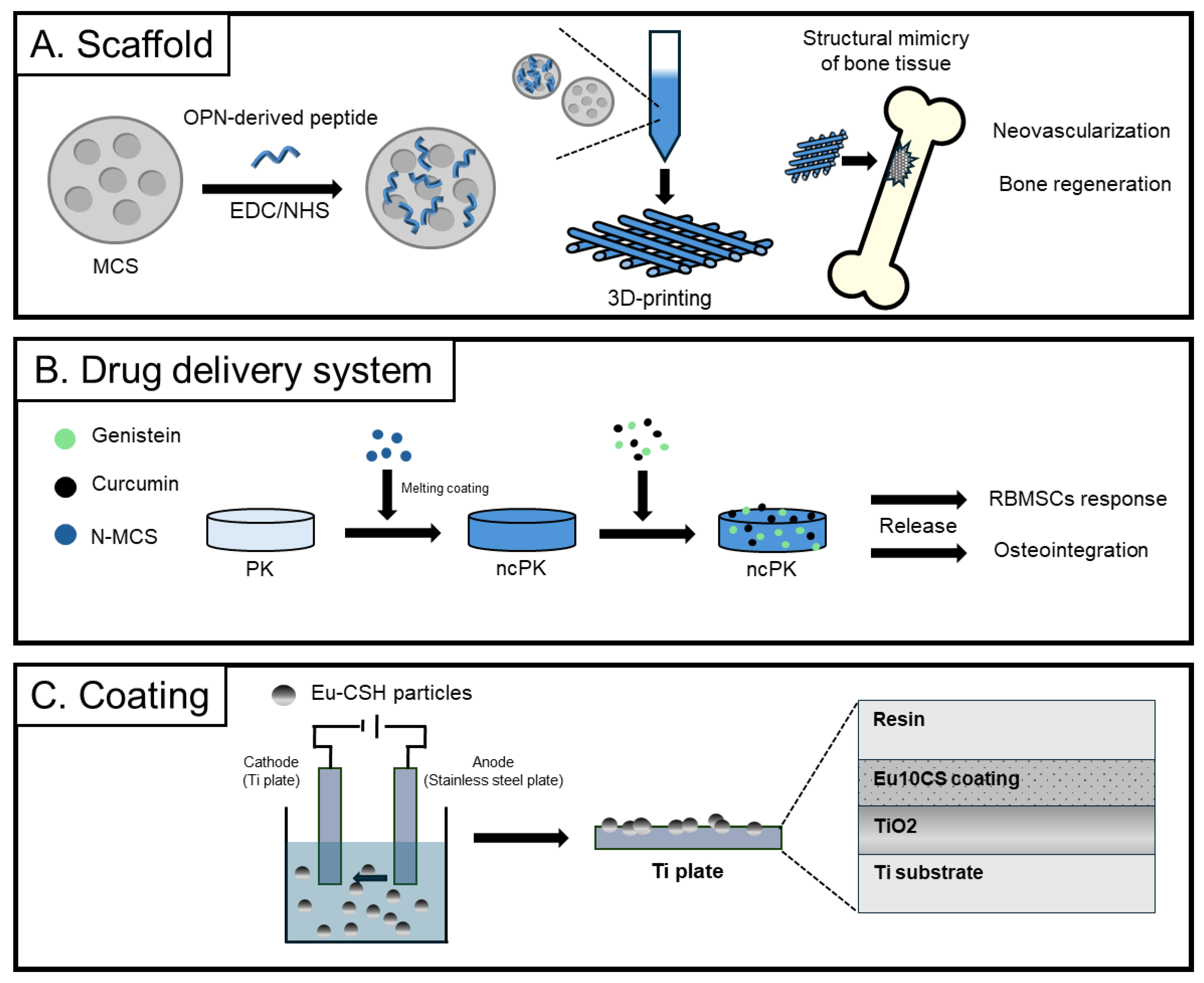
3.3. Calcium-Silicate-Based Applications
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, X.Y.; Fu, S.Y.; Zhu, Y.F. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzade, S.; Liu, J.Y.; Wang, H.R.; Li, X.; Cao, J.R.; Cao, H.L.; Tang, B.; Yuan, H.Y. Recent advances on bioactive baghdadite ceramic for bone tissue engineering applications: 20 years of research and innovation (a review). Mater. Today Bio 2022, 17, 100473. [Google Scholar] [CrossRef]
- Youness, R.A.; El-deen, D.M.T.; Taha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2023, 15, 2493–2505. [Google Scholar] [CrossRef]
- Blokhuis, T.J.; Arts, J.J.C. Bioactive and osteoinductive bone graft substitutes: Definitions, facts and myths. Inj.-Int. J. Care Inj. 2011, 42, S26–S29. [Google Scholar] [CrossRef]
- Liendo, F.; Arduino, M.; Deorsola, F.A.A.; Bensaid, S. Factors controlling and influencing polymorphism, morphology and size of calcium carbonate synthesized through the carbonation route: A review. Powder Technol. 2022, 398, 117050. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.S.; Jin, B.A.; Liu, Z.M.; Shao, C.Y.; Zhao, R.B.; Wang, X.Y.; Tang, R.K. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29, 1605903. [Google Scholar] [CrossRef]
- Huang, D.; He, B.; Mi, P. Calcium phosphate nanocarriers for drug delivery to tumors: Imaging, therapy and theranostics. Biomater. Sci. 2019, 7, 3942–3960. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.Z.; Ma, Y.H.; Jiang, Y.Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef]
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Laurenti, M.; Al Subaie, A.; Abdallah, M.N.; Cortes, A.R.G.; Ackerman, J.L.; Vali, H.; Basu, K.; Zhang, Y.L.; Murshed, M.; Strandman, S.; et al. Two-Dimensional Magnesium Phosphate Nanosheets Form Highly Thixotropic Gels That Up-Regulate Bone Formation. Nano Lett. 2016, 16, 4779–4787. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and Medical Applications of Calcium Phosphate Nanoparticles. Chem.-Eur. J. 2021, 27, 7471–7488. [Google Scholar] [CrossRef]
- Kong, C.H.; Steffi, C.; Shi, Z.L.; Wang, W. Development of mesoporous bioactive glass nanoparticles and its use in bone tissue engineering. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2018, 106, 2878–2887. [Google Scholar] [CrossRef]
- Liu, Z.H.; He, X.Y.; Chen, S.P.; Yu, H.M. Advances in the use of calcium silicate-based materials in bone tissue engineering. Ceram. Int. 2023, 49, 19355–19363. [Google Scholar] [CrossRef]
- Drevet, R.; Faure, J.; Benhayoune, H. Bioactive Calcium Phosphate Coatings for Bone Implant Applications: A Review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tian, Y.; You, J.; Hu, X.; Liu, Y.N. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium carbonate nano- and microparticles: Synthesis methods and biological applications. 3 Biotech 2021, 11, 457. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.Q.; Li, W.D.; Fan, Y.; Li, Z.H.; Wei, J.C. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.J.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.Y.; Chen, Q.M.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.D.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Qu, H.W.; Fu, H.Y.; Han, Z.Y.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Hong, M.H.; Lee, J.H.; Jung, H.S.; Shin, H.; Shin, H. Biomineralization of bone tissue: Calcium phosphate-based inorganics in collagen fibrillar organic matrices. Biomater. Res. 2022, 26, 42. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.S.; Volodkin, D. CaCO3 crystals as versatile carriers for controlled delivery of antimicrobials. J. Control. Release 2020, 328, 470–489. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Choudhary, N.; Gnanamoorthy, G.; Tirth, V.; Prasad, S.; Khan, A.H.; Islam, S.; Khan, N.A. The Processing of Calcium Rich Agricultural and Industrial Waste for Recovery of Calcium Carbonate and Calcium Oxide and Their Application for Environmental Cleanup: A Review. Appl. Sci. 2021, 11, 4212. [Google Scholar] [CrossRef]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.; Ringu, T.; Pramanik, N. A Focus on Biomaterials Based on Calcium Phosphate Nanoparticles: An Indispensable Tool for Emerging Biomedical Applications. Bionanoscience 2023, 13, 795–818. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Shu, T.Y.; Wang, S.L.; Liu, Z.B.; Cheng, Y.L.; Li, A.; Pei, D.D. The Osteoinductivity of Calcium Phosphate-Based Biomaterials: A Tight Interaction With Bone Healing. Front. Bioeng. Biotechnol. 2022, 10, 911180. [Google Scholar] [CrossRef] [PubMed]
- Majeed, R.; Elnawawy, H.M.; Kutty, M.G.; Yahya, N.A.; Azami, N.H.; Abu Kasim, N.H.; Nabhan, M.S.; Cooper, P.R.; Camilleri, J.; Ahmed, H.M.A. Physicochemical, mechanical and biological properties of nano-calcium silicate-based cements: A systematic review. Odontology 2023, 111, 759–776. [Google Scholar] [CrossRef]
- Singh, P.; Yu, X.J.; Kumar, A.; Dubey, A.K. Recent advances in silicate-based crystalline bioceramics for orthopedic applications: A review. J. Mater. Sci. 2022, 57, 13109–13151. [Google Scholar] [CrossRef]
- Yin, H.; Yang, X.L.; Peng, L.S.; Xia, C.C.; Zhang, D.Y.; Cui, F.; Huang, H.J.; Li, Z.S. Trends of calcium silicate biomaterials in medical research and applications: A bibliometric analysis from 1990 to 2020. Front. Pharmacol. 2022, 13, 991377. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.L.; Lee, B.T. A Combination of Biphasic Calcium Phosphate Scaffold with Hyaluronic Acid-Gelatin Hydrogel as a New Tool for Bone Regeneration. Tissue Eng. Part A 2014, 20, 1993–2004. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Zhou, X.T.; Wang, Q.H.; Lei, Z.P.; Zhang, K.; Zhen, S.X.; Yao, H.Q.; Zu, Y. Calcium Carbonate-Based Nanoplatforms for Cancer Therapeutics: Current State of Art and Future Breakthroughs. ACS Omega 2024, 9, 12539–12552. [Google Scholar] [CrossRef]
- Zafar, B.; Campbell, J.; Cooke, J.; Skirtach, A.G.; Volodkin, D. Modification of Surfaces with Vaterite CaCO3 Particles. Micromachines 2022, 13, 473. [Google Scholar] [CrossRef]
- Ye, P.L.; Xiao, F.R.; Wei, S.P. Biomineralization and Characterization of Calcite and Vaterite Induced by the Fungus Cladosporium sp. YPLJS-14. Minerals 2023, 13, 1344. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Nanoscale analysis of the morphology and surface stability of calcium carbonate polymorphs. Sci. Rep. 2013, 3, 1587. [Google Scholar] [CrossRef]
- Declet, A.; Reyes, E.; Suárez, O.M. Calcium Carbonate Precipitation: A Review of the Carbonate Crystallization Process and Applications in Bioinspired Composites. Rev. Adv. Mater. Sci. 2016, 44, 87–107. [Google Scholar]
- Min, K.H.; Kim, D.H.; Pack, S.P. Size Control of Biomimetic Curved-Edge Vaterite with Chiral Toroid Morphology via Sonochemical Synthesis. Biomimetics 2024, 9, 174. [Google Scholar] [CrossRef]
- Udrea, I.; Capat, C.; Olaru, E.A.; Isopescu, R.; Mihai, M.; Mateescu, C.D.; Bradu, C. Vaterite Synthesis via Gas-Liquid Route under Controlled pH Conditions. Ind. Eng. Chem. Res. 2012, 51, 8185–8193. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Z.; Li, M.H.; Qu, Q.Y.; Ma, X.; Yu, S.H.; Zhao, Y.L. A Preloaded Amorphous Calcium Carbonate/Doxorubicin@Silica Nanoreactor for pH-Responsive Delivery of an Anticancer Drug. Angew. Chem.-Int. Ed. 2015, 54, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gan, M.; Fan, X.; Sun, Z.; Wei, J.; Li, J.; Ji, Z. Synthesis of high-value CaCO3 via indirect CO2 fixation utilized blast furnace slag. J. Environ. Chem. Eng. 2023, 11, 110655. [Google Scholar] [CrossRef]
- Yan, W.; Liang, B.; Li, W.; Huang, H.; Shi, D.; Chen, Z.; Li, Z.; Yu, M.; Wei, G.; Huang, K. Study on the growth mechanism of porous spherical calcium carbonate synthesized by carbonization controlled by amino acids. J. Solid State Chem. 2024, 329, 124370. [Google Scholar] [CrossRef]
- Ma, J.; Tang, Y.; Yaseen, M.; Qin, L.; Chen, X.; Xiong, S.; Liao, D.; Tong, Z. Hollow spherical vaterite calcium carbonate prepared by spray-bubble template method for immobilization of papain. Sep. Purif. Technol. 2023, 322, 124278. [Google Scholar] [CrossRef]
- Qi, C.; Lin, J.; Fu, L.H.; Huang, P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018, 47, 357–403. [Google Scholar] [CrossRef]
- Ueno, Y.; Futagawa, H.; Takagi, Y.; Ueno, A.; Mizushima, Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Control. Release 2005, 103, 93–98. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Yang, Y.S.; Su, S.H.; Liu, S.C.; Liu, W.L.; Yang, Q.F.; Tian, L.J.; Tan, Z.L.; Fan, L.; Yu, B.; Wang, J.; et al. Triple-functional bone adhesive with enhanced internal fixation, bacteriostasis and osteoinductive properties for open fracture repair. Bioact. Mater. 2023, 25, 273–290. [Google Scholar] [CrossRef]
- Idaszek, J.; Jaroszewicz, J.; Choinska, E.; Górecka, Z.; Hyc, A.; Osiecka-Iwan, A.; Wielunska-Kus, B.; Swieszkowski, W.; Moskalewski, S. Toward osteomimetic formation of calcium phosphate coatings with carbonated hydroxyapatite. Biomater. Adv. 2023, 149, 213403. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolanthai, E.; Nivethaa, E.A.K.; Pandian, M.S.; Ramasamy, P.; Catalani, L.H.; Kalkura, S.N. Enhanced in vitro inhibition of MCF-7 and magnetic properties of cobalt incorporated calcium phosphate (HAp and ß-TCP) nanoparticles. Ceram. Int. 2023, 49, 855–861. [Google Scholar] [CrossRef]
- Choi, B.Y.; Cui, Z.K.; Kim, S.; Fan, J.B.; Wu, B.M.; Lee, M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J. Mater. Chem. B 2015, 3, 6448–6455. [Google Scholar] [CrossRef]
- Latocha, J.; Wojasinski, M.; Janowska, O.; Chojnacka, U.; Gierlotka, S.; Ciach, T.; Sobieszuk, P. Morphology-controlled precipitation/remodeling of plate and rod-shaped hydroxyapatite nanoparticles. Aiche J. 2022, 68, e17897. [Google Scholar] [CrossRef]
- da Silva Brum, I.; de Carvalho, J.J.; da Silva Pires, J.L.; de Carvalho, M.A.A.; Dos Santos, L.B.F.; Elias, C.N. Nanosized hydroxyapatite and beta-tricalcium phosphate composite: Physico-chemical, cytotoxicity, morphological properties and in vivo trial. Sci. Rep. 2019, 9, 19602. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Neira, I.S.; Kolen’ko, Y.V.; Lebedev, O.I.; Van Tendeloo, G.; Gupta, H.S.; Guitián, F.; Yoshimura, M. An Effective Morphology Control of Hydroxyapatite Crystals via Hydrothermal Synthesis. Cryst. Growth Des. 2009, 9, 466–474. [Google Scholar] [CrossRef]
- Ganesan, K.; Kovtun, A.; Neumann, S.; Heumann, R.; Epple, M. Calcium phosphate nanoparticles: Colloidally stabilized and made fluorescent by a phosphate-functionalized porphyrin. J. Mater. Chem. 2008, 18, 3655–3661. [Google Scholar] [CrossRef]
- Gausterer, J.C.; Schüssler, C.; Gabor, F. The impact of calcium phosphate on FITC-BSA loading of sonochemically prepared PLGA nanoparticles for inner ear drug delivery elucidated by two different fluorimetric quantification methods. Ultrason. Sonochem. 2021, 79, 105783. [Google Scholar] [CrossRef] [PubMed]
- Guibert, C.; Landoulsi, J. Enzymatic Approach in Calcium Phosphate Biomineralization: A Contribution to Reconcile the Physicochemical with the Physiological View. Int. J. Mol. Sci. 2021, 22, 12957. [Google Scholar] [CrossRef] [PubMed]
- Mutalib, A.A.A.; Jaafar, N.F. Potential of deep eutectic solvent in photocatalyst fabrication methods for water pollutant degradation: A review. J. Environ. Chem. Eng. 2022, 10, 107422. [Google Scholar] [CrossRef]
- Dördelmann, G.; Kozlova, D.; Karczewski, S.; Lizio, R.; Knauer, S.; Epple, M. Calcium phosphate increases the encapsulation efficiency of hydrophilic drugs (proteins, nucleic acids) into poly(D,L-lactide-co-glycolide acid) nanoparticles for intracellular delivery. J. Mater. Chem. B 2014, 2, 7250–7259. [Google Scholar] [CrossRef]
- Sikder, P.; Ren, Y.F.; Bhaduri, S.B. Microwave processing of calcium phosphate and magnesium phosphate based orthopedic bioceramics: A state-of-the-art review. Acta Biomater. 2020, 111, 29–53. [Google Scholar] [CrossRef]
- Chahal, H.K.; Matthews, S.; Jones, M.I. Effect of process conditions on spray dried calcium carbonate powders for thermal spraying. Ceram. Int. 2021, 47, 351–360. [Google Scholar] [CrossRef]
- Singh, Y.P.; Mishra, B.; Gupta, M.K.; Bhaskar, R.; Han, S.S.; Mishra, N.C.; Dasgupta, S. Gelatin/monetite electrospun scaffolds to regenerate bone tissue: Fabrication, characterization, and in-vitro evaluation. J. Mech. Behav. Biomed. Mater. 2023, 137, 105524. [Google Scholar] [CrossRef]
- Kahar, N.N.F.N.M.N.; Ahmad, N.; Jaafar, M.; Yahaya, B.H.; Sulaiman, A.R.; Hamid, Z.A.A. A review of bioceramics scaffolds for bone defects in different types of animal models: HA and β-TCP. Biomed. Phys. Eng. Express 2022, 8, 052002. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium Phosphate Nanoparticles for Therapeutic Applications in Bone Regeneration. Nanomaterials 2019, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cho, I.S.; Kim, J.Y.; Jang, H.L.; Han, G.S.; Ryu, H.S.; Shin, H.; Jung, H.S.; Kim, H.; Hong, K.S. Simple Large-Scale Synthesis of Hydroxyapatite Nanoparticles: In Situ Observation of Crystallization Process. Langmuir 2010, 26, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.N.; Ding, X.F.; Huang, R.Z.; Jiang, R.H.; Huang, H.Y.; Pan, X.; Min, W.; Chen, J.; Duan, J.A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Fernandes, G.V.D.; de Oliveira, A.M.; Granjeiro, J.M. Calcium silicate as a graft material for bone fractures: A systematic review. J. Int. Med. Res. 2018, 46, 2537–2548. [Google Scholar] [CrossRef]
- Yu, B.; Fu, S.Y.; Kang, Z.R.; Zhu, M.; Ding, H.F.; Luo, T.; Zhu, Y.F.; Zhang, Y.T. Enhanced bone regeneration of 3D printed β-CaSiO4 scaffolds by aluminum ions solid solution. Ceram. Int. 2020, 46, 7783–7791. [Google Scholar] [CrossRef]
- Kang, Z.R.; Yu, B.; Fu, S.Y.; Li, D.J.; Zhang, X.; Qian, Z.; Zhong, Z.Y.; Yu, B.Q.; Ding, H.F.; Zhu, Y.F.; et al. Three-dimensional printing of CaTiO3 incorporated porous β-Ca2SiO4 composite scaffolds for bone regeneration. Appl. Mater. Today 2019, 16, 132–140. [Google Scholar] [CrossRef]
- Zhang, N.L.; Molenda, J.A.; Fournelle, J.H.; Murphy, W.L.; Sahai, N. Effects of pseudowollastonite (CaSiO3) bioceramic on in vitro activity of human mesenchymal stem cells. Biomaterials 2010, 31, 7653–7665. [Google Scholar] [CrossRef]
- Du, Z.Y.; Zhao, Z.D.; Liu, H.H.; Liu, X.; Zhang, X.; Huang, Y.Q.; Leng, H.J.; Cai, Q.; Yang, X.P. Macroporous scaffolds developed from CaSiO3 nanofibers regulating bone regeneration via controlled calcination. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 113, 111005. [Google Scholar] [CrossRef]
- Han, F.; Li, T.; Li, M.M.; Zhang, B.J.; Wang, Y.F.; Zhu, Y.F.; Wu, C.T. Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Bioact. Mater. 2023, 20, 29–40. [Google Scholar] [CrossRef]
- Sathain, A.; Monvisade, P.; Siriphannon, P. Bioactive alginate/carrageenan/calcium silicate porous scaffolds for bone tissue engineering. Mater. Today Commun. 2021, 26, 102165. [Google Scholar] [CrossRef]
- Fiocco, L.; Li, S.; Stevens, M.M.; Bernardo, E.; Jones, J.R. Biocompatibility and bioactivity of porous polymer-derived Ca-Mg silicate ceramics. Acta Biomater. 2017, 50, 56–67. [Google Scholar] [CrossRef]
- Zheng, T.Y.; Guo, L.Y.; Du, Z.Y.; Leng, H.J.; Cai, Q.; Yang, X.P. Bioceramic fibrous scaffolds built with calcium silicate/hydroxyapatite nanofibers showing advantages for bone regeneration. Ceram. Int. 2021, 47, 18920–18930. [Google Scholar] [CrossRef]
- Wu, T.T.; Lu, T.L.; Shi, H.S.; Wang, J.C.; Ye, J.D. Enhanced osteogenesis, angiogenesis and inhibited osteoclastogenesis of a calcium phosphate cement incorporated with strontium doped calcium silicate bioceramic. Ceram. Int. 2023, 49, 6630–6645. [Google Scholar] [CrossRef]
- Ressler, A.; Bauer, L.; Prebeg, T.; Ledinski, M.; Hussainova, I.; Urlic, I.; Ivankovic, M.; Ivankovic, H. PCL/Si-Doped Multi-Phase Calcium Phosphate Scaffolds Derived from Cuttlefish Bone. Materials 2022, 15, 3348. [Google Scholar] [CrossRef] [PubMed]
- Solonenko, A.P.; Blesman, A.I.; Polonyankin, D.A. Synthesis and physicochemical investigation of calcium silicate hydrate with different stoichiometric composition. J. Phys. Conf. Ser. 2019, 1210, 012132. [Google Scholar] [CrossRef]
- Vakalova, T.V.; Pogrebenkov, V.M.; Karionova, N.P. Solid-phase synthesis of wollastonite in natural and technogenic siliceous stock mixtures with varying levels of calcium carbonate component. Ceram. Int. 2016, 42, 16453–16462. [Google Scholar] [CrossRef]
- Kee, C.C.; Ang, B.C.; Metselaar, H.S.C. Synthesis of europium-doped calcium silicate hydrate via hydrothermal and coprecipitation method. Ceram. Int. 2021, 47, 4803–4812. [Google Scholar] [CrossRef]
- Balbinot, G.D.; Leitune, V.C.B.; Nunes, J.S.; Visioli, F.; Collares, F.M. Synthesis of sol-gel derived calcium silicate particles and development of a bioactive endodontic cement. Dent. Mater. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Zhang, N.; Zhai, D.; Chen, L.; Zou, Z.Y.; Lin, K.L.; Chang, J. Hydrothermal synthesis and characterization of Si and Sr co-substituted hydroxyapatite nanowires using strontium containing calcium silicate as precursors. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 37, 286–291. [Google Scholar] [CrossRef]
- Betancur-Granados, N.; Restrepo, J.C.; Tobón, J.I.; Restrepo-Baena, O.J. Dicalcium silicate (2CaO•SiO2) synthesized through flame spray pyrolysis and solution combustion synthesis methods. Ceram. Int. 2019, 45, 9589–9595. [Google Scholar] [CrossRef]
- Bouatrous, M.; Bouzerara, F.; Bhakta, A.K.; Delobel, F.; Delhalle, J.; Mekhalif, Z. A modified wet chemical synthesis of Wollastonite ceramic nanopowders and their characterizations. Ceram. Int. 2020, 46, 12618–12625. [Google Scholar] [CrossRef]
- Palakurthy, S.; Reddy, K.V.G.; Samudrala, R.K.; Azeem, P.A. bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 98, 109–117. [Google Scholar] [CrossRef]
- Lin, Y.T.; Shie, M.Y.; Lin, Y.H.; Ho, C.C.; Kao, C.Z.; Huang, S.H. The Development of Light-Curable Calcium-Silicate-Containing Composites Used in Odontogenic Regeneration. Polymers 2021, 13, 3107. [Google Scholar] [CrossRef]
- Gao, S.J.; Li, J.W.; Lei, Q.J.; Chen, Y.; Huang, H.Y.; Yan, F.F.; Xiao, L.F.; Zhang, T.; Wang, L.L.; Wei, R.X.; et al. Calcium sulfate-Cu2+ delivery system improves 3D-Printed calcium silicate artificial bone to repair large bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1224557. [Google Scholar] [CrossRef]
- Ifegwu, O.C.; Awale, G.; Rajpura, K.; Lo, K.W.; Laurencin, C.T. Harnessing cAMP signaling in musculoskeletal regenerative engineering. Drug Discov. Today 2017, 22, 1027–1044. [Google Scholar] [CrossRef]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]
- Oral, M.; Ercan, B.; Kapusuz, D. Calcium carbonate polymorph dictates in vitro osteoblast proliferation. J. Aust. Ceram. Soc. 2020, 56, 1421–1426. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamula, E.; Reilly, G.C.; Douglas, T.E.L. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Gong, Y.H.; Zhang, Y.L.; Cao, Z.N.; Ye, F.; Lin, Z.F.; Li, Y. Development of CaCO3 microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration. Biomater. Sci. 2019, 7, 3614–3626. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Lapa, A.; Mendes, A.C.; Balcaen, L.; Samal, S.K.; Chai, F.; Van der Voort, P.; Stevens, C.V.; Parakhonskiy, B.V.; Chronakis, I.S.; et al. Bioinspired, biomimetic, double-enzymatic mineralization of hydrogels for bone regeneration with calcium carbonate. Mater. Lett. 2017, 190, 13–16. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z. Chitosan-calcium carbonate scaffold with high mineral content and hierarchical structure for bone regeneration. Smart Mater. Med. 2023, 4, 552–561. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Ji, Y.R.; Kang, Z.W.; Li, F.; Ge, S.F.; Yang, D.P.; Ruan, J.; Fan, X.Q. Integrating eggshell-derived CaCO3/MgO nanocomposites and chitosan into a biomimetic scaffold for bone regeneration. Chem. Eng. J. 2020, 395, 125098. [Google Scholar] [CrossRef]
- Unger, R.E.; Stojanovic, S.; Besch, L.; Alkildani, S.; Schröder, R.; Jung, O.; Bogram, C.; Görke, O.; Najman, S.; Tremel, W.; et al. In Vivo Biocompatibility Investigation of an Injectable Calcium Carbonate (Vaterite) as a Bone Substitute including Compositional Analysis via SEM-EDX Technology. Int. J. Mol. Sci. 2022, 23, 1196. [Google Scholar] [CrossRef]
- Bao, Z.T.; Gu, Z.P.; Xu, J.B.; Zhao, M.; Liu, G.T.; Wu, J. Acid-responsive composite hydrogel platform with space-controllable stiffness and calcium supply for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125353. [Google Scholar] [CrossRef]
- Abd-Elkawi, M.; Sharshar, A.; Misk, T.; Elgohary, I.; Gadallah, S. Effect of calcium carbonate nanoparticles, silver nanoparticles and advanced platelet-rich fibrin for enhancing bone healing in a rabbit model. Sci. Rep. 2023, 13, 15232. [Google Scholar] [CrossRef]
- Lu, K.; Wang, D.L.; Zou, G.Y.; Wu, Y.; Li, F.; Song, Q.S.; Sun, Y.M. A multifunctional composite hydrogel that sequentially modulates the process of bone healing and guides the repair of bone defects. Biomed. Mater. 2024, 19, 035010. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, M.W.; Shen, Y.D.; Shen, X.K.; Li, M.H.; Li, Y.A.; Liu, Y.; Cai, K.Y.; Luo, Z.; Hu, Y. Bone-targeting cell membrane-engineered CaCO3-based nanoparticles restore local bone homeostasis for microenvironment-responsive osteoporosis treatment. Chem. Eng. J. 2023, 470, 144145. [Google Scholar] [CrossRef]
- Jia, F.; Ruan, L.F.; Du, C.C.; Liu, Y.; Cai, X.M.; Dou, R.; Zhang, J.Y.; Liu, X.G.; Chen, J.; Zhang, X.C.; et al. The nanoformula of zoledronic acid and calcium carbonate targets osteoclasts and reverses osteoporosis. Biomaterials 2023, 296, 122059. [Google Scholar] [CrossRef]
- Li, X.N.; Yang, X.; Liu, X.J.; He, W.; Huang, Q.L.; Li, S.R.; Feng, Q.L. Calcium carbonate nanoparticles promote osteogenesis compared to adipogenesis in human bone-marrow mesenchymal stem cells. Prog. Nat. Sci.-Mater. Int. 2018, 28, 598–608. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Zhao, L.; Chang, Y.H.; Zhang, Y.; Cui, Y.; Zhang, Z.Y. Calcium-mineralized polypeptide nanoparticle for intracellular drug delivery in osteosarcoma chemotherapy. Bioact. Mater. 2020, 5, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Chernozem, R.V.; Surmeneva, M.A.; Abalymov, A.A.; Parakhonskiy, B.V.; Rigole, P.; Coenye, T.; Surmenev, R.A.; Skirtach, A.G. Piezoelectric hybrid scaffolds mineralized with calcium carbonate for tissue engineering: Analysis of local enzyme and small-molecule drug delivery, cell response and antibacterial performance. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 122, 111909. [Google Scholar] [CrossRef]
- Memar, M.Y.; Rezaee, M.A.; Barzegar-Jalali, M.; Gholikhani, T.; Adibkia, K. The Antibacterial Effect of Ciprofloxacin Loaded Calcium Carbonate (CaCO3) Nanoparticles Against the Common Bacterial Agents of Osteomyelitis. Curr. Microbiol. 2023, 80, 173. [Google Scholar] [CrossRef]
- Liu, Z.H.; Liu, Q.L.; He, X.Y.; Zhu, Y.H.; Pan, Q.L.; Zhang, R.M.; Yu, H.M. Polydopamine and CuS/CaCO3 nanocomposites coated titanium alloy screw as efficient antibacterial device. Vacuum 2023, 214, 112208. [Google Scholar] [CrossRef]
- Jiang, P.L.; Hou, R.Q.; Chen, T.; Bai, L.C.; Li, J.A.; Zhu, S.J.; Wang, L.G.; Willumeit-Römer, R.; Guan, S.K. Enhanced degradation performance and promoted bone regeneration of novel CaCO3-based hybrid coatings on magnesium alloy as bioresorbable orthopedic implants. Chem. Eng. J. 2023, 467, 143460. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Chudinova, E.A.; Chernozem, R.V.; Lapanje, A.; Koptyug, A.V.; Rijavec, T.; Loza, K.; Prymak, O.; Epple, M.; Wittmar, A.; et al. Development of a bone substitute material based on additive manufactured Ti6Al4V alloys modified with bioceramic calcium carbonate coating: Characterization and antimicrobial properties. Ceram. Int. 2020, 46, 25661–25670. [Google Scholar] [CrossRef]
- Thi, B.L.; Shi, R.; Long, B.D.; Ramesh, S.; Shi, X.L.; Sugiura, Y.; Ishikawa, K. Biological responses of MC3T3-E1 on calcium carbonate coatings fabricated by hydrothermal reaction on titanium. Biomed. Mater. 2020, 15, 035004. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.L.; Li, P.; Hou, R.X.; Shavandi, A.; Yang, S.F. Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration. ACS Omega 2020, 5, 10948–10957. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.S.; Liang, H.W.; Liu, Y.; Bai, J.M.; Wang, M. Digital light processing (DLP) of nano biphasic calcium phosphate bioceramic for making bone tissue engineering scaffolds. Ceram. Int. 2022, 48, 27681–27692. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 107, 110347. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Bilgin, S.; Isik, G.; Motameni, A.; Tezcaner, A.; Evis, Z. Biomechanical Evaluation of an Injectable Alginate/Dicalcium Phosphate Cement Composites for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2021, 118, 104439. [Google Scholar] [CrossRef]
- Feng, P.; Wang, K.; Shuai, Y.; Peng, S.P.; Hu, Y.B.; Shuai, C.J. Hydroxyapatite nanoparticles in situ grown on carbon nanotube as a reinforcement for poly (ε-caprolactone) bone scaffold. Mater. Today Adv. 2022, 15, 100272. [Google Scholar] [CrossRef]
- Zerankeshi, M.M.; Mofakhami, S.; Salahinejad, E. 3D porous HA/TCP composite scaffolds for bone tissue engineering. Ceram. Int. 2022, 48, 22647–22663. [Google Scholar] [CrossRef]
- Rezk, A.I.; Kim, K.S.; Kim, C.S. Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) Composite Nanofibers Incorporating Hydroxyapatite Nanoparticles and Simvastatin for Bone Tissue Regeneration and Drug Delivery Applications. Polymers 2020, 12, 2667. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, M.; Szewczyk, A.; Skwira, A.; Sadej, R.; Walker, G. Biphasic composite of calcium phosphate-based mesoporous silica as a novel bone drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 455–470. [Google Scholar] [CrossRef]
- Qiu, C.; Wu, Y.Y.; Guo, Q.Y.; Shi, Q.L.; Zhang, J.Z.; Meng, Y.Q.; Xia, F.; Wang, J.G. Preparation and application of calcium phosphate nanocarriers in drug delivery. Mater. Today Bio 2022, 17, 100501. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Mahmood, M.S.; Abdul-Ameer, F.M.A.; Fatalla, A.A. Effect of commercially pure titanium implant coated with calcium carbonate and nanohydroxyapatite mixture on osseointegration. J. Med. Life 2023, 16, 52–61. [Google Scholar] [CrossRef]
- Sapino, S.; Chindamo, G.; Chirio, D.; Manzoli, M.; Peira, E.; Riganti, C.; Gallarate, M. Calcium Phosphate-Coated Lipid Nanoparticles as a Potential Tool in Bone Diseases Therapy. Nanomaterials 2021, 11, 2983. [Google Scholar] [CrossRef]
- Kaviya, M.; Ramakrishnan, P.; Mohamed, S.B.; Ramakrishnan, R.; Gimbun, J.; Veerabadran, K.M.; Kuppusamy, M.R.; Kaviyarasu, K.; Sridhar, T.M. Synthesis and characterization of nano-hydroxyapatite/graphene oxide composite materials for medical implant coating applications. Mater. Today-Proc. 2021, 36, 204–207. [Google Scholar] [CrossRef]
- Dai, W.Y.; Zheng, Y.F.; Li, B.; Yang, F.; Chen, W.X.; Li, Y.F.; Deng, Y.; Bai, D.; Shu, R. A 3D-printed orthopedic implant with dual-effect synergy based on MoS2 and hydroxyapatite nanoparticles for tumor therapy and bone regeneration. Colloids Surf. B-Biointerfaces 2023, 228, 113384. [Google Scholar] [CrossRef] [PubMed]
- Tenkumo, T.; Kruse, B.; Kostka, K.; Sokolova, V.; Ogawa, T.; Yoda, N.; Prymak, O.; Suzuki, O.; Sasaki, K.; Epple, M. Development of triple-functionalized calcium phosphate nanoparticles as an advanced drug delivery system for bone tissue repair. Regen. Ther. 2024, 25, 49–60. [Google Scholar] [CrossRef]
- Akiyama, N.; Patel, K.D.; Jang, E.J.; Shannon, M.R.; Patel, R.; Patel, M.; Perriman, A.W. Tubular nanomaterials for bone tissue engineering. J. Mater. Chem. B 2023, 11, 6225–6248. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Chu, Y.H.; Chen, P.T. Mechanical Biocompatibility, Osteogenic Activity, and Antibacterial Efficacy of Calcium Silicate-Zirconia Biocomposites. ACS Omega 2021, 6, 7106–7118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; He, H.; Meng, Q.X.; Zhu, Y.F.; Ye, X.J.; Xu, N.; Yu, J.M. Osteopontin sequence modified mesoporous calcium silicate scaffolds to promote angiogenesis in bone tissue regeneration. J. Mater. Chem. B 2020, 8, 5849–5861. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Y.; Hu, M.; Liao, F.; Yang, F.; Ke, Q.F.; Guo, Y.P.; Zhu, Z.H. La-Doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Biomater. Sci. 2019, 7, 1565–1573. [Google Scholar] [CrossRef]
- Xu, K.F.; Meng, Q.X.; Li, L.; Zhu, M. Mesoporous calcium silicate and titanium composite scaffolds via 3D-printing for improved properties in bone repair. Ceram. Int. 2021, 47, 18905–18912. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Highly bioactive bone cement microspheres based on α-tricalcium phosphate microparticles/mesoporous bioactive glass nanoparticles: Formulation, physico-chemical characterization and in vivo bone regeneration. Colloids Surf. B-Biointerfaces 2022, 217, 112650. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shie, M.Y.; Lee, A.K.X.; Chou, Y.T.; Chiang, C.; Lin, C.P. 3D-Printed Ginsenoside Rb1-Loaded Mesoporous Calcium Silicate/Calcium Sulfate Scaffolds for Inflammation Inhibition and Bone Regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Jadidi, A.; Salahinejad, E.; Sharifi, E.; Tayebi, L. Drug-delivery Ca-Mg silicate scaffolds encapsulated in PLGA. Int. J. Pharm. 2020, 589, 119855. [Google Scholar] [CrossRef]
- Duan, M.T.; Fan, W.; Fan, B. Mesoporous Calcium-Silicate Nanoparticles Loaded with Low-Dose Triton-100+Ag+ to Achieve Both Enhanced Antibacterial Properties and Low Cytotoxicity for Dentin Disinfection of Human Teeth. Pharmaceutics 2021, 13, 1518. [Google Scholar] [CrossRef]
- Huang, K.H.; Wang, C.Y.; Chen, C.Y.; Hsu, T.T.; Lin, C.P. Incorporation of Calcium Sulfate Dihydrate into a Mesoporous Calcium Silicate/Poly-ε-Caprolactone Scaffold to Regulate the Release of Bone Morphogenetic Protein-2 and Accelerate Bone Regeneration. Biomedicines 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Chen, Y.J.; Huang, T.H.; Lin, Y.H.; Hsu, T.T.; Ho, C.C. Assessment of the Release Profile of Fibroblast Growth Factor-2-Load Mesoporous Calcium Silicate/Poly-ε-caprolactone 3D Scaffold for Regulate Bone Regeneration. Processes 2020, 8, 1249. [Google Scholar] [CrossRef]
- Gao, Y.X.; Huang, P.; Chen, R.Y.; Wang, M.; Wang, Y.N.; Sa, Y.; Jiang, T. Mesoporous calcium silicate nanoparticles for superficial dental tissue reconstruction, in vitro and in vivo. RSC Adv. 2021, 11, 24681–24693. [Google Scholar] [CrossRef]
- Zou, F.; Lv, F.Z.; Ma, X.S.; Xia, X.L.; Cai, L.; Mei, S.Q.; Wei, J.; Niu, Y.F.; Jiang, J.Y. Dual drugs release from nanoporously bioactive coating on polyetheretherketone for enhancement of antibacterial activity, rBMSCs responses and osseointegration. Mater. Des. 2020, 188, 108433. [Google Scholar] [CrossRef]
- Sasireka, A.; Suganthi, S.; Vignesh, S.; Raj, V.; Oh, T.H. Preparation and characterization of zinc modified calcium silicate/ polycaprolactone with graphene oxide composite coating for bone repair applications. Ceram. Int. 2023, 49, 20251–20260. [Google Scholar] [CrossRef]
- Kee, C.C.; Ng, K.; Ang, B.C.; Metselaar, H.S.C. Synthesis, characterization and in-vitro biocompatibility of electrophoretic deposited europium-doped calcium silicate on titanium substrate. J. Eur. Ceram. Soc. 2023, 43, 1189–1204. [Google Scholar] [CrossRef]
- Tang, G.K.; Liu, Z.Q.; Liu, Y.; Yu, J.M.; Wang, X.; Tan, Z.H.; Ye, X.J. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.W.; Heo, J.H.; Park, C.; Kim, D.H.; Yi, G.S.; Kang, H.C.; Jung, H.S.; Shin, H.; Lee, J.H. Natural bone-mimicking nanopore-incorporated hydroxyapatite scaffolds for enhanced bone tissue regeneration. Biomater. Res. 2022, 26, 7. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]


| Property | Calcium Carbonate | Calcium Phosphate | Calcium Silicate |
|---|---|---|---|
| Chemical composition | CaCO3 | Ca3(PO4)2 | Ca2SiO4 |
| Biocompatibility | Generally high | Generally high | Generally high |
| Biodegradability | Biodegradable | Biodegradable | Biodegradable |
| Solubility in water | Limited solubility | Limited solubility | Insoluble |
| Bone mimicking | Limited bone mimicking | Exceptional | Superior |
| Osteoinductivity | Low | High | Moderate to high |
| Thermal stability | Decomposes at high temperatures | Stable at high temperatures | Stable at high temperatures |
| Applications | Delivery vehicles, supplements | Bone grafts, dental implants | Supplements, biomedical devices |
| Uses in tissue engineering | Limited applications | Mainly bone regeneration | Limited applications |
| Method | Pros | Cons | Challenges |
| Spontaneous precipitation | Biocompatibility Versatility Ease of implementation | Limited scalability Uniformity issues Limited control over properties | Need for an additive to control the size and CaCO3 phase Difficulty synthesizing at an upscale level |
| Slow carbonation | Biocompatibility Ease of scale-up Environmental sustainability | Long processing time Complexity Regulatory considerations | Difficulty synthesizing uniform CaCO3 Extended synthesis time |
| Reverse emulsion | Controlled particle size and morphology Uniformity and monodispersed nature Encapsulation of active ingredients | Complexity of emulsion formation Limited scalability Potential for residual surfactants | Various factors controlling the size and morphology Surfactant removal steps Difficulty synthesizing at an upscale level |
| Hydrothermal and solvothermal synthesis | Enhanced reactivity Versatility High purity | Equipment complexity Limited solvent compatibility Safety concerns Energy-intensive nature | Optimization of reaction conditions Need for contaminant control |
| CO2 bubbling | Environmentally friendly Ease of implementation Biocompatibility | Long processing time Limited control over particle properties Scale-up challenges | Difficulty synthesizing uniform CaCO3 Need for contaminant control |
| Name | Abbreviation | Chemical Formula | Ca/P Ratio |
|---|---|---|---|
| Hydroxyapatite | HA | Ca10(PO4)6(OH)2 | 1.67 |
| Calcium-deficient hydroxyapatite | CDHA | Ca10−x(HPO4)x(PO4)6−x (OH)2 (0 < x < 1) | 1.5–1.67 |
| Dicalcium phosphate dihydrate | DCPD | CaHPO4∙2H2O | 1 |
| α-Tricalcium phosphate | α-TCP | α-Ca3(PO4)2 | 1.5 |
| β-Tricalcium phosphate | β-TCP | β-Ca3(PO4)2 | 1.5 |
| Octacalcium phosphate | OCP | Ca8(HPO4)2(PO4)4·5H2O | 1.33 |
| Fluorapatite | FA | Ca10(PO4)F2 | 1.67 |
| Name | Chemical Formula | Components | Chemical Equation |
|---|---|---|---|
| Calcium metasilicate | CaSiO3 | CaO·SiO2 | r CaX2 + m M2SiO3 + n H2O → rCaO·mSiO2·nH2O + MX [86] |
| Dicalcium silicate | Ca2SiO4 | 2CaO·SiO2 | |
| Tricalcium silicate | Ca3SiO5 | 3CaO·SiO2 | |
| Rankinite | Ca3Si2O7 | 3CaO·SiO2 | |
| Tobermorite | Ca5Si6O16(OH)2·4H2O | 5CaO·6SiO2·5H2O | |
| Pseudowollastonite | β-CaSiO3 | CaO·SiO2 | |
| Wollastonite | α-CaSiO3 | CaO·SiO2 |
| Biomedical Application | Materials | Primary Function | Ca-Based Effect | Biomedical Results | Ref. |
|---|---|---|---|---|---|
| Scaffold | CaCO3 (calcite), chitosan | Osteogenesis | To promote a bone-like environment Calcium ions for bone regeneration | MSC migration and osteogenic differentiation ↑ Calvaria defect repair (rats) | [102] |
| CaCO3 (eggshell derived), MgO, chitosan, BMP-2 | Bone substitute | Sustainable release of MgO Calcium ions for bone regeneration | Inducing osteoinductive effects (hADSCs) Calvaria defect repair (SD rats) | [103] | |
| CaCO3 (vaterite), PEG | Bone substitute | Microsized precursor of hydroxyapatite for mineralization | Bone tissue regeneration ↑(BALB/c mice) | [104] | |
| CaCO3 (vaterite), Pluronic F127 diacrylate | Bone substitute | Acid-responsive property for spaced controlled distribution of scaffolds Calcium ions for expression of osteogenesis-related genes | Biocompatibility Osteogenic differentiation ↑ Skull defect repair (New Zealand white rabbits) | [105] | |
| CaCO3 (vaterite), Ag nanoparticle, advanced platelet-rich fibrin | Bone substitute | Calcium ions for osteoconductivity Nanosized precursor of β-tricalcium phosphate and hydroxyapatite | Forearm defect repair (New Zealand white rabbits) | [106] | |
| Drug delivery system | CaCO3 (vaterite), BMP-2, GelMA | Bone substitute | pH-sensitive release of BMP-2 in the weakly acidic environment of bone injury Bone filling materials by promoting calcium ions | Skull defect repair (SD rats) | [107] |
| CaCO3 (vaterite), β-estradiol, AC4ManNAz | Osteoporosis | Control of β-estradiol release in the acidic osteoporotic microenvironment Regulating the activity of osteoclasts and osteoblasts by promoting calcium ions Incresaing β-estradiol in vivo stability and availability by nanostructure | Osteoclast proliferation, bone resorption activity ↓ Osteoblast proliferation, differentiation, bone generation ↑ Osteoporosis treatment (C57BL/6J mice) | [108] | |
| CaCO3 (vaterite), HA-MC, ZOL, PBAE-SA | Osteoporosis | Synergistically enhanced antiosteoporotic effects on zoledronic acid H+ consumption for inhibiting osteoclasts | Whole-body bone mass ↑ (C57BL/6 mice) | [109] | |
| CaCO3 (rhombohedron-like/fusiform) | Osteogenesis | Dose- and shape-dependent osteogenesis effect Enhanced osteogenesis for promoting calcium ions | Alkaline phosphatase activity ↑ Collagen secretion ↑ Osteogenesis ↑Adipogenesis ↓ (hADSCs) | [110] | |
| CaCO3 (vaterite), methoxy poly(ethylene glycol)-block-poly(L-glutamic acid), doxorubicin, | Osteosarcoma | Increasing stability of doxorubicin loading by nanostructure pH-dependent release of drugs Biocompatibility | Anti-tumor effect Anti-bone destruction effect (BALB/c mice) | [111] | |
| CaCO3 (vaterite), alkaline phosphatase, vancomycin | Antibacterial effect | High surface area and porous structure for drug- and biomolecule-loading microcarriers Promotes bioactivity by releasing calcium ions | Cell attachment induction (MC3T3-E1) Antibacterial effect (S. aureus) | [112] | |
| CaCO3 (vaterite), ciprofloxacin | Osteomyelitis | Sustainable ciprofloxacin release through porous structure and biodegradability Uniformly entrapped ciprofloxacin by nanostructure | Antibacterial effect (methicillin-resistant and methicillin-susceptible S. aureus, E. faecalis, A. baumannii, P. aeruginosa, E. coli, and K. pneumonia; methicillin-resistant and methicillin-susceptible coagulase-negative staphylococci) | [113] | |
| Coating | CaCO3 (calcite), CuS, polydopamine, Ti alloy screws | Antibacterial implant | Releasing calcium ions for bioactivity High surface area and porous structure for CuS loading by microsized template | Antibacterial effect (S. aureus, E. coli) | [114] |
| CaCO3 (calcite, aragonite, vaterite), glutamate acid, dopamine, Mg alloy | Orthopedic implant | Increasing the surface roughness of template to enhance cell attachment | MC3T3-E1 proliferation, differentiation ↑ Osteogenesis promotion (SD rats) | [115] | |
| CaCO3 (calcite, vaterite), Ti6Al4V alloy | Antibacterial implant | Superhydrophilic template surface with reduced average surface roughness by nanostructure | Antibacterial effect by reducing bacterial adhesion on the template surface (S. aureus) | [116] | |
| CaCO3 (vaterite), Ti film | Bone implant | To improve biological response to osteoblast cells Increasing bone-like nodule formation on the surface | MC3T3-E1 proliferation and differentiation ↑ Osteogenesis promotion (MC3T3-E1) | [117] |
| Biomedical Application | Materials | Primary Function | Ca-Based Effects | Biomedical Results | Ref. |
|---|---|---|---|---|---|
| Scaffold | Calcium phosphate (BCP), hydroxyethyl chitosan, polyvinyl alcohol | Bone regeneration | To promote hydrogel bonds, physical crosslinking, and biomineralization | Improved the compressive strength of the HECS/PVA/BCP hydrogel without sacrificing the porous structure Further improved cytocompatibility via the addition of HECS and in vitro biomineralization | [118] |
| Calcium phosphate (BCP) | Bone tissue engineering | To promote osteoconductivity and bioresorbable | Presented comparable mechanical properties with human cancellous bone and higher cell proliferation rates (rat bone mesenchymal stem cells) | [119] | |
| Calcium phosphate (hydroxyapatite), chitosan, polyvinyl alcohol | Bone tissue engineering | To provide larger surface areas for ion exchange | Facilitated osteoblast cells to attach and proliferate (mouse osteoblast cells) | [120] | |
| Calcium phosphate (DCP), sodium alginate | Bone substitute | To provide an alternative option for PMMA | Increased cell (DPSCs) availability ratio, with no influence observed on cell shape, confirming the in vitro biocompatibility of the materials | [121] | |
| Calcium phosphate (hydroxyapatite), carbon nanotube | Bone substitute | To attract HPO42− by Ca2+ via electrovalent bonding to synthesize HA nanocrystals | Presented a Ca/P ratio of the apatite layer on the surface of the scaffold as 1.66, which was close to the ratio of normal bone | [122] | |
| Calcium phosphate (hydroxyapatite, TCP) | Bone regeneration | To adjust bio-performance by the HA/TCP ratio and pores | Presented an adjustable biodegradation rate by the HA/TCP ratio at an inverse relation, which is promising for designing patient-specific scaffolds | [123] | |
| Drug delivery system | Calcium phosphate (hydroxyapatite), poly(ε-caprolactone)/poly(glycerol sebacate) | Bone tissue regeneration | To act as a simvastatin-loading nanocarrier | MC3T3E1 osteoblast cells/enhanced osteoblast cell growth, proliferation, and adhesion | [124] |
| Calcium phosphate (hydroxyapatite), mesoporous silica material | Bone therapy | To act as a doxycycline-hydrochloride-loading nanocarriers | The 30%CaP@MSi allowing completion of 5-day release of the drug | [125] | |
| Calcium phosphate | Bone regeneration | Plasmid-DNA-encoding VEGF/siRNA inhibiting TNF-α-loading nanocarriers | Increased levels of bone-formation-related markers at the protein and gene levels in 3mixCaP after 10 days | [126] | |
| Coating | Calcium phosphate (hydroxyapatite), titanium | Bone regeneration | To contribute to the control of cell adhesion and mineral binding | New bone beginning to develop at the implant interface after 2 weeks (rabbit femurs) | [127] |
| Calcium phosphate, lipid nanoparticle | Bone therapy | To provide better cell accumulation than uncoated nanoparticles (NPs) | More dye delivered by CaP NPs to the cells within 24 h than the uncoated NPs | [128] | |
| Calcium phosphate (hydroxyapatite), graphene oxide | Bone tissue engineering | To find out its efficacy as an osteoinductive material | Catalyst for dye degradation and water treatment purposes | [129] | |
| Calcium phosphate (hydroxyapatite), molybdenum disulfide | Bone therapy and bone regeneration | To boost bone regeneration and integration around the implant | Exhibited adequate in vivo tissue compatibility and outstanding bone regeneration ability in the rat tibia defect model | [130] |
| Biomedical Application | Materials | Primary Function | Ca-Based Effects | Biomedical Results | Ref. |
|---|---|---|---|---|---|
| Scaffold | CaSi-ZrO2 | Load-bearing implants | Improvement of mechanical biocompatibility Concentration-dependent antibiotic effect Promotion of osteogenic activity | Long-term stability Antibacterial ability against E. coli and S. aureus hMSC osteogenesis ↑ | [133] |
| Osteopontin motif-modified MCS | Bone regeneration scaffold | Bone-mimicking structure by mesoporosity Enhanced apatite formation by the large surface area | HUVEC adhesion and proliferation ↑ hBMSC osteogenic differentiation ↑ Vessel formation and bone growth ↑ in rabbits | [134] | |
| Lanthanum, MCS, chitosan | Bone defect repair | Proliferation and osteogenic differentiation by Ca2+ release | Cell adhesion, spreading, and proliferation ↑ of hBMSCs New bone formulation ↑ in rats | [135] | |
| Amorphous calcium silicate, titanium | Bone tissue engineering | Porosity suitable for bone tissue engineering Apatite deposition on the scaffold by the Ca2+ release and large surface area | Inhibition of rapid degradation Enhanced compressive strengths by MCS (no cell and in vivo data) | [136] | |
| α-Tricalcium phosphate, mesoporous calcium silicate nanoparticle | Bone regeneration cement | Decrease in inflammation by the alkaline dissolution reaction of MCS Mesoporosity-induced hydroxyapatite mineralization | Bone-like hydroxyapatite formation ability by mesoporosity New bone formulation ↑ in rats | [137] | |
| Drug delivery system | Ginsenoside Rb1, polycaprolactone, MCS, calcium sulfate | Bone substitute scaffold | Uniform porous structure and suitable environment provided for cells | Anti-inflammation, depending on the drug concentration Cell proliferation and mineralization ↑ of hDPSCs Bone regeneration in rabbits | [138] |
| Vancomycin, PLGA, Bredigite (Ca7MgSi4O16) | Bone tissue regeneration Local antibiotic Delivery | Drug loading and implementation template of porous scaffold | Local pH buffering by PLGA Sustained drug release Cytocompatibility ↑ of 3500 hDPSCs | [139] | |
| Triton-100, silver ion, MCS nanoparticle | Bone defect filling material | Sustained-release scaffold by interaction Biomineralization increase by release of Ca2+ and SiO32− | Sustained release for 7 days Antibacterial effects against E. faecalis Low toxicity by MC3T3-E1 | [140] | |
| BMP-2, MCS, calcium sulfate, polycaprolactone | Bone regeneration | Enhanced hydroxyapatite precipitation and crystallization Cell differentiation by released Ca and Si ions | Prolonged and controlled drug release over 6 months Proliferation and osteogenesis ↑ of hDPSCs Angiogenesis ↑ in rabbits | [141] | |
| FGF-2, MCS, polycaprolactone | Bone-healing composite filler | Apatite deposition on the MCS scaffold Enhancement of bone cell differentiation by sustained release of Ca and the drug | Cell proliferation and ALP activity ↑ of hWJMSCs Healing of femur bone defect in rabbist | [142] | |
| Chlorhexidine, MCS nanoparticle | Dental care biomaterial | Barrier layer formation for dentin by continuous apatite deposition Antibacterial activity by sustained release of chlorhexidine binding MCS’s Si ion | Sustained release Antibacterial activity against E. faecalis Low cytotoxicity by HDPCs Low dentin permeability and inflammation in rats | [143] | |
| Genistein, curcumin, Mg-CS, polyetheretherketone | Implant for bone substitutes | Increase surface roughness and wettability by mesoporous nanoparticles Stimulation of cell proliferation and differentiation by release of Ca, Mg, and Si ions | Apatite mineralization Cell adhesion and proliferation ↑ of rBMSCs Antibacterial activity against E. coli and S. aureus Osteogenesis and osseointegration in rabbits | [144] | |
| Coating | Zinc-modified CS, polycaprolactone, graphene oxide | Orthopedic implant | Bone-like apatite growing on the implant surface by forming amorphous Ca | Antibacterial activity Cell viability and differentiation ↑ of MG63 human osteoblast cells | [145] |
| Europium, calcium silicate, titanium | Biomedical implant coating | Enhanced wettability by hydrophilicity of CS Improvement of apatite formation on the titanium implant by Ca release | Biologically similar apatite-forming ability Cell adhesion, proliferation, and ALP activity ↑ of hFOB | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.H.; Kim, D.H.; Kim, K.H.; Seo, J.-H.; Pack, S.P. Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics 2024, 9, 511. https://doi.org/10.3390/biomimetics9090511
Min KH, Kim DH, Kim KH, Seo J-H, Pack SP. Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics. 2024; 9(9):511. https://doi.org/10.3390/biomimetics9090511
Chicago/Turabian StyleMin, Ki Ha, Dong Hyun Kim, Koung Hee Kim, Joo-Hyung Seo, and Seung Pil Pack. 2024. "Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration" Biomimetics 9, no. 9: 511. https://doi.org/10.3390/biomimetics9090511
APA StyleMin, K. H., Kim, D. H., Kim, K. H., Seo, J.-H., & Pack, S. P. (2024). Biomimetic Scaffolds of Calcium-Based Materials for Bone Regeneration. Biomimetics, 9(9), 511. https://doi.org/10.3390/biomimetics9090511







