Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function
Abstract
1. Introduction
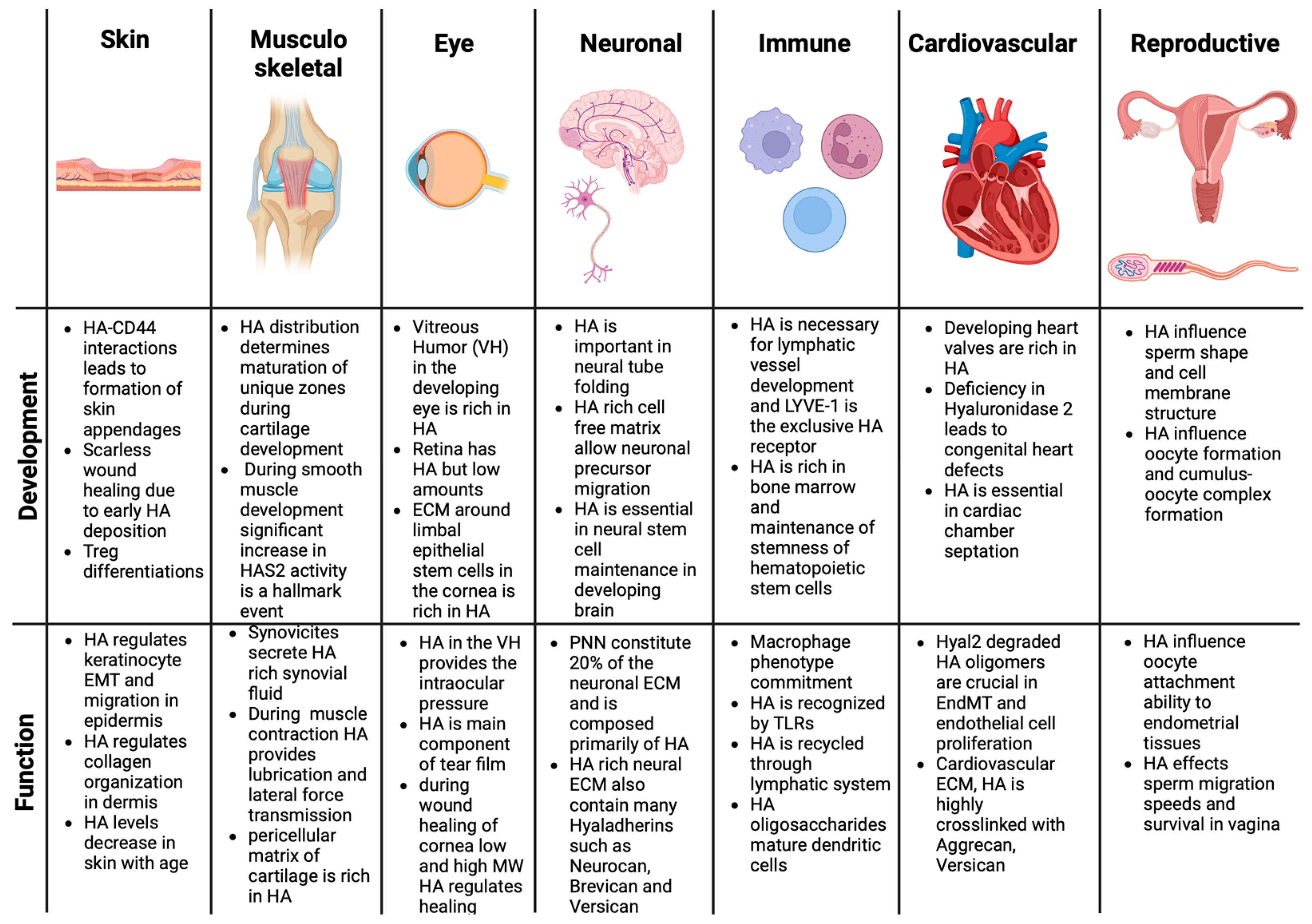
2. Regulatory Roles of HA in Tissue Function and Development
2.1. Role of HA in Skin Tissue Development and Function
2.2. Role of HA in Cartilage Tissue Development and Function
2.3. Role of HA in Musculoskeletal Tissue Development and Function
2.4. Role of HA in Eye Development and Function
2.5. Role of HA in Neural Tissue Development and Function
2.6. Role of HA in Cardiovascular Tissue Development and Function
2.7. Role of HA in Immune System Development and Function
2.8. Role of HA in Reproductive System Development and Function
2.9. Role of HA in Respiratory Tissue Development and Function
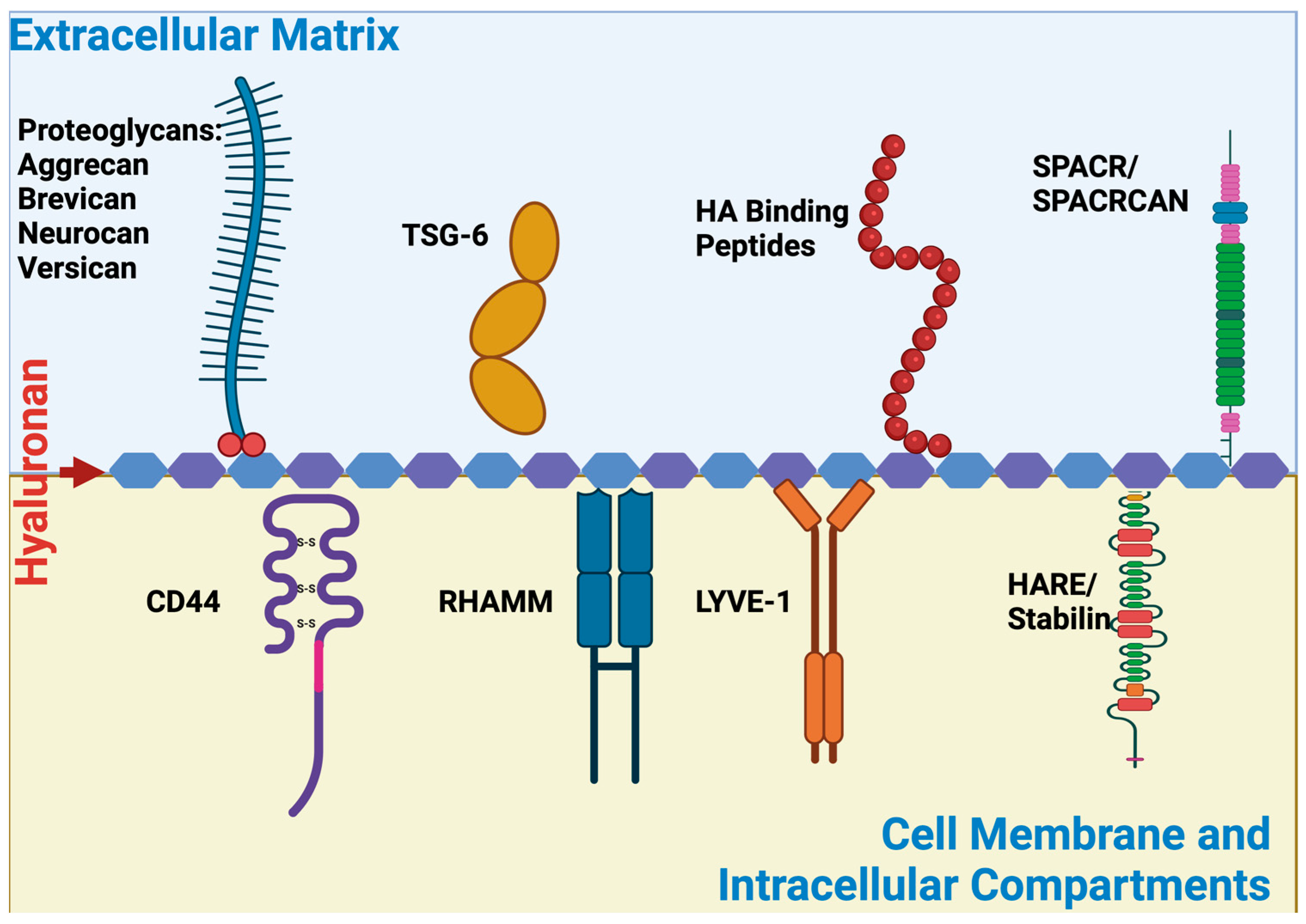
3. HA Binding Proteins and Discovery of HA Binding Peptides
3.1. HA Binding Proteins (Hyaladherins)
3.1.1. CD 44
3.1.2. RHAMM
3.1.3. LYVE-1
3.1.4. Toll-like Receptors
3.1.5. Stabilin
3.1.6. TSG-6-TNFIP6
3.1.7. Aggrecan
3.1.8. Brevican
3.1.9. Neurocan
3.1.10. Versican
3.1.11. HABP1/C1QBP
3.1.12. HARE
3.1.13. SHAP
3.1.14. SPACR
3.1.15. HA Binding Peptides (HABPs)
3.2. HA Binding Domains Located on HA Binding Molecules
3.2.1. LINK Domains
3.2.2. RHAMM-Derived HA Binding Domains and Their Roles
3.2.3. CD44-Derived HA Binding Domains and Their Roles
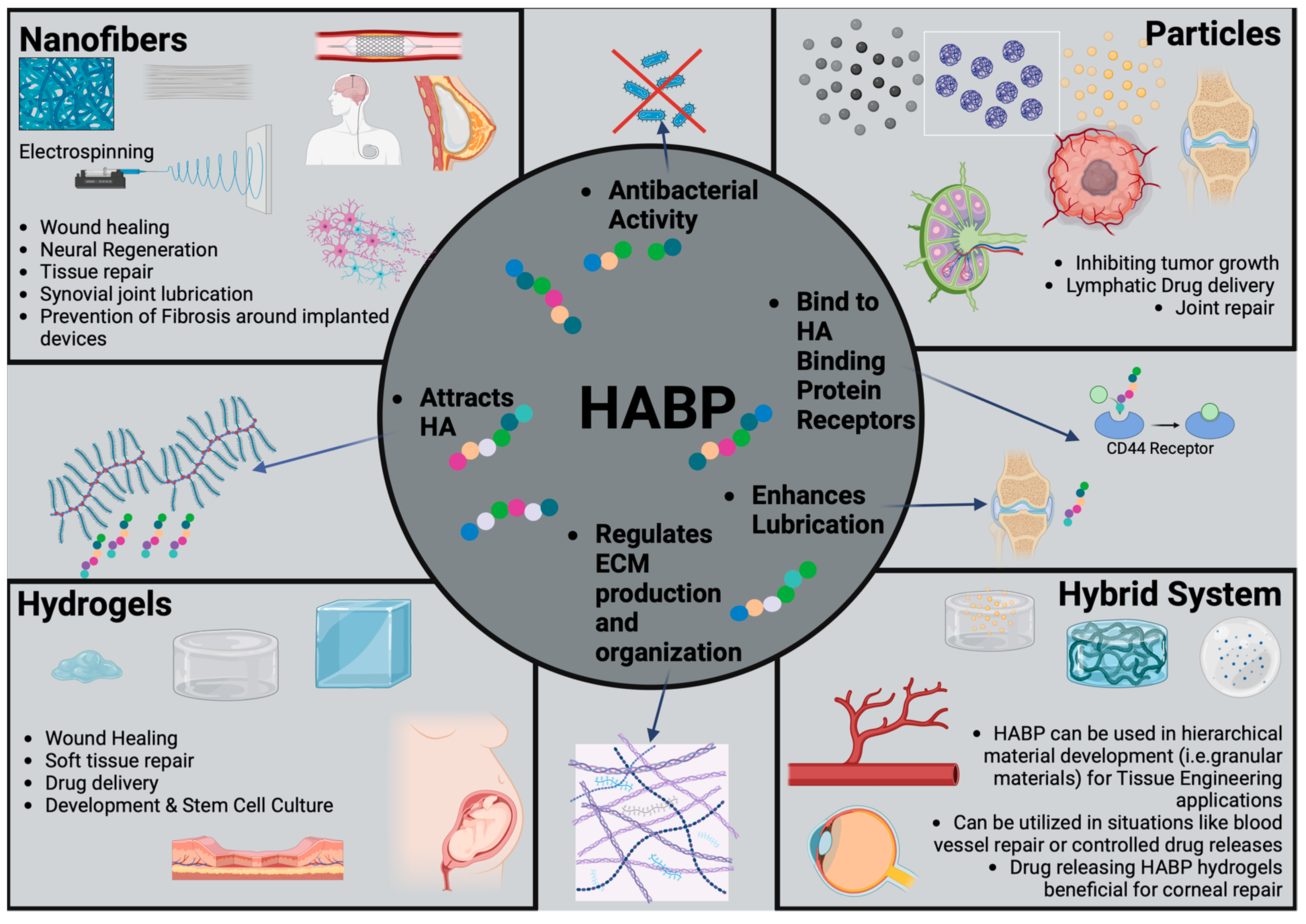
4. HABP-Based Biomaterials
4.1. HABP Biomaterials in Wound-Healing Research
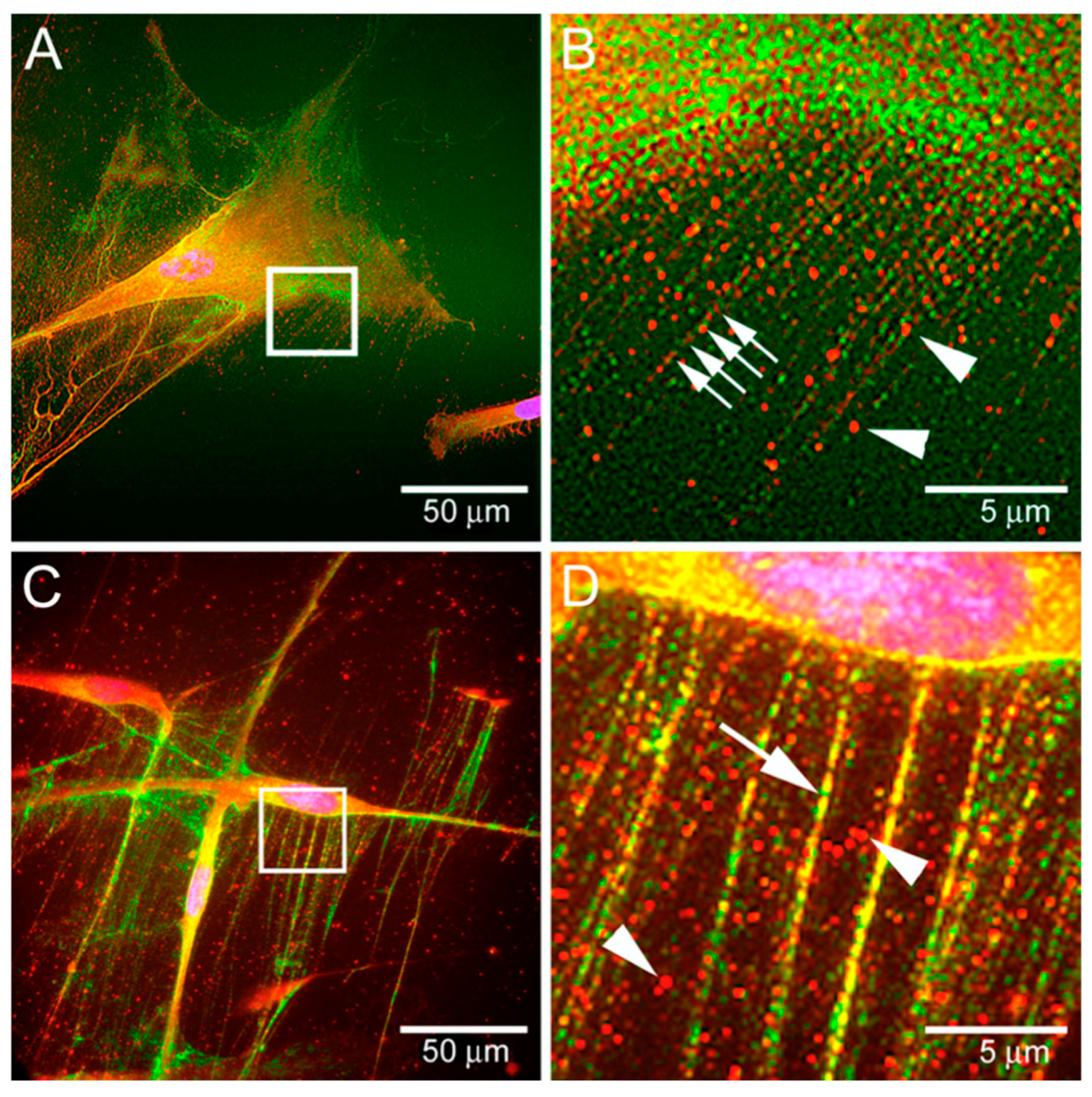
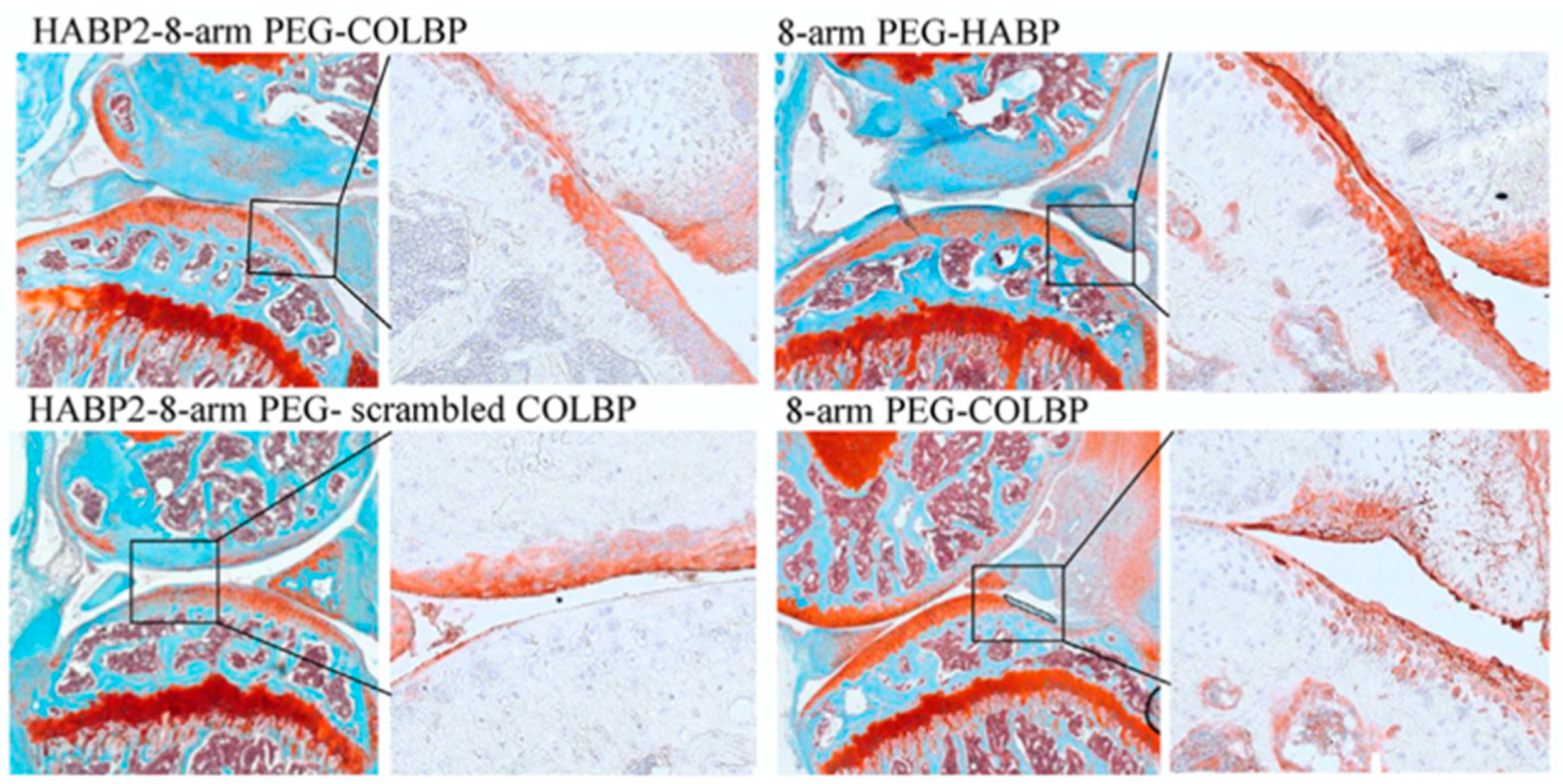
4.2. HABP Biomaterials in Musculoskeletal Research
4.3. HABP Biomaterials as Biological Lubrication Agents
4.4. HABP Biomaterials in Cancer Research
4.5. HABP Biomaterials in Tissue Engineering
5. Future Potential of HA Binding Biomaterials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Day, A.J.; Prestwich, G.D. Hyaluronan-binding proteins: Tying up the giant. J. Biol. Chem. 2002, 277, 4585–4588. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Evered, D.; Whelan, J. The Biology of Hyaluronan; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Toole, B.P. Hyaluronan in morphogenesis. J. Intern. Med. 1997, 242, 35–40. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Hong, W.X.; Malhotra, S.; Cheung, A.T.M.; Ransom, R.C.; Rennert, R.C.; Morrison, S.D.; Lorenz, H.P.; Longaker, M.T. Embryonic skin development and repair. Organogenesis 2018, 14, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.J.; Stern, R. Age-dependent changes of hyaluronan in human skin. J. Investig. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef]
- Hanke-Roos, M.; Fuchs, K.; Maleschlijski, S.; Sleeman, J.; Orian-Rousseau, V.; Rosenhahn, A. CD44 mediates the catch-bond acti-vated rolling of HEPG2Iso epithelial cancer cells on hyaluronan. Cell Adh. Migr. 2017, 11, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Lee, H.-G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 6, 138552. [Google Scholar] [CrossRef] [PubMed]
- Huffer, A.; Ozdemir, T. Substrate stiffness regulates type II diabetic fibroblast phenotype and metabolic activity. Biochem. Biophys. Res. Commun. 2024, 709, 149833. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2016, 62, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Faust, H.J.; Sommerfeld, S.D.; Rathod, S.; Rittenbach, A.; Banerjee, S.R.; Tsui, B.M.; Pomper, M.; Amzel, M.L.; Singh, A.; Elisseeff, J.H. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018, 183, 93–101. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2018, 78–79, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D. The Normal Synovium. Open Rheumatol. J. 2012, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Hong, X.; Wong, M.M.; Karamariti, E.; Bhaloo, S.I.; Warren, D.; Kong, W.; Hu, Y.; Xu, Q. Hyaluronan is crucial for stem cell differentiation into smooth muscle lineage. Stem Cells 2016, 34, 1225–1238. [Google Scholar] [CrossRef]
- Hunt, L.C.; Gorman, C.; Kintakas, C.; McCulloch, D.R.; Mackie, E.J.; White, J.D. Hyaluronan synthesis and myogenesis: A re-quirement for hyaluronan synthesis during myogenic differentiation independent of pericellular matrix formation. J. Biol. Chem. 2013, 288, 13006–13021. [Google Scholar] [CrossRef]
- Kosher, R.A.; Savage, M.P.; Walker, K.H. A gradation of hyaluronate accumulation along the proximodistal axis of the embryonic chick limb bud. Development 1981, 63, 85–98. [Google Scholar] [CrossRef]
- Yu, Q.; Grammatikakis, N.; Toole, B.P. Expression of multiple CD44 isoforms in the apical ectodermal ridge of the embryonic mouse limb. Dev. Dyn. 1996, 207, 204–214. [Google Scholar] [CrossRef]
- Culty, M.; Nguyen, H.A.; Underhill, C.B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyalu-ronan. J. Cell Biol. 1992, 116, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Knudson, C.B.; Knudson, W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J. Cell Sci. 1993, 106, 365–375. [Google Scholar] [CrossRef]
- Bastow, E.R.; Byers, S.; Golub, S.B.; Clarkin, C.E.; Pitsillides, A.A.; Fosang, A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2007, 65, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Prince, C.W. Roles of hyaluronan in bone resorption. BMC Musculoskelet. Disord. 2004, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Stancíková, M.; Svík, K.; Istok, R.; Rovenský, J.; Velebný, V. The effects of hyaluronan on bone resorption and bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Int. J. Tissue React. 2004, 26, 9–16. [Google Scholar]
- Sherwood, L.M.; Parris, E.E.; Raisz, L.G. Physiologic and pharmacologic regulation of bone resorption. N. Engl. J. Med. 1970, 282, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cheng, Y.Y.; Koo, P.L.; Lee, K.M.; Qin, L.; Cheng, J.C.Y.; Kumta, S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. Part A 2003, 66, 880–884. [Google Scholar] [CrossRef]
- Pilloni, A.; Bernard, G.W. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res. 1998, 294, 323–333. [Google Scholar] [CrossRef]
- Nagahata, M.; Tsuchiya, T.; Ishiguro, T.; Matsuda, N.; Nakatsuchi, Y.; Teramoto, A.; Hachimori, A.; Abe, K. A novel function of N-cadherin and Connexin43: Marked enhancement of alkaline phosphatase activity in rat calvarial osteoblast exposed to sulfated hyaluronan. Biochem. Biophys. Res. Commun. 2004, 315, 603–611. [Google Scholar] [CrossRef]
- Itoh, S.; Matubara, M.; Kawauchi, T.; Nakamura, H.; Yukitake, S.; Ichinose, S.; Shinomiya, K. Enhancement of bone ingrowth in a titanium fiber mesh implant by rhBMP-2 and hyaluronic acid. J. Mater. Sci. Mater. Med. 2001, 12, 575–581. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef]
- Cho, B.C.; Park, J.W.; Baik, B.S.; Kwon, I.C.; Kim, I.S. The role of hyaluronic acid, chitosan, and calcium sulfate and their combined effect on early bony consolidation in distraction osteogenesis of a canine model. J. Craniofacial Surg. 2002, 13, 783–793. [Google Scholar] [CrossRef]
- Cochran, D.; Wisner, L.; Richards, M.; Rouse, C. The induction of specific metabolic alterations in mouse calvarial organ cultures by glycosaminoglycans. Arch. Oral Biol. 1990, 35, 515–522. [Google Scholar] [CrossRef]
- Hurley, M.M.; Kream, B.E.; Raisz, L.G. Structural determinants of the capacity of heparin to inhibit collagen synthesis in 21-day fetal rat calvariae. J. Bone Miner. Res. 1990, 5, 1127–1133. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Muscle fascia and force transmission. J. Bodyw. Mov. Ther. 2010, 14, 411–417. [Google Scholar] [CrossRef]
- Amir, A.; Kim, S.; Stecco, A.; Jankowski, M.P.; Raghavan, P. Hyaluronan homeostasis and its role in pain and muscle stiffness. PM&R 2022, 14, 1490–1496. [Google Scholar]
- Rasool, G.; Wang, A.B.; Rymer, W.Z.; Lee, S.S.M. Shear waves reveal viscoelastic changes in skeletal muscles after hemispheric stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.B.; Perreault, E.J.; Royston, T.J.; Lee, S.S. Changes in shear wave propagation within skeletal muscle during active and passive force generation. J. Biomech. 2019, 94, 115–122. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C. Turnover and metabolism of hyaluronan. In Ciba Foundation Symposium 143-The Biology of Hyaluronan: The Biology of Hyaluronan: Ciba Foundation Symposium 143; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 41–59. [Google Scholar]
- Boskey, A.L.; Robey, P.G. The regulatory role of matrix proteins in mineralization of bone. In Osteoporosis, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 235–255. [Google Scholar]
- Bremer, F.M.; Rasquin, F. Histochemical localization of hyaluronic acid in vitreous during embryonic development. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2466–2469. [Google Scholar]
- Le Goff, M.M.; Bishop, P.N. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Swindle-Reilly, K.E. Rheological properties and age-related changes of the human vitreous humor. Front. Bioeng. Biotechnol. 2018, 6, 199. [Google Scholar] [CrossRef] [PubMed]
- Gesteira, T.F.; Sun, M.; Coulson-Thomas, Y.M.; Yamaguchi, Y.; Yeh, L.-K.; Hascall, V.; Coulson-Thomas, V.J. Hyaluronan rich microenvironment in the limbal stem cell niche regulates limbal stem cell differentiation. Investig. Opthalmology Vis. Sci. 2017, 58, 4407–4421. [Google Scholar] [CrossRef]
- Puri, S.; Moreno, I.Y.; Sun, M.; Verma, S.; Lin, X.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan supports the limbal stem cell phenotype during ex vivo culture. Stem Cell Res. Ther. 2022, 13, 1–21. [Google Scholar] [CrossRef]
- Chiu, H.-I.; Wu, S.-B.; Tsai, C.-C. The Role of Fibrogenesis and Extracellular Matrix Proteins in the Pathogenesis of Graves’ Ophthalmopathy. Int. J. Mol. Sci. 2024, 25, 3288. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Amankwah, R.; Powell-Richards, A.; Dua, H.S. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Deng, Y.; Tian, B.; Wang, B.; Sun, Y.; Huang, H.; Chen, L.; Ling, S.; Yuan, J. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J. Ophthalmol. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Müller-Lierheim, W.G.K. Why chain length of hyaluronan in eye drops matters. Diagnostics 2020, 10, 511. [Google Scholar] [CrossRef]
- Morris-Wiman, J.; Brinkley, L.L. The role of the mesenchyme in mouse neural fold elevation. II. Patterns of hyaluronate synthesis and distribution in embryos developing in vitro. Am. J. Anat. 1990, 188, 133–147. [Google Scholar] [CrossRef] [PubMed]
- GSchoenwolf, C.; Fisher, M. Analysis of the effects of Streptomyces hyaluronidase on formation of the neural tube. Development 1983, 73, 1–15. [Google Scholar] [CrossRef]
- Bignami, A.; Hosley, M.; Dahl, D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. 1993, 188, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, O.; Akiyama, H.; McGeer, E.G.; McGeer, P.L. Immunohistochemical localization of hyaluronic acid in rat and human brain. Brain Res. 1994, 635, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.K.; Chang, L.B.; Preti, C. Glycosaminoglycans of brain during development. Biochemistry 1975, 14, 85–88. [Google Scholar] [CrossRef]
- Forster, E.; Zhao, S.; Frotscher, M. Hyaluronan-associated adhesive cues control fiber segregation in the hippocampus. Development 2001, 128, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-K.; Wang, J.; Lin, L.; Hao, Y.; Chan, S.-O. Enzymatic removal of hyaluronan affects routing of axons in the mouse optic chiasm. NeuroReport 2007, 18, 1533–1538. [Google Scholar] [CrossRef]
- Nagy, J.; Price, M.; Staines, W.; Lynn, B.; Granholm, A.-C. The hyaluronan receptor for RHAMM in noradrenergic fibers contributes to axon growth capacity of locus coeruleus neurons in an intraocular transplant model. Neuroscience 1998, 86, 241–255. [Google Scholar] [CrossRef]
- Liu, X.Y.; Seh, C.C.; Cheung, P.C. HSP90 is required for TAK1 stability but not for its activation in the pro-inflammatory signaling pathway. FEBS Lett. 2008, 582, 4023–4031. [Google Scholar] [CrossRef]
- Maxwell, C.A.; McCarthy, J.; Turley, E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? J. Cell Sci. 2008, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Kawakatsu, T.; Tayama, S.; Kobayashi, Y.; Sugiura, N.; Kimata, K.; Takai, Y. Hyaluronan–CD44 pathway regulates orientation of mitotic spindle in normal epithelial cells. Genes Cells 2008, 13, 759–770. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 2014, 258, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U. Brain matrix: Structure, turnover and necessity. Biochem. Soc. Trans. 2007, 35, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U. Extracellular matrix components associated with remodeling processes in brain. Cell. Mol. Life Sci. 2004, 61, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Tammi, M.I.; Day, A.J.; Turley, E.A. Hyaluronan and homeostasis: A balancing act. J. Biol. Chem. 2002, 277, 4581–4584. [Google Scholar] [CrossRef]
- Stichel, C.C.; Kappler, J.; Junghans, U.; Koops, A.; Kresse, H.; Muller, H.W. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 1995, 704, 263–274. [Google Scholar] [CrossRef]
- Ding, H.-Y.; Xie, Y.-N.; Dong, Q.; Kimata, K.; Nishida, Y.; Ishiguro, N.; Zhuo, L.-S. Roles of hyaluronan in cardiovascular and nervous system disorders. J. Zhejiang Univ. B 2019, 20, 428–436. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Schroeder, J.A.; Bradley, J.; Klewer, S.E.; McDonald, J.A. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2–ErbB3 receptors. Nat. Med. 2002, 8, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Xiang, B.; Liu, M.; Hemming, R.; Dolinsky, V.W.; Triggs-Raine, B. Hyaluronidase 2 deficiency causes increased mesenchymal cells, congenital heart defects, and heart failure. Circ. Cardiovasc. Genet. 2017, 10, e001598. [Google Scholar] [CrossRef]
- Lei, Y.; Bortolin, L.; Benesch-Lee, F.; Oguntolu, T.; Dong, Z.; Bondah, N.; Billiar, K. Hyaluronic acid regulates heart valve interstitial cell contraction in fibrin-based scaffolds. Acta Biomater. 2021, 136, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Genasetti, A.; Vigetti, D.; Viola, M.; Karousou, E.; Moretto, P.; Rizzi, M.; Bartolini, B.; Clerici, M.; Pallotti, F.; De Luca, G.; et al. Hyaluronan and human endothelial cell behavior. Connect. Tissue Res. 2008, 49, 120–123. [Google Scholar] [CrossRef]
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin. Investig. Med. 2008, 31, E106–E116. [Google Scholar] [CrossRef] [PubMed]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef]
- Walsh, E.C.; Stainier, D.Y.R. UDP-Glucose Dehydrogenase Required for Cardiac Valve Formation in Zebrafish. Science 2001, 293, 1670–1673. [Google Scholar] [CrossRef]
- Camenisch, T.D.; McDonald, J.A. Hyaluronan is bigger better? Am. J. Respir. Cell Mol. Biol. 2000, 23, 431–433. [Google Scholar] [CrossRef]
- Baranova, N.S.; Nilebäck, E.; Haller, F.M.; Briggs, D.C.; Svedhem, S.; Day, A.J.; Richter, R.P. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 2011, 286, 25675–25686. [Google Scholar] [CrossRef]
- Lee-Sayer, S.S.M.; Dong, Y.; Arif, A.A.; Olsson, M.; Brown, K.L.; Johnson, P. The Where, When, How, and Why of Hyaluronan Binding by Immune Cells. Front. Immunol. 2015, 6, 135556. [Google Scholar] [CrossRef]
- Jackson, D.G. Hyaluronan in the lymphatics: The key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 2019, 78–79, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; de la Motte, C.A. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic roles of hyaluronan in dermal wound healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Tesar, B.M.; Jiang, D.; Liang, J.; Palmer, S.M.; Noble, P.W.; Goldstein, D.R. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 2006, 6, 2622–2635. [Google Scholar] [CrossRef]
- DeGrendele, H.C.; Estess, P.; Picker, L.J.; Siegelman, M.H. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: A novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 1996, 183, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- De Grendele, H.C.; Estess, P.; Siegelman, M.H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278, 672–675. [Google Scholar] [CrossRef]
- De Grendele, H.C.; Kosfiszer, M.; Estess, P.; Siegelman, M.H. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J. Immunol. 1997, 159, 2549–2553. [Google Scholar] [CrossRef]
- Do, Y.; Nagarkatti, P.S.; Nagarkatti, M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J. Immunother. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Sleeman, J.P.; Simon, J.C. Hyaluronan–magic glue for the regulation of the immune response? Trends Immunol. 2003, 24, 112–114. [Google Scholar] [CrossRef]
- McKee, C.M.; Penno, M.B.; Cowman, M.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996, 98, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Eggli, P.S.; Graber, W. Cytochemical localization of hyaluronan in rat and human skin mast cell granules. J. Investig. Dermatol. 1993, 100, 121–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukui, M.; Whittlesey, K.; Metcalfe, D.D.; Dastych, J. Human mast cells express the hyaluronic-acid-binding isoform of CD44 and adhere to hyaluronic acid. Clin. Immunol. 2000, 94, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ghaleno, L.R.; Pennisi, C.P.; Shahverdi, A.; Dardmeh, F.; Alipour, H.; Valojerdi, M.R. Exploring the Role of Hyaluronic Acid in Reproductive Biology and Beyond: Applications in Assisted Reproduction and Tissue Engineering. Adv. Biol. 2024, 8, e2300621. [Google Scholar] [CrossRef]
- Mahdavinezhad, F.; Gharaei, R.; Farmani, A.R.; Hashemi, F.; Kouhestani, M.; Amidi, F. The Potential Relationship Between Different Human Female Reproductive Disorders and Sperm Quality in Female Genital Tract. Reprod. Sci. 2021, 29, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.E.; Dweik, R.A.; Garantziotis, S.; Aronica, M.A. The Rise and Fall of Hyaluronan in Respiratory Diseases. Int. J. Cell Biol. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, D.; Jung, Y.; Xie, T.; Ingram, J.; Church, T.; Degan, S.; Leonard, M.; Kraft, M.; Noble, P.W. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J. Allergy Clin. Immunol. 2011, 128, 403–411.e3. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.M.; Martínez-García, M.A. Use of hyaluronic acid (HA) in chronic airway diseases. Cells 2020, 9, 2210. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Radovits, T.; Fava, M.; Mayr, U.; Lin, W.-Y.; Ermolaeva, E.; Martínez-López, D.; Lindberg, E.L.; Duregotti, E.; Daróczi, L.; et al. Extracellular Matrix in Heart Failure: Role of ADAMTS5 in Proteoglycan Remodeling. Circulation 2021, 144, 2021–2034. [Google Scholar] [CrossRef]
- Didangelos, A.; Yin, X.; Mandal, K.; Baumert, M.; Jahangiri, M.; Mayr, M. Proteomics characterization of extracellular space components in the human aorta. Mol. Cell. Proteom. 2010, 9, 2048–2062. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Herndon, M.E.; Patel, N.; Hecht, J.T.; Tuan, R.S.; Lawler, J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J. Biol. Chem. 2007, 282, 24591–24598. [Google Scholar] [CrossRef]
- Retzler, C.; Göhring, W.; Rauch, U. Analysis of neurocan structures interacting with the neural cell adhesion molecule N-CAM. 1996, 271, 27304–27310. J. Biol. Chem. 1996, 271, 27304–27310. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Naba, A.; Larson, B.L.; Zhou, F.; Prijić, M.; Whittaker, C.A.; Del Rosario, A.; Langer, R.; Hynes, R.O.; Anderson, D.G. Comprehensive proteomic characterization of stem cell-derived extracellular matrices. Biomaterials 2017, 128, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Gupta, S.K.; Zoccarato, A.; Kitazume-Taneike, R.; Fava, M.; Yin, X.; Werner, T.; Hirt, M.N.; Zampetaki, A.; Viviano, A.; et al. Glycoproteomics Reveals Decorin Peptides with Anti-Myostatin Activity in Human Atrial Fibrillation. Circulation 2016, 134, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Whittaker, C.A.; Carr, S.A.; Tanabe, K.K.; Hynes, R.O. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer 2014, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Zimmermann, D.R.; Iozzo, R.V. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J. Biol. Chem. 1994, 269, 32999–33008. [Google Scholar] [CrossRef] [PubMed]
- Malla, N.; Berg, E.; Theocharis, A.D.; Svineng, G.; Uhlin-Hansen, L.; Winberg, J. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013, 280, 2870–2887. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, S.A.; Mahoney, D.J.; Martinmanso, G.; Ali, T.; Nentwich, H.A.; Sipes, J.M.; Zeng, B.; Vogel, T.; Day, A.J.; Roberts, D.D. TSG-6 binds via its CUB_C domain to the cell-binding domain of fibronectin and increases fibronectin matrix assembly. Matrix Biol. 2008, 27, 201–210. [Google Scholar] [CrossRef][Green Version]
- Briggs, D.C.; Birchenough, H.L.; Ali, T.; Rugg, M.S.; Waltho, J.P.; Ievoli, E.; Jowitt, T.A.; Enghild, J.J.; Richter, R.P.; Salustri, A.; et al. Metal ion-dependent heavy chain transfer activity of TSG-6 mediates assembly of the cumulus-oocyte matrix. J. Biol. Chem. 2015, 290, 28708–28723. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.I.; Day, A.J.; Milner, C.M. TSG-6 Inhibits Neutrophil Migration via Direct Interaction with the Chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef]
- Leali, D.; Inforzato, A.; Ronca, R.; Bianchi, R.; Belleri, M.; Coltrini, D.; Di Salle, E.; Sironi, M.; Norata, G.D.; Bottazzi, B.; et al. Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction. Arter. Thromb. Vasc. Biol. 2012, 32, 696–703. [Google Scholar] [CrossRef]
- Mahoney, D.J.; Mikecz, K.; Ali, T.; Mabilleau, G.; Benayahu, D.; Plaas, A.; Milner, C.M.; Day, A.J.; Sabokbar, A. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J. Biol. Chem. 2008, 283, 25952–25962. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Ikeda, R.; Goto, S.; Yoshida, K.; Mitsumori, R.; Sakamoto, Y.; Tajima, A.; Yokoyama, T.; Toh, S.; Furukawa, K.-I.; et al. Tumour necrosis factor α-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem. J. 2006, 398, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Gallatin, W.M.; Wayner, E.A.; Hoffman, P.A.; John, T.S.; Butcher, E.C.; Carter, W.G. Structural homology between lymphocyte receptors for high endothelium and class III extracellular matrix receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 4654–4658. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I.; Aruffo, A.; Amiot, M.; Seed, B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991, 10, 343–348. [Google Scholar] [CrossRef]
- Li, L.; Asteriou, T.; Bernert, B.; Heldin, C.-H.; Heldin, P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem. J. 2007, 404, 327–336. [Google Scholar] [CrossRef]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef]
- Martín-Villar, E.; Fernández-Muñoz, B.; Parsons, M.; Yurrita, M.M.; Megías, D.; Pérez-Gómez, E.; Jones, G.E.; Quintanilla, M. Podoplanin associates with cd44 to promote directional cell migration. Mol. Biol. Cell 2010, 21, 4387–4399. [Google Scholar] [CrossRef]
- Lawrance, W.; Banerji, S.; Day, A.J.; Bhattacharjee, S.; Jackson, D.G. Binding of hyaluronan to the native lymphatic vessel endothelial receptor LYVE-1 is critically dependent on receptor clustering and hyaluronan organization. J. Biol. Chem. 2016, 291, 8014–8030. [Google Scholar] [CrossRef]
- Yang, B.; Savani, R.; Turley, E. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef]
- Adachi, H.; Tsujimoto, M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating Activities. J. Biol. Chem. 2002, 277, 34264–34270. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; So, I.-S.; Park, S.-Y.; Kim, I.-S. Thymosin β4 is involved in stabilin-2-mediated apoptotic cell engulfment. FEBS Lett. 2008, 582, 2161–2166. [Google Scholar] [CrossRef]
- Felbor, U.; Gehrig, A.; Sauer, C.; Marquardt, A.; Köhler, M.; Schmid, M.; Weber, B. Genomic organization and chromosomal localization of the interphotoreceptor matrix proteoglycan-1 (IMPG1) gene: A candidate for 6q-linked retinopathies. Cytogenet. Genome Res. 1998, 81, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Foletta, V.C.; Lee, J.W.; Rayborn, M.E.; Rodriguez, I.R.; Young, W.; Hollyfield, J.G. SPACRCAN, a novel human interphotoreceptor matrix hyaluronan-binding proteoglycan synthesized by photoreceptors and pinealocytes. J. Biol. Chem. 2000, 275, 6945–6955. [Google Scholar] [CrossRef]
- Choi-Miura, N.-H.; Tobe, T.; Sumiya, J.-I.; Nakano, Y.; Sano, Y.; Mazda, T.; Tomita, M. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: It has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J. Biochem. 1996, 119, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Goetinck, P.F.; Stirpe, N.S.; Tsonis, P.A.; Carlone, D. The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J. Cell Biol. 1987, 105, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J. Understanding Hyaluronan-Protein Interactions, Glycoforum. 2001. Available online: https://www.glycoforum.gr.jp/article/05A1.html (accessed on 14 April 2024).
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Baaten, B.J.; Li, C.-R.; Bradley, L.M. Multifaceted regulation of T cells by CD44. Commun. Integr. Biol. 2010, 3, 508–512. [Google Scholar] [CrossRef]
- Hinneh, J.A.; Gillis, J.L.; Moore, N.L.; Butler, L.M.; Centenera, M.M. The role of RHAMM in cancer: Exposing novel therapeutic vulnerabilities. Front. Oncol. 2022, 12, 982231. [Google Scholar] [CrossRef]
- Tolg, C.; Hamilton, S.R.; Morningstar, L.; Zhang, J.; Zhang, S.; Esguerra, K.V.; Telmer, P.G.; Luyt, L.G.; Harrison, R.; McCarthy, J.B.; et al. RHAMM Promotes Interphase Microtubule Instability and Mitotic Spindle Integrity through MEK1/ERK1/2 Activity. J. Biol. Chem. 2010, 285, 26461–26474. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.; Harrison, R. RHAMM, a Member of the Hyaladherins, Glycoforum. 1999. Available online: https://www.glycoforum.gr.jp/article/03A3.html (accessed on 14 April 2024).
- Banerji, S.; Ni, J.; Wang, S.X.; Clasper, S.; Su, J.; Tammi, R.; Jones, M.; Jackson, D.G. LYVE-1, a new homologue of the cd44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999, 144, 789–801. [Google Scholar] [CrossRef]
- Jackson, D.G. Leucocyte trafficking via the lymphatic vasculature—Mechanisms and consequences. Front. Immunol. 2019, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef]
- Makkar, S.; Riehl, T.E.; Chen, B.; Yan, Y.; Alvarado, D.M.; Ciorba, M.A.; Stenson, W.F. Hyaluronic acid binding to TLR4 promotes proliferation and blocks apoptosis in colon cancer. Mol. Cancer Ther. 2019, 18, 2446–2456. [Google Scholar] [CrossRef]
- Ebid, R.; Lichtnekert, J.; Anders, H.-J. Hyaluronan Is Not a Ligand but a Regulator of Toll-Like Receptor Signaling in Mesangial Cells: Role of Extracellular Matrix in Innate Immunity. ISRN Nephrol. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lesley, J.; Gál, I.; Mahoney, D.J.; Cordell, M.R.; Rugg, M.S.; Hyman, R.; Day, A.J.; Mikecz, K. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J. Biol. Chem. 2004, 279, 25745–25754. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H. Aggrecan and versican: Two brothers close or apart. Am. J. Physiol. Physiol. 2022, 322, C967–C976. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Aggrecan, the primary weight-bearing cartilage proteoglycan, has context-dependent, cell-directive properties in embryonic development and neurogenesis: Aggrecan glycan side chain modifications convey interactive biodiversity. Biomolecules 2020, 10, 1244. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Brevican: A major proteoglycan in adult brain. Perspect. Dev. Neurobiol. 1996, 3, 307–317. [Google Scholar]
- Hamel, M.G.; Mayer, J.; Gottschall, P.E. Altered production and proteolytic processing of brevican by transforming growth factor β in cultured astrocytes. J. Neurochem. 2005, 93, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wu, C.; Guo, L.; Liu, Y.; Mo, W.; Wang, H.; Ding, J.; Wong, E.T.; Yu, M. The role of brevican in glioma: Promoting tumor cell motility in vitro and in vivo. BMC Cancer 2012, 12, 607. [Google Scholar] [CrossRef]
- Maeda, N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 2015, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, M.; Datta, K. A novel glycoprotein that binds to hyaluronic-acid. Biochem. Int. 1986, 13, 79–88. [Google Scholar]
- Dsouza, M.; Datta, K. Evidence for naturally-occurring hyaluronic-acid binding-protein in rat-liver. Biochem. Biophys. Res. Commun. 1985, 10, 43–51. [Google Scholar]
- Majumdar, M.; Meenakshi, J.; Goswami, S.K.; Datta, K. Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem. Biophys. Res. Commun. 2002, 291, 829–837. [Google Scholar] [CrossRef]
- Kamal, A.; Datta, K. Upregulation of hyaluronan binding protein 1 (HABP1/p32/gC1qR) is associated with Cisplatin induced apoptosis. Apoptosis 2006, 11, 861–874. [Google Scholar] [CrossRef]
- Zhou, B.; Weigel, J.A.; Fauss, L.; Weigel, P.H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2000, 275, 37733–37741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Oka, J.A.; Singh, A.; Weigel, P.H. Purification and subunit characterization of the rat liver endocytic hyaluronan receptor. J. Biol. Chem. 1999, 274, 33831–33834. [Google Scholar] [CrossRef]
- Politz, O.; Gratchev, A.; McCOURT, P.A.G.; Schledzewski, K.; Guillot, P.; Johansson, S.; Svineng, G.; Franke, P.; Kannicht, C.; Kzhyshkowska, J. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem. J. 2002, 362, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Tanaka, M.; Suzuki, K.; Miyajima, A. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Dev. Dyn. 2007, 236, 2258–2267. [Google Scholar] [CrossRef]
- Falkowski, M.; Schledzewski, K.; Hansen, B.; Goerdt, S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. 2003, 120, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Noda, H.; Okaniwa, N.; Adachi, K.; Shinmura, T.; Nakagawa, S.; Ebi, M.; Ogasawara, N.; Funaki, Y.; Zhuo, L. Se-rum-derived hyaluronan-associated protein is a novel biomarker for inflammatory bowel diseases. Digestion 1961, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Suzuki, S.; Kimata, K. Hyaluronic acid associated with the surfaces of cultured fibroblasts is linked to a serum-derived 85-kDa protein. J. Biol. Chem. 1990, 265, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yoneda, M.; Kimata, K. A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter alpha-trypsin inhibitor. J. Biol. Chem. 1993, 268, 26725–26730. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, T.E. Cartilage; aggrecan-link protein-hyaluronan aggregates. Glycoforum 1998, 2, A5. [Google Scholar]
- Zaleski, K.J.; Kolodka, T.; Cywes-Bentley, C.; McLoughlin, R.M.; Delaney, M.L.; Charlton, B.T.; Johnson, W.; Tzianabos, A.O. Hyaluronic acid binding peptides prevent experimental staphylococcal wound infection. Antimicrob. Agents Chemother. 2006, 50, 3856–3860. [Google Scholar] [CrossRef] [PubMed]
- Mummert, M.E.; Mohamadzadeh, M.; Mummert, D.I.; Mizumoto, N.; Takashima, A. Development of a Peptide Inhibitor of Hy-aluronan-Mediated Leukocyte Trafficking; Rockefeller University Press: New York City, NY, USA, 2000; Available online: http://www.jem.org/cgi/content/full/192/6/769 (accessed on 1 April 2024).
- Unterman, S.A.; Gibson, M.; Lee, J.H.; Crist, J.; Chansakul, T.; Yang, E.C.; Elisseeff, J.H. Hyaluronic acid-binding scaffold for articular cartilage repair. Tissue Eng. Part A 2012, 18, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Li, W.; Chang, L.; Gautrot, J.E.; Wang, W.; Azevedo, H.S. Hyaluronan (HA) Immobilized on Surfaces via Self-Assembled Monolayers of HA-Binding Peptide Modulates Endothelial Cell Spreading and Migration through Focal Adhesion. ACS Appl. Mater. Interfaces 2021, 13, 25792–25804. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.J.; Elder, R.M.; Neumann, A.J.; Jayaraman, A.; Bryant, S.J. Interaction of hyaluronan binding peptides with glycosaminoglycans in poly(ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, Y.; Hu, H.; Zhang, C.; Wang, Y.; Xu, X.; Zhang, T.; Su, R.; Luo, X. Enhanced Transdermal Absorption of Hyaluronic Acid via Fusion with Pep-1 and a Hyaluronic Acid Binding Peptide. Macromol. Biosci. 2023, 23, e2200173. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.Y.; Wang, C.; Galarraga, J.H.; Puré, E.; Han, L.; Burdick, J.A. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 2019, 222, 119451. [Google Scholar] [CrossRef] [PubMed]
- Deloney, M.; Garoosi, P.; Dartora, V.F.C.; Christiansen, B.A.; Panitch, A. Hyaluronic acid-binding, anionic, nanoparticles inhibit ECM degradation and restore compressive stiffness in aggrecan-depleted articular cartilage explants. Pharmaceutics 2021, 13, 1503. [Google Scholar] [CrossRef]
- Liu, N.; Xu, X.; Chen, J.; Wang, L.; Yang, S.; Underhill, C.B.; Zhang, L. Hyaluronan-binding peptide can inhibit tumor growth by interacting with Bcl-2. Int. J. Cancer 2003, 109, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Chen, Y.; Chen, J.; Yang, S.; Gao, F.; Underhill, C.B.; Creswell, K.; Zhang, L. A Peptide with Three Hyaluronan Binding Motifs Inhibits Tumor Growth and Induces Apoptosis. Cancer Res. 2003, 63, 5685–5690. Available online: http://aacrjournals.org/cancerres/article-pdf/63/18/5685/2507948/ch1803005685.pdf (accessed on 30 March 2024). [PubMed]


| Protein | NCBI Gene ID | Function | Expression in Normal Tissues (Reads per Kilobase of Transcript per Million Mapped Reads) * |
|---|---|---|---|
| Aggrecan | 176 | An integral part of the ECM in cartilaginous tissue and it withstands compression in cartilage Enables carbohydrate binding Enables ECM structural constituent conferring compression resistance [101,102] Enables HA binding Enables metal ion binding Enables protein binding [103] | 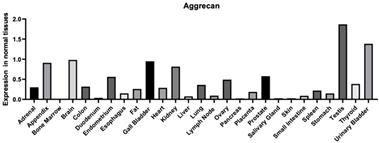 |
| Neurocan | 1463 | Chondroitin sulfate proteoglycan that is involved in the regulation of cell adhesion and migration Enables calcium ion binding Enables carbohydrate binding Enables HA binding [104] | 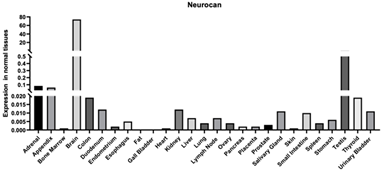 |
| Versican | 1462 | The protein encoded is a large chondroitin sulfate proteoglycan and is a major component of the extracellular matrix. This protein is involved in cell adhesion, proliferation, migration, and angiogenesis and plays a central role in tissue morphogenesis and maintenance. Enables calcium ion binding Enables carbohydrate binding Enables ECM structural constituent conferring compression resistance [102,105,106,107] Enables glycosaminoglycan binding [108] Enables HA binding [108] Enables protein binding [109] | 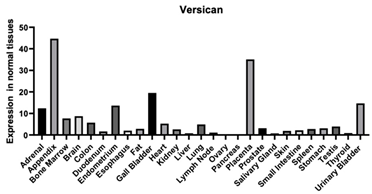 |
| Brevican | 63827 | This gene encodes a member of the lectican family of chondroitin sulfate proteoglycans that is specifically expressed in the central nervous system. May function in the formation of the brain’s extracellular matrix Enables carbohydrate binding Enables HA binding Enables protein binding |  |
| TSG-6 | 7130 | A secretory protein that contains a hyaluronan binding domain. This protein has been shown to form a stable complex with inter-alpha-inhibitor (I alpha I), and thus enhance the serine protease inhibitory activity of I alpha I, which is important in the protease network associated with inflammation. Enables calcium ion binding [110] Enables carboxylesterase activity Enables fibronectin binding [110] Enables HA binding [111] Enables protein binding [111,112,113,114,115] | 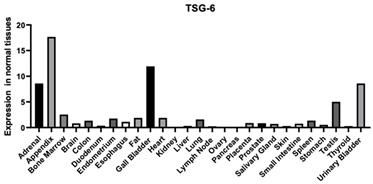 |
| CD44 | 960 | CD44 is found in a variety of tissues with the highest expression found in the appendix and the skin A cell-surface glycoprotein is involved in cell-cell interactions, cell adhesion, and migration. It is a receptor for HA and can also interact with other ligands, such as osteopontin, collagens, and matrix metalloproteinases (MMPs). This protein participates in a wide variety of cellular functions including lymphocyte activation, recirculation and homing, hematopoiesis, and tumor metastasis. Enables collagen binding [116] Contributes to cytokine receptor activity Enables HA binding [117,118,119] Enables protein binding [120] Enables transmembrane signaling receptor activity | 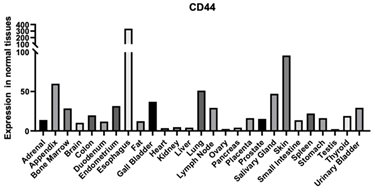 |
| LYVE | 10894 | A type I integral membrane glycoprotein. The encoded protein acts as a receptor and binds to both soluble and immobilized hyaluronan. This protein may function in lymphatic hyaluronan transport and have a role in tumor metastasis. Enables cargo receptor activity Enables HA binding [121] Enables protein binding Enables signaling receptor activity Enables transmembrane signaling receptor activity |  |
| RHAMM | 3161 | Involved in cell motility. It is expressed in breast tissue and together with other proteins, it forms a complex with BRCA1 and BRCA2, thus is potentially associated with a higher risk of breast cancer. Cargo receptor activity—binding to a specific substance to deliver it to a transport vesicle HA binding [122] Protein binding [63,64] |  |
| Stabilin | 55576 | This protein is a large, transmembrane receptor protein that may function in angiogenesis, lymphocyte homing, cell adhesion, or receptor scavenging. The protein contains 7 fasciclin, 15 epidermal growth factor (EGF)-like, and 2 laminin-type EGF-like domains as well as a C-type lectin-like hyaluronan binding LINK module. The receptor has been shown to bind and endocytose ligands such as hyaluronan, low-density lipoprotein, Gram-positive and Gram-negative bacteria, and advanced glycosylation end products. Enables calcium ion binding Enables HA binding Enables low-density lipoprotein particle binding [123] Enables protein binding [124] Enables protein-disulfide reductase activity [123] Enables scavenger receptor activity [118] |  |
| SPACR | 3617 | A protein that is a major component of the retinal interphotoreceptor matrix. The encoded protein is a proteoglycan that is thought to play a role in maintaining the viability of photoreceptor cells and in the adhesion of the neural retina to the retinal pigment epithelium. Enables chondroitin sulfate binding Enables extracellular matrix structural constituent [125] Enables heparin binding Enables HA binding | 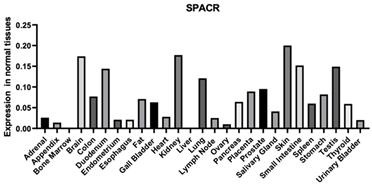 |
| SPACRCAN | 50939 | This protein binds chondroitin sulfate and hyaluronan and is a proteoglycan. The encoded protein plays a role in the organization of the interphotoreceptor matrix and may promote the growth and maintenance of the light-sensitive photoreceptor outer segment. Enables ECM structural component [126] Enables heparin binding Enables HA binding | 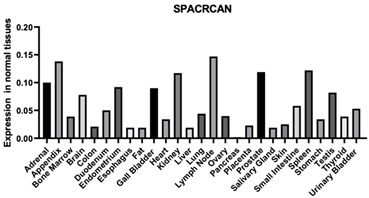 |
| PHBP HA binding peptide 2 | 3026 | A member of the peptidase S1 family of serine proteases. The encoded preproprotein is secreted by hepatocytes and proteolytically processed to generate heavy and light chains that form the mature heterodimer. Further autoproteolysis leads to smaller, inactive peptides. This extracellular protease binds hyaluronic acid and may play a role in the coagulation and fibrinolysis systems Enables calcium ion binding Enables GAG binding Enables peptidase activity [127] Enables serine-type endopeptidase activity |  |
| HABP Sequence | Results |
|---|---|
| HABP35 LKQKIKHVVKLKVVVKLRSQLVKRKQN | Decease bacterial burden at the wound site [161] |
| HABP42 (all D-amino acids; STMMSRSHKTRSHHV) | Decease bacterial burden at the wound site [161] |
| GAHWQFNALTVRGGGS (HABP52) | Decease bacterial burden at the wound site [161] Binds to HA with high affinity and inhibits leukocyte adhesion to HA |
| GAHWQFNALTVR | High HA binding, inhibited the adhesion of leukocytes and inhibited hypersensitivity responses [162] HA interactive PEG hydrogels increased the cartilage tissue production in the defects (reduced cartilage degradation) [163] More hydrophilic and attracted more HA than other SAMs [164] |
| TLRAIWPMWMSS (“Pep-4”) | Not successful in HA binding [162] |
| IPLTANYQGDFT (“Pep-5”) | Not successful in HA binding [162] |
| (TSYGRPALLPAA “Pep-2”) | Not successful in HA binding [162] |
| (MDHLAPTFRPAI “Pep-3”) | Not successful in HA binding [162] |
| RYPISRPRKRC | measuring sGAGs through biochemical assays and it was found that the incorporation of HA improves sGSG content in the hydrogel while minimizing its loss from the hydrogel indicating an improvement in neocartilage deposition [165] |
| GYPISGPGGGC (charge control peptide) | HABP bind negatively charged GAGs largely through electrostatic interactions, but interactions typically occur in a physiological environment [165] |
| WRHGFALTAVNQ (scrambled) | HABP bind negatively charged GAGs largely through electrostatic interactions, but interactions typically occurred in a physiological environment [120] |
| CNGRCGGKQKIKHVVKLKVVVKLKSQLVKRKVVVRRRKKIQGRSKR | the tumor cells transfected with P4 grew slower [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huffer, A.; Mao, M.; Ballard, K.; Ozdemir, T. Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function. Biomimetics 2024, 9, 499. https://doi.org/10.3390/biomimetics9080499
Huffer A, Mao M, Ballard K, Ozdemir T. Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function. Biomimetics. 2024; 9(8):499. https://doi.org/10.3390/biomimetics9080499
Chicago/Turabian StyleHuffer, Amelia, Mingyang Mao, Katherine Ballard, and Tugba Ozdemir. 2024. "Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function" Biomimetics 9, no. 8: 499. https://doi.org/10.3390/biomimetics9080499
APA StyleHuffer, A., Mao, M., Ballard, K., & Ozdemir, T. (2024). Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function. Biomimetics, 9(8), 499. https://doi.org/10.3390/biomimetics9080499






