Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin
Abstract
1. Introduction
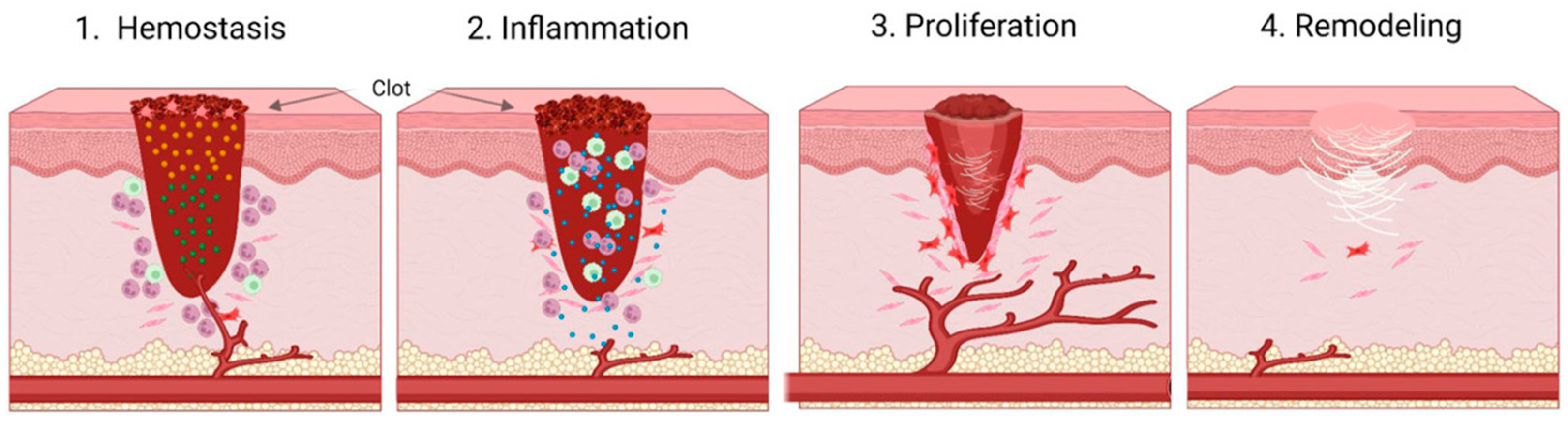
1.1. Physiological Stages of Wound Healing
1.2. Advancement in Wound Management Techniques
1.3. Concept of Biomimetic Materials
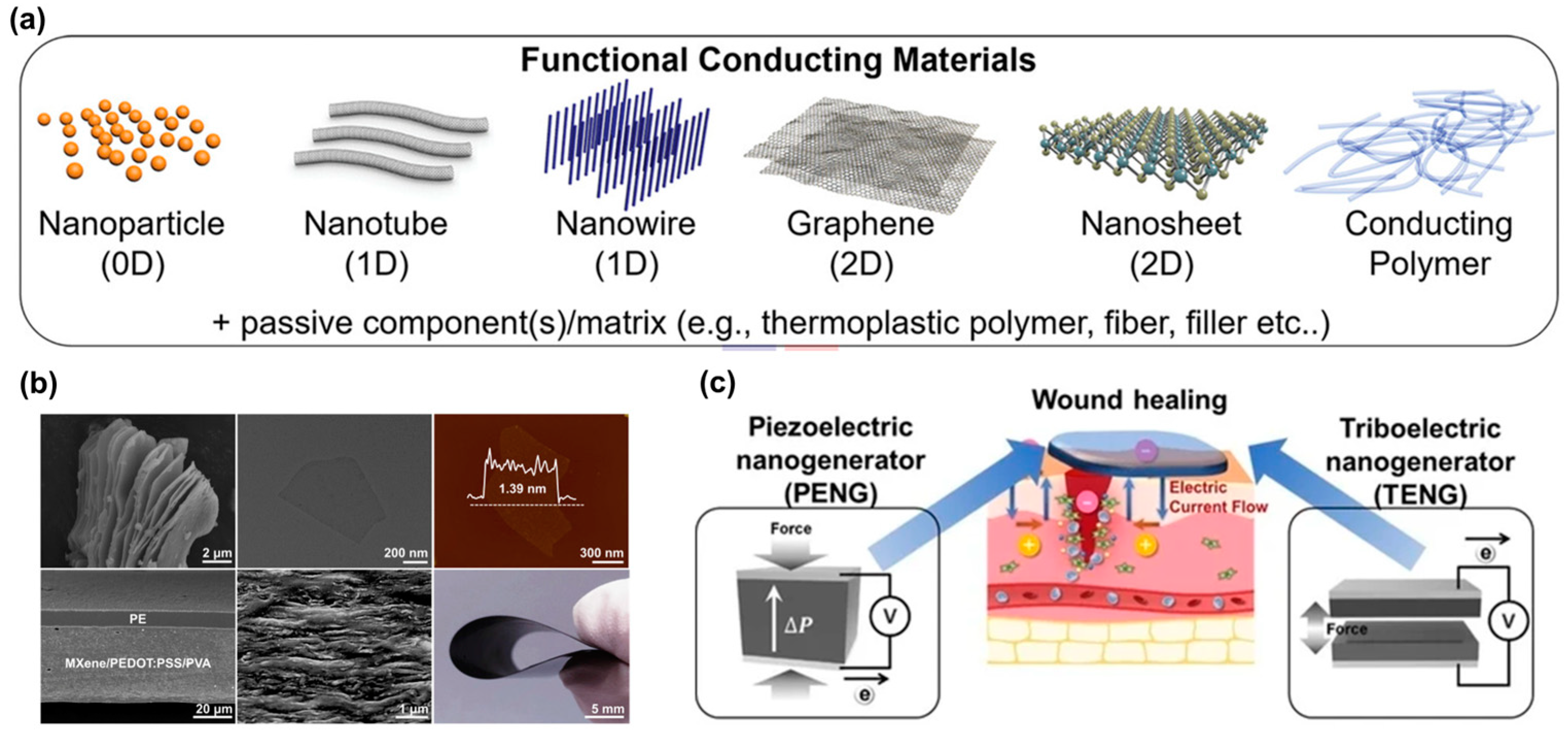
1.4. Electronic Skin
2. Biomimetics Skin for Wound Care Techniques
| Application | Inspiration | Material | Function | Ref. |
|---|---|---|---|---|
| Wound healing | Skin biological structure | mPCL | Skin regeneration, scar reduction, cell therapy | [85] |
| Skin cells | PCL, collagen | Promotes mechanical strength, wound healing, suture | [86] | |
| Fetal skin in early pregnancy | Hyaluronan, vitamin E, dopamine, β-cyclodextrin | Anti-inflammatory, cell migration, collagen synthesis, distribution, reconstruction, scar minimization | [87] | |
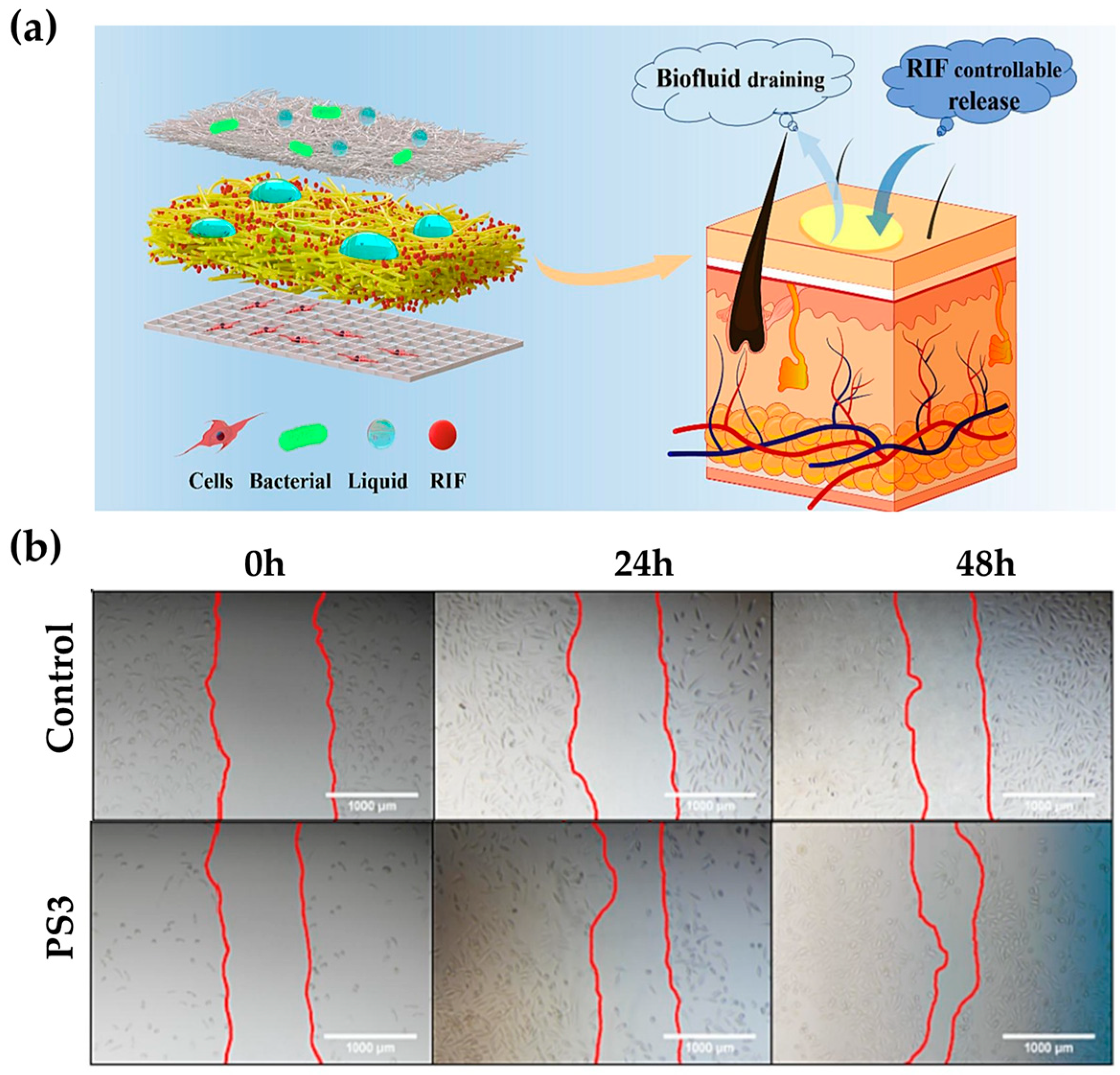
| Skin (endothelium/dermis/epidermis) | PCL, RIF | Antibacterial, moisture retention, penetration gradient, moisture absorption, breathability, hydrophobicity, drug release according to pH changes, wound healing | [88] | |
| Wound healing of oral mucosa | Chitosan, sodium alginate, EDTA, EGF | Wound healing, scar minimization, sterilization, moist environment, growth factor expression | [94] | |
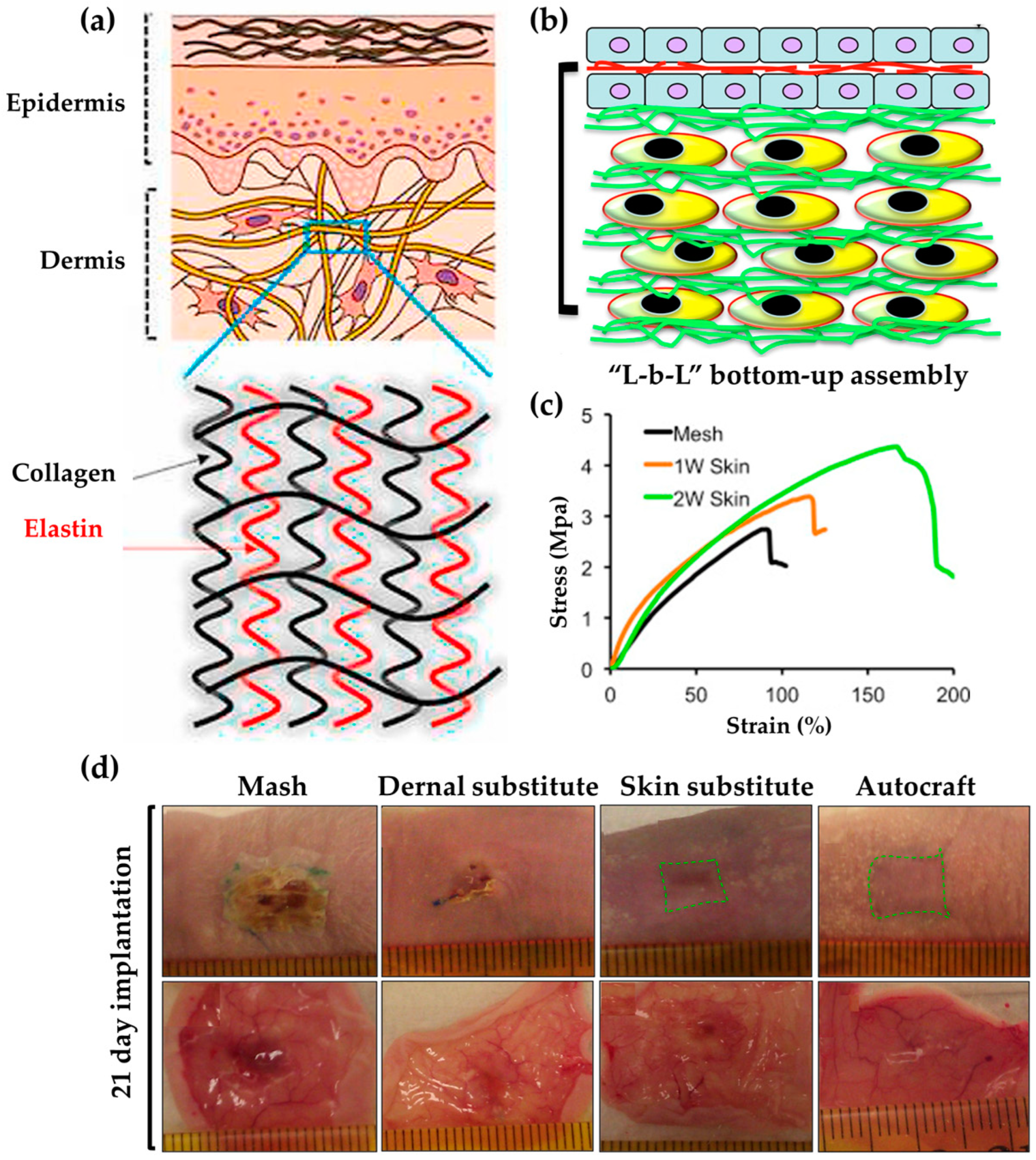
| ECM of the dermis | Bovine collagen type I, tropoelastin | Regenerated skin | [95] | |
| ECM | PCL, cellulose acetate, chitosan, collagen I | Biocompatibility, cell adhesion, spreading, regenerative skin, blood vessel formation, protein upregulation, skin regeneration | [96] | |
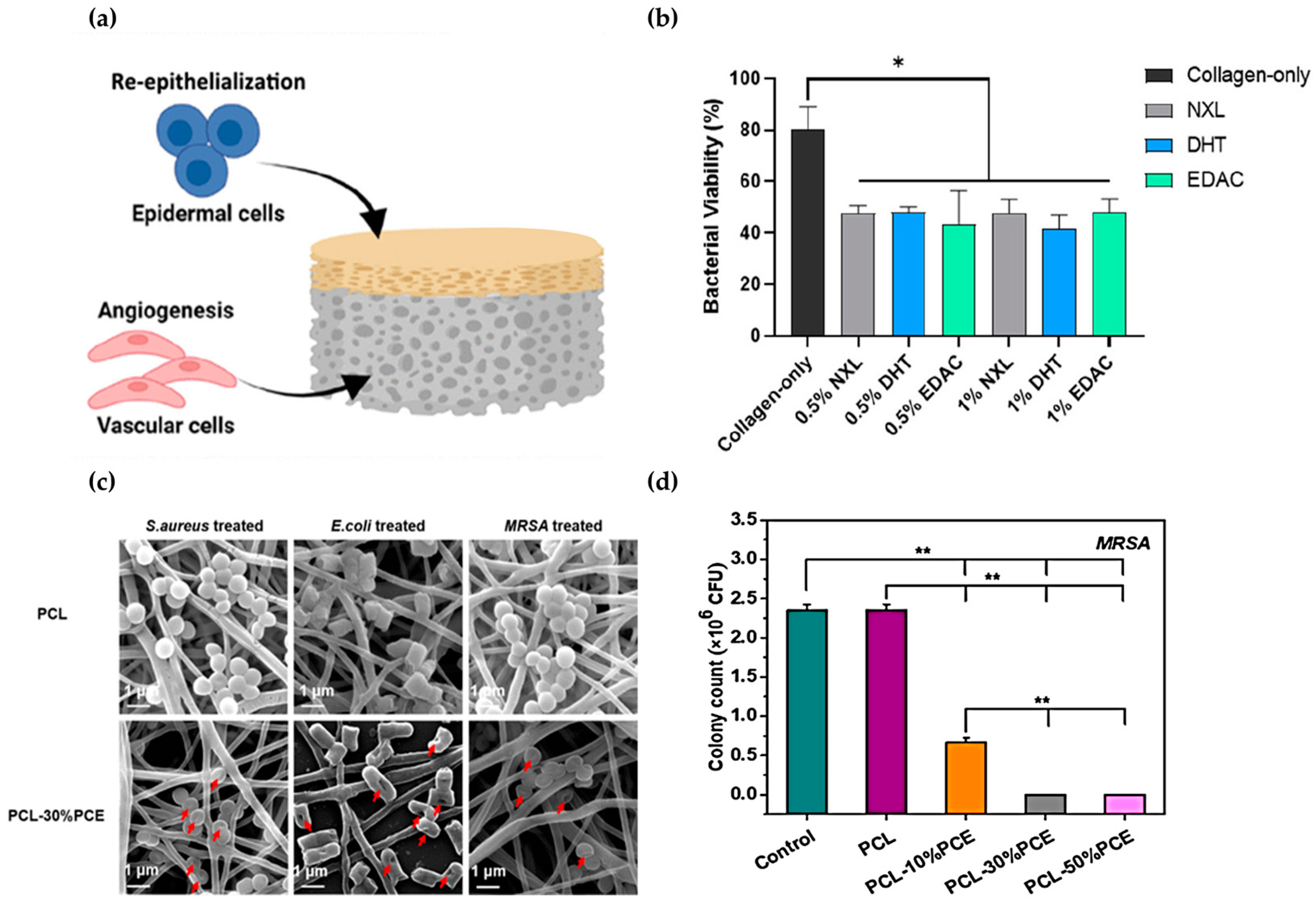
| Antibacterial | Skin (epidermis/dermis) | Collagen, chitosan | Angiogenesis, structural stability, antibacterial, cell proliferation, diabetic wound healing | [90] |
| ECM | Collagen, PCL, bioactive glass nanoparticles | Diabetic wounds, cell proliferation, angiogenesis, collagen remodeling | [92] | |
| Skin cells, matrix structure | PPL, PCL | Biocompatibility, wound healing, skin regeneration | [91] | |
| ECM | AgNPs, gelatin, GPTMS | Wound healing, tissue regeneration | [93] |
3. Biomimetics of Animal- and Plant-Driven Wound Care Techniques
3.1. Wound Care Techniques Inspired by Animals
3.2. Wound Care Techniques Inspired by Plants
| Inspired Organism | Inspired Species | Inspiration | Application | Material | Function | Ref. |
|---|---|---|---|---|---|---|
| Animal | lizard | Small nano-tipped hairs (thorns) | Antibacterial | Chitosan, alginate, silk fibroin | Antibacterial, hydrophobicity | [102] |
| Directional fluid flow skin | Sensing | Graphene, gold, graphite | Sensing, exudate collection | [104] | ||
| mussel | Adhesion | Hemostasis, wound healing | Collagen, starch | Adhesion, sealing, hemostasis, healing | [103] | |
| Adhesion | Drug release | Methacrylated laminarin, hydrogel | Adhesion, drug content, drug release, antibacterial | [109] | ||
| native blood vessels | plant cellulose-based blood vessel | Artificial blood vessels | Cellulose hydrogel, HEMA | Mechanical performance, cytocompatibility, hemocompatibility, artificial blood vessels | [114] | |
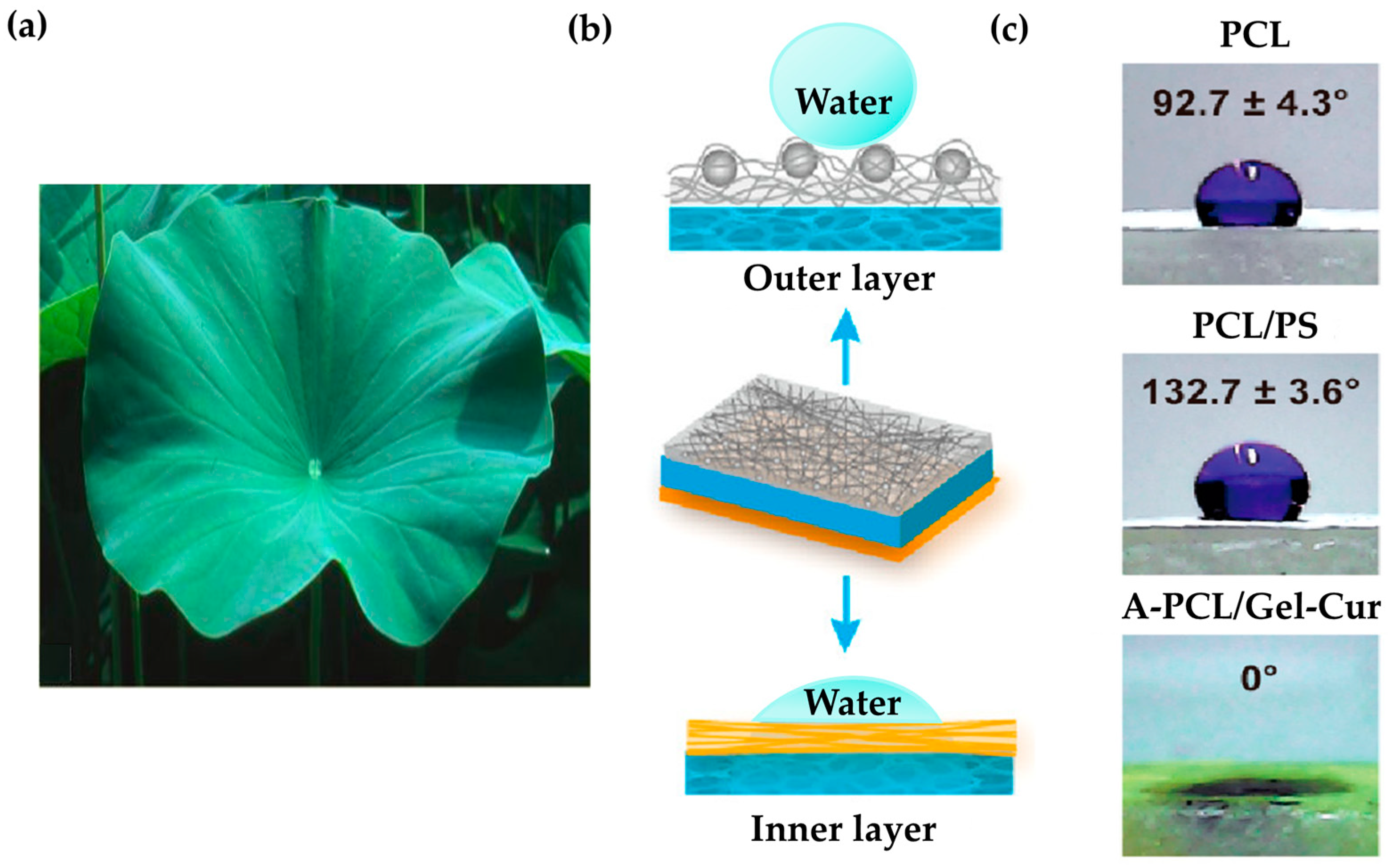
| Plant | lotus leaf | Skin structure, superhydrophobic structure | Wound healing | Collagen, chitosan, PCL, PS, Curcumin, gelatin | Antibacterial, healing, cell growth, hydrophobicity | [113] |
| fine structure of leaves | Sensing | PDMS, PPy/Ag hybrid | Electrical conductivity, pressure, stretch and bend sensors | [115] | ||
| Delosperma cooperi | Centripetal multilayer structure | Self-sealing | PDMS, shape memory polymer | Self-sealing | [116] |
4. Biomimetic Nanoparticles for Wound Care Techniques
4.1. Wound Care Techniques Inspired by Nanostructure
4.2. Wound Care Techniques Inspired by Diatom and Diatomite
| Inspired Organism | Inspired Species | Inspiration | Application | Material | Function | Ref. |
|---|---|---|---|---|---|---|
| Nanostructure | Nanovesicles | Endothelial cell-derived nanovesicles | Infection diabetic wound | Extruded endothelium, rhamnolipid liposome | Antibacterial, targeting, penetration | [122] |
| Nanozyme | Homing mechanism of natural macrophages | Wound healing | RAW 264.7, IL-4, Cobalt | Targeting, anti-inflammatory, antibacterial, antioxidant, immunomodulatory | [123] | |
| Glucose oxidase, peroxidase | Infection diabetic wound | Fe2+, AuNPs | Antibacterial, healing | [124] | ||
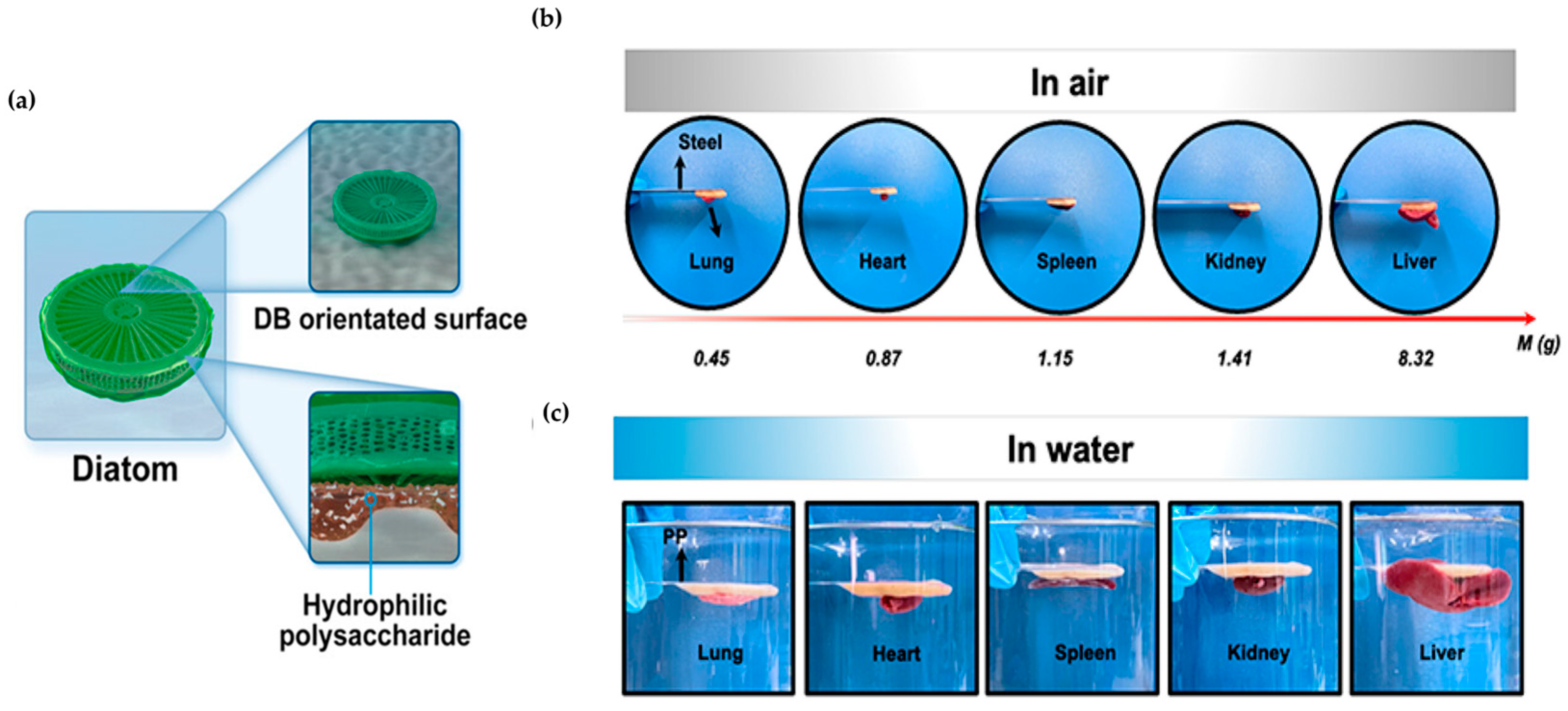
| Diatomaceous | Diatom | Adhesion | Hemostasis | DB, BSP | Adhesion, hemostasis | [133] |
| Adhesion | Adhesion | THMA, MA | Adhesion | [134] | ||
| Diatomite | Structure | Antibacterial, hemostasis | Silver, hollow polymerized silicon NPs | Antibacterial, hemostasis | [136] | |
| Siliceous frustule | Antibacterial | Graphen oxide, TEOS, MPTMS | Antibacterial, controlled release | [137] |
5. Electronic Skin (E-Skin)
5.1. Concept of E-Skin and Its Multifunctional Capabilities
5.2. E-skin Integration with Wound Monitoring and Management
5.2.1. Skin-Inspired Antibacterial E-Skin
5.2.2. Adhesion and Visualization Techniques Inspired by Nature
5.2.3. Electrospun Nanofibers for E-Skin Dressings
5.2.4. Biofuel Cell Patch
5.2.5. Wound Monitoring and Treatment System
5.2.6. Wireless Smart Dressings with Closed-Loop Systems
5.2.7. Remote Digital Postoperative Wound Monitoring and Machine Learning Applications
5.3. Challenges and Future Perspective on E-Skin for Wound Monitoring and Management
| Polymer | Composites/ Components | Properties/ Function | Monitoring | Treatment | Ref. |
|---|---|---|---|---|---|
| Polyaniline, PVA | PDA decorated AgNPs | Conductive antimicrobial self-healing repeatable adhesiveness | Epidermal sensor | Diabetic Foot Wound Dressings | [182] |
| P(AM-co-SBMA) | MXene PDA AgNPs | Conductive, antibacterial, biocompatible, self-adhesive, stretchable | Epidermal sensor | Diabetic Wound Healing | [183] |
| Carboxyl Methyl chitosan (CMCS), phenylboronic acid grafted sodium alginate | MXene nanosheets network catechol-rich Tea polyphenol | Self-healing and shear-thinning properties injectable self-healable, adhesive conductive antibacterial and hemostatic properties | Epidermal sensor electrophysiological signals like electromyogram (EMG) and electrocardiogram (ECG) signals | Hemostatic potential in mouse liver hemorrhage and mouse tail amputation models | [189] |
| Polyurethane P(MMA-BA) | LM PDA Recombinant spidroin protein | Stretchability pressure sensitivity | Motion Tactile sensing Biochemical sensing | Promoting wound healing | [138] |
| Gelatin, dextran | Hydroxyapatite curcumin | Antimicrobial antioxidant, pH-responsive color change | pH | Rapid wound healing | [197] |
| GelMA | CNT, graphene | Breathability, conductivity, stretchability, moisture absorption capacity | Moisture sensor strain sensor | Wound management and monitoring | [201] |
| PLA/PVP | MXene F sensor (PANI and Ag/AgCl electrode) | Stretchability, bendability, breathability, anti-infection | Wound pH | Photothermal therapy Faster wound healing | [205] |
| Paper and inkjet printing Binder: ethylcellulose polymer | Oxygen sensing dye Oxygen generation catalyst (KMnO4) surfactant | Low-cost Continuous oxygen delivery and sensing Flexible Scalable, biocompatible | Oxygen | Oxygen delivering Increase in oxygen levels to clinically relevant levels. | [206] |
| Alginate/CaCl2 | Gentamicin Growth factors Dowex bead conjugated with dye Growth factors | User-friendly colorimetric sensor | pH glucose | Faster wound healing, diabetic wounds | [167] |
| PDMS | LM heater NFC-integrated circuit PNIPAM-co-AM hydrogel bead antibiotics | Microfluidic channel flexible and wearable on-demand drug release | Temperature humidity | Faster healing of an infected wound | [213] |
| PEDOT:PSS dry pellet NIPAM/AAM/ MBAA | NFC transponder Ag/AgCl electrode Platinum electrode H2O2, ascorbic acid | Conductive Flexible Biocompatible Attachable/detachable on demand Delivering electrical stimulation | Temperature Skin impedance | Wound care and healing | [69] |
| Collagen Fibrin, thrombin | Neural interface electrodes | Pressure temperature | Wound healing and restoring skin tactile function | [214] |
6. Applications and Future Directions
6.1. Description and Limitations of Wound Care in Biomimicry
6.2. Consider the Challenges and Opportunities for Clinical Adoption
6.3. Propose Future Directions for Research and Development
- (1)
- Biomimetic wound dressings have shown great promise in promoting wound healing. The traditional approach to wound dressings has limitations in supporting complex wound healing processes, as they primarily focus on sealing the wound, absorbing exudate, and maintaining a moist environment. However, by incorporating ECM components like collagen, glycosaminoglycan, or hyaluronic acid into traditional dressings, it can actively promote epithelialization, angiogenesis, and collagen deposition, thereby enhancing the overall wound healing process [90,237,238].
- (2)
- Biomimetics presents a promising approach to wound care technology and has the potential to extend its benefits to the treatment of other diseases and conditions. The field of biomedicine can benefit greatly from mimicking components or micro- and nanostructures found in various living organisms such as animals, plants, and humans [239]. Materials that utilize these biomimetic components or structures offer numerous advantages, including biocompatibility, biodegradability, targeting efficiency, low toxicity, and antioxidant and anti-inflammatory properties. These biomimetic materials have proven effective in wound healing technologies and show promise in the treatment of a variety of diseases, particularly cancer [119,240].
- (3)
- Traditional wound dressings lack the ability to provide real-time information about wound conditions, resulting in missed opportunities to adjust treatment. Therefore, there is a need for versatile smart E-skin patches that can accurately monitor wound conditions and accelerate wound healing. For this, E-skin materials are required to possess properties such as elasticity, self-healing capabilities, biocompatibility, skin-like softness, and the ability to generate electrical signals for rapid sensory transmission [241,242]. Researchers are exploring new materials and structural designs to achieve these properties using hydrogels, liquid metals, conductive polymers, and nanomaterials [243]. Scientists at Stanford University have made a significant breakthrough in synthetic skin technology. They developed a multi-layered thin film sensor that heals by auto-realignment, closely resembling the natural healing process of our skin [244].
- (4)
- The advancement of E-skin, with its soft, stretchable, biocompatible, and adhesive properties, enables continuous health monitoring as a wearable device that adheres well to the body due to its soft and flexible nature [245]. In addition, for continuous signal sensing and monitoring, E-skin devices need to operate wirelessly and continuously [156,246]. Energy self-generation, storage, and power efficiency are crucial to provide enough power without compromising comfort or fit. Furthermore, advances in E-skin’s strain sensors can act as a sensitive touch interface or even detect gestures and movements, enabling a seamless human–machine interface. This human–machine interface can be applied to sensitive health monitoring [247,248]. Wearable self-powered sensors can be used to monitor vital signs (heart rate, temperature, blood pressure, etc.), detect skin conditions such as pressure ulcers and diabetic patients, track wound healing progress, and so on, for the healthcare of the elderly population and patients with cardiovascular disease and diabetes [248].
- (5)
- Future research will focus on the development of E-skins that closely mimic human skin in terms of functionality and properties, including mechanoreceptors, softness, flexibility, self-healing ability, and environmental adaptability, and even develop E-skins that can outperform human skin in certain areas [249,250]. Meanwhile, making artificial skin that acts like real skin is hard due to material issues, but using AI and machine learning could help find and improve materials, making the skin better and safer [251]. Future developments in E-Skin sensors will include increased accuracy, improved battery life, and integration of more sensors for comprehensive health monitoring. Integrating AI using machine learning algorithms into the E-skin for independent data analysis can help us to understand the user’s personalized health profile, enabling the creation of precise personalized treatment plans. This will enable the creation of personalized and accurate monitoring and treatment plans. Additionally, drug delivery mechanisms can be integrated directly into E-skins for targeted therapy. It is important to consider the growing concern for the environment, leading to the development of sustainable and recyclable E-skins as an important future direction.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Multifaceted roles of mitochondria in wound healing and chronic wound pathogenesis. Front. Cell Dev. Biol. 2023, 11, 1252318. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, F.; Zhang, Q.; Huang, X.; Wang, Z. Autophagy and skin wound healing. Burn. Trauma. 2022, 10, tkac003. [Google Scholar] [CrossRef]
- Dorgalaleh, A.; Daneshi, M.; Rashidpanah, J.; Roshani Yasaghi, E. An Overview of Hemostasis. In Congenital Bleeding Disorders; Dorgalaleh, A., Ed.; Springer: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K. Basics of wound healing. In Innovations and Advances in Wound Healing; Springer: Singapore, 2023; pp. 1–42. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Turabelidze, A.; Dipietro, L.A. Inflammation and wound healing. Endod. Top. 2011, 24, 26–38. [Google Scholar] [CrossRef]
- Adderley, U.J. Managing wound exudate and promoting healing. Br. J. Community Nurs. 2010, 15, S15–S20. [Google Scholar] [CrossRef] [PubMed]
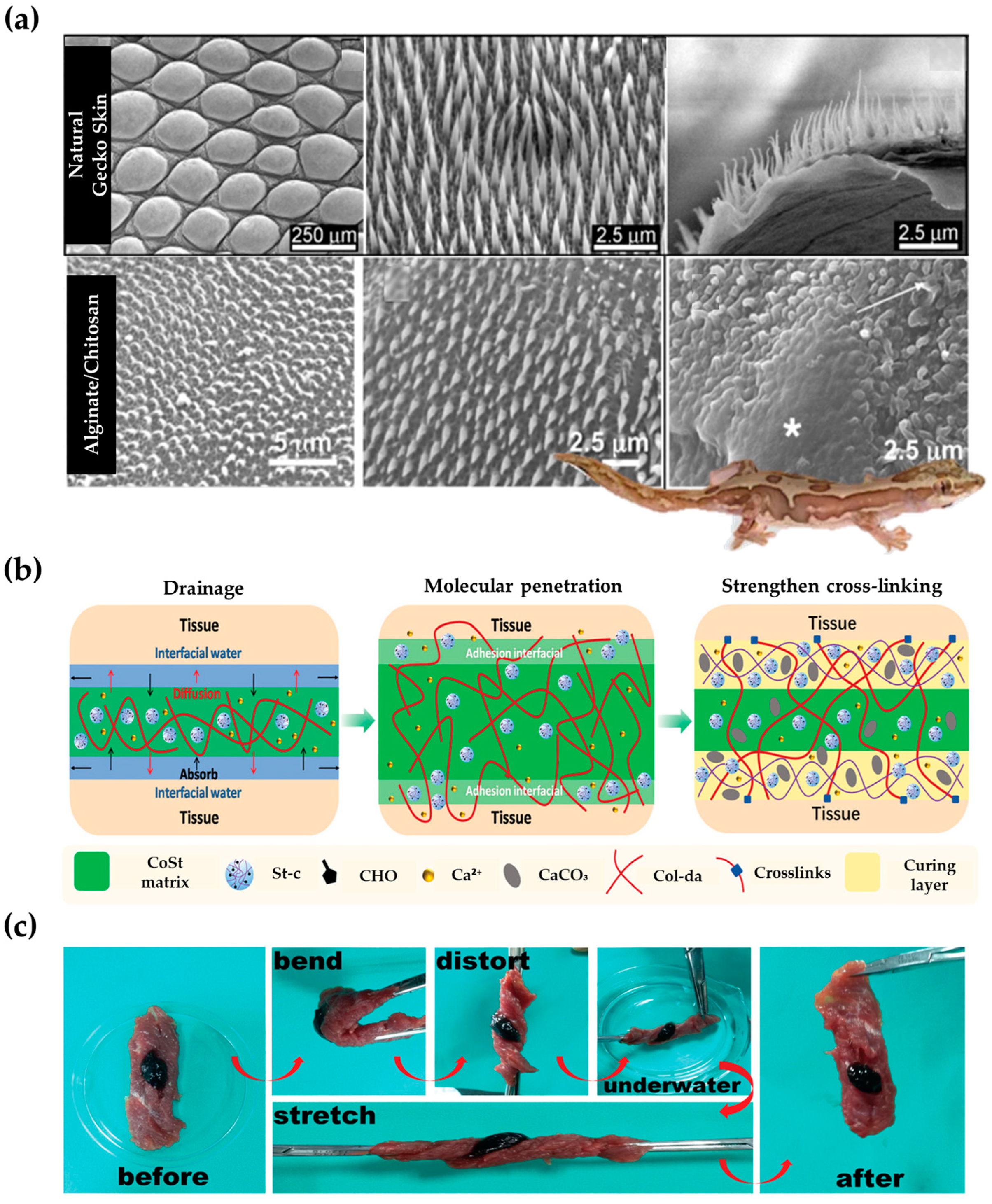
- Spear, M. Wound exudate—The good, the bad, and the ugly. Plast. Surg. Nurs. 2012, 32, 77–79. [Google Scholar] [CrossRef]
- Gethin, G. Understanding the inflammatory process in wound healing. Br. J. Community Nurs. 2012, 7, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K. The physiology of wound healing. Ann. Emerg. Med. 1988, 17, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar]
- Soliman, A.M.; Das, S.; Abd Ghafar, N.; Teoh, S.L. Role of MicroRNA in Proliferation Phase of Wound Healing. Front. Genet. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.K.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing an overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ge, X.; Xiang, Y.; Qi, X.; Li, Y.; Xu, H.; Cai, E.; Zhang, C.; Lan, Y.; Chen, X.; et al. An ionic liquid functionalized sericin hydrogel for drug-resistant bacteria-infected diabetic wound healing. Chin. Chem. Lett. 2024, 109819. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. 2), 5–15. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Mirhaj, M.; Labbaf, S.; Tavakoli, M.; Seifalian, A.M. Emerging treatment strategies in wound care. Int. Wound J. 2022, 19, 1934–1954. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, K.M.; Ilyas, S.; Tamrin, T.; Radecka, I.; Swingler, S.; Gupta, A.; Stamboulis, A.G.; Gea, S. Bioactive bacterial cellulose wound dressings for burns with collagen in-situ and chitosan ex-situ impregnation. Int. J. Biol. Macromol. 2023, 230, 123118. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gao, Y.; Li, T.; Xiao, S.; Adeli, M.; Rodriguez, R.D.; Geng, W.; Chen, Q.; Cheng, C.; Zhao, C. Amorphizing Metal Selenides-Based ROS Biocatalysts at Surface Nanolayer toward Ultrafast Inflammatory Diabetic Wound Healing. ACS Nano 2023, 17, 2943–2957. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; He, S.; Ye, K.; Shao, X.; Yang, Q.; Yang, G. Photopolymerizable, immunomodulatory hydrogels of gelatin methacryloyl and carboxymethyl chitosan as all-in-one strategic dressing for wound healing. Int. J. Biol. Macromol. 2023, 253, 127151. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Eberlein, T.; Gerke, P.; Lorenz, H.; Ammer, R. Advantages in wound healing by a topical easy to use wound healing lipo-gel for abrasive wounds—Evidence from a randomized, controlled experimental clinical study. Wound Med. 2016, 15, 11–19. [Google Scholar] [CrossRef]
- Ramazan, E. 18—Advances in fabric structures for wound care. In Advanced Textiles for Wound Care, 2nd ed.; Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2019; pp. 509–540. [Google Scholar] [CrossRef]
- Kus, K.J.; Ruiz, E.S. Wound dressings—A practical review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Lakhani, A.; Jamel, W.; Riddiough, G.E.; Cabalag, C.S.; Stevens, S.; Liu, D.S. Prophylactic negative pressure wound dressings reduces wound complications following emergency laparotomies: A systematic review and meta-analysis. Surgery 2022, 172, 949–954. [Google Scholar] [CrossRef]
- Natarajan, S.; Williamson, D.; Stiltz, A.J.; Harding, K. Advances in wound care and healing technology. Am. J. Clin. Dermatol. 2000, 1, 269–275. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 1443–1444. [Google Scholar] [CrossRef]
- Vincent, J.F.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, O.H. Some interesting and useful biomimetic transforms. In Proceedings of the Third International Biophysics Congress, Boston, MA, USA, 29 August–3 September 1969; p. 197. [Google Scholar]
- Hesselberg, T. Biomimetics and the case of the remarkable ragworms. Naturwissenschaften 2007, 94, 613–621. [Google Scholar] [CrossRef]
- Gabler-Smith, M.-R.K.; Lauder, G.V. Ridges and riblets: Shark skin surfaces versus biomimetic models. Front. Mar. Sci. 2022, 9, 975062. [Google Scholar] [CrossRef]
- Dean, B.; Bhushan, B. Shark-skin surfaces for fluid-drag reduction in turbulent flow: A review. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4775–4806. [Google Scholar] [PubMed]
- Chen, J.; Fan, Y.; Dong, G.; Zhou, H.; Du, R.; Tang, X.; Ying, Y.; Li, J. Designing biomimetic scaffolds for skin tissue engineering. Biomater. Sci. 2023, 11, 3051–3076. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhou, Q.; Ma, X.; Gao, B. Review of biomimetic ordered microstructures in advancing synergistic integration of adhesion and microfluidics. RSC Adv. 2024, 14, 11643–11658. [Google Scholar] [CrossRef]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar]
- Hammock, M.L.; Chortos, A.; Tee, B.C.; Tok, J.B.; Bao, Z. 25th anniversary article: The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human–Machine Interfaces. Adv. Mater. 2022, 35, e2203193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, X.; Yang, Y.; Zhou, J.; Yao, K.; Li, J.; Zhou, Y.; Li, M.; Wong, T.H.; Yu, X. Wearable and battery-free wound dressing system for wireless and early sepsis diagnosis. Bioeng. Transl. Med. 2023, 8, e10445. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.J.; Lai, Q.T.; Tang, Z.; Tang, X.G.; Zhao, X.H.; Roy, V.A.L. Advanced Functional Composite Materials toward E-Skin for Health Monitoring and Artificial Intelligence. Adv. Mater. Technol. 2022, 8, 2201088. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, e1904765. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kashiwagi, T.; Arie, T.; Akita, S.; Takei, K. Human-Like Electronic Skin-Integrated Soft Robotic Hand. Adv. Intell. Syst. 2019, 1, 1900018. [Google Scholar] [CrossRef]
- Li, J.-A.; Ma, Z.; Wang, H.-T.; Gao, X.-X.; Zhou, Z.; Tao, R.-W.; Pan, L.-J.; Shi, Y. Skin-Inspired Electronics and Its Applications in Advanced Intelligent Systems. Adv. Intell. Syst. 2019, 1, 1900063. [Google Scholar] [CrossRef]
- Arcangeli, D.; Gualandi, I.; Mariani, F.; Tessarolo, M.; Ceccardi, F.; Decataldo, F.; Melandri, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real-Time Monitoring in Wound Exudate. ACS Sens. 2023, 8, 1593–1608. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef]
- Garland, N.T.; Song, J.W.; Ma, T.; Kim, Y.J.; Vázquez-Guardado, A.; Hashkavayi, A.B.; Ganeshan, S.K.; Sharma, N.; Ryu, H.; Lee, M.-R.K.; et al. A Miniaturized, Battery-Free, Wireless Wound Monitor That Predicts Wound Closure Rate Early. Adv. Health Mater. 2023, 12, e2301280. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, J.; Wu, X.; Zeng, M.; Tian, Y.; Wu, K.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Flexible and temperature-responsive hydrogel dressing for real-time and remote wound healing monitoring. J. Mater. Chem. B 2023, 11, 4934–4945. [Google Scholar] [CrossRef]
- McLean, K.A.; Sgro, A.; Brown, L.R.; Buijs, L.F.; Daines, L.; Potter, M.A.; Bouamrane, M.M.; Harrison, E.M. Evaluation of remote digital postoperative wound monitoring in routine surgical practice. NPJ Digit. Med. 2023, 6, 85. [Google Scholar] [CrossRef]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sens. Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.-Y.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Ding, B.; Yu, J.; Sun, G.; Wang, M. Biomimicry via Electrospinning. Crit. Rev. Solid. State Mater. Sci. 2012, 37, 94–114. [Google Scholar] [CrossRef]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Polym. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Thakar, C.M.; Parkhe, S.S.; Jain, A.; Phasinam, K.; Murugesan, G.; Ventayen, R.J.M. 3d Printing: Basic principles and applications. Mater. Today Proc. 2022, 51, 842–849. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef]
- Wang, C.; Park, M.J.; Yu, H.; Matsuyama, H.; Drioli, E.; Shon, H.K. Recent advances of nanocomposite membranes using layer-by-layer assembly. J. Membr. Sci. 2022, 661, 120926. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-layer assembly methods and their biomedical applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef]
- Jang, H.J.; Tiruneh, D.M.; Ryu, H.; Yoon, J.K. Piezoelectric and Triboelectric Nanogenerators for Enhanced Wound Healing. Biomimetics 2023, 8, 517. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.M.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral. Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Biomedical Applications of Biodegradable Polymers in Wound Care. In Wound Healing Research; Springer: Singapore, 2021; pp. 509–597. [Google Scholar] [CrossRef]
- Shafiee, A.; Cavalcanti, A.S.; Saidy, N.T.; Schneidereit, D.; Friedrich, O.; Ravichandran, A.; De-Juan-Pardo, E.M.; Hutmacher, D.W. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials 2021, 268, 120558. [Google Scholar] [CrossRef] [PubMed]
- Mahjour, S.B.; Fu, X.; Yang, X.; Fong, J.; Sefat, F.; Wang, H. Rapid creation of skin substitutes from human skin cells and biomimetic nanofibers for acute full-thickness wound repair. Burns 2015, 41, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, G.; Mukherjee, S.; Sun, Y.; Wang, C.; Mai, B.; Liu, K.; Yang, C.; Chen, Y. Elastic, Persistently Moisture-Retentive, and Wearable Biomimetic Film Inspired by Fetal Scarless Repair for Promoting Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 5542–5556. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lang, Y.; Li, C.; Liu, S.; Chang, M.-W. Biomimetic 3D composite scaffold with pH-Responsive micropatterns for wound healing. Chem. Eng. J. 2024, 485, 149646. [Google Scholar] [CrossRef]
- Li, S.; Renick, P.; Senkowsky, J.; Nair, A.; Tang, L. Diagnostics for Wound Infections. Adv Wound Care (New Rochelle) 2021, 10, 317–327. [Google Scholar] [CrossRef]
- McGrath, M.; Zimkowska, K.; Genoud, K.J.; Maughan, J.; Gutierrez Gonzalez, J.; Browne, S.; O’Brien, F.J. A Biomimetic, Bilayered Antimicrobial Collagen-Based Scaffold for Enhanced Healing of Complex Wound Conditions. ACS Appl. Mater. Interfaces 2023, 15, 17444–17458. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ge, J.; Guo, Y.; Lei, B.; Ma, P.X. Biomimetic Elastomeric Polypeptide-Based Nanofibrous Matrix for Overcoming Multidrug-Resistant Bacteria and Enhancing Full-Thickness Wound Healing/Skin Regeneration. ACS Nano 2018, 12, 10772–10784. [Google Scholar] [CrossRef]
- Gao, W.; Jin, W.; Li, Y.; Wan, L.; Wang, C.; Lin, C.; Chen, X.; Lei, B.; Mao, C. A highly bioactive bone extracellular matrix-biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J. Mater. Chem. B 2017, 5, 7285–7296. [Google Scholar] [CrossRef] [PubMed]
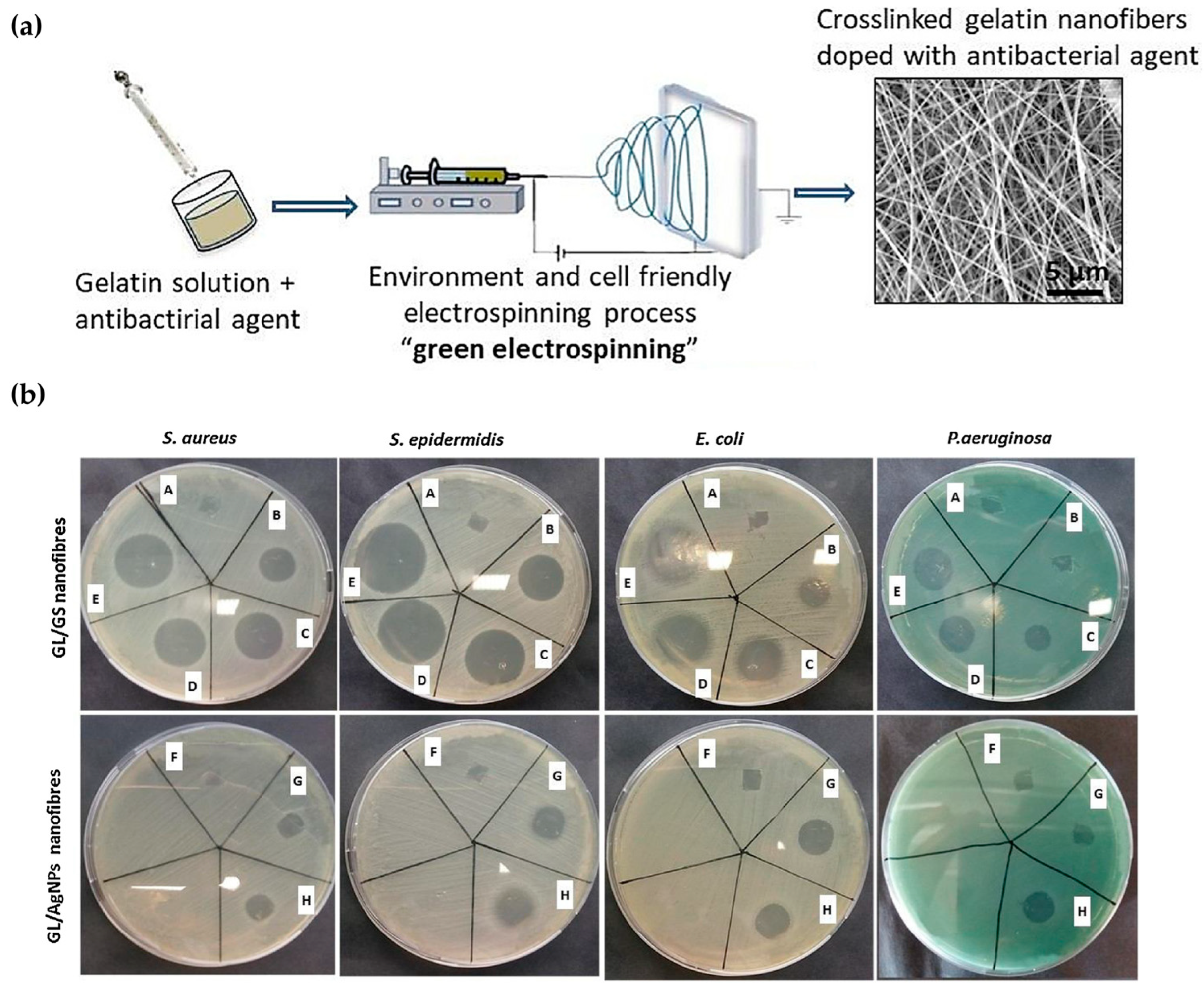
- Tonda-Turo, C.; Ruini, F.; Ceresa, C.; Gentile, P.; Varela, P.; Ferreira, A.M.; Fracchia, L.; Ciardelli, G. Nanostructured scaffold with biomimetic and antibacterial properties for wound healing produced by ‘green electrospinning’. Colloids Surf. B Biointerfaces 2018, 172, 233–243. [Google Scholar] [CrossRef]
- Kong, X.; Fu, J.; Shao, K.; Wang, L.; Lan, X.; Shi, J. Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 2019, 100, 255–269. [Google Scholar] [CrossRef]
- Diller, R.B.; Kellar, R.S. An acellular tissue engineered biomimetic wound healing device created using collagen and tropoelastin accelerates wound healing. J. Tissue Viability 2022, 31, 485–490. [Google Scholar] [CrossRef]
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef]
- Eslahi, N.; Soleimani, F.; Lotfi, R.; Mohandes, F.; Simchi, A.; Razavi, M. How biomimetic nanofibers advance the realm of cutaneous wound management: The state-of-the-art and future prospects. Prog. Mater. Sci. 2024, 145, 101293. [Google Scholar] [CrossRef]
- Gao, Z.; Shi, Q.; Fukuda, T.; Li, C.; Huang, Q. An overview of biomimetic robots with animal behaviors. Neurocomputing 2019, 332, 339–350. [Google Scholar] [CrossRef]
- Baban, N.S.; Orozaliev, A.; Kirchhof, S.; Stubbs, C.J.; Song, Y.A. Biomimetic fracture model of lizard tail autotomy. Science 2022, 375, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, K.K.; Watson, J.A.; Kim, H.Y.; Yoon, K.S.; Kim, E.J.; Lee, J.M.; Watson, G.S.; Jung, H.S. High Quality Bioreplication of Intricate Nanostructures from a Fragile Gecko Skin Surface with Bactericidal Properties. Sci. Rep. 2017, 7, 41023. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kang, X.; Gao, X.; Zhuang, Y.; Fan, C.; Shen, H.; Chen, Y.; Dai, J. Biomimetic Natural Biopolymer-Based Wet-Tissue Adhesive for Tough Adhesion, Seamless Sealed, Emergency/Nonpressing Hemostasis, and Promoted Wound Healing. Adv. Funct. Mater. 2023, 33, 2211340. [Google Scholar] [CrossRef]
- Gao, Y.; Nguyen, D.T.; Yeo, T.; Lim, S.B.; Tan, W.X.; Madden, L.E.; Jin, L.; Long, J.Y.K.; Aloweni, F.A.B.; Liew, Y.J.A.; et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 2021, 7, eabg9614. [Google Scholar] [CrossRef] [PubMed]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Klode, J.; Schottler, L.; Stoffels, I.; Korber, A.; Schadendorf, D.; Dissemond, J. Investigation of adhesion of modern wound dressings: A comparative analysis of 56 different wound dressings. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 933–939. [Google Scholar] [CrossRef]
- Xu, M.; Miao, Y.; Yu, J.; Zhang, L. Physiologically-Regulated Adhesion of Hydrogels for Wound Dressing. Adv. Mater. Interfaces 2021, 8, 2101131. [Google Scholar] [CrossRef]
- Peng, X.; Xia, X.; Xu, X.; Yang, X.; Yang, B.; Zhao, P.; Yuan, W.; Chiu, P.W.Y.; Bian, L. Ultrafast self-gelling powder mediates robust wet adhesion to promote healing of gastrointestinal perforations. Sci. Adv. 2021, 7, eabe8739. [Google Scholar] [CrossRef] [PubMed]
- Amaral, K.R.; Silva, A.S.; Santos, L.F.; Castanheira, E.J.; Mendes, M.C.; Costa, D.C.S.; Rodrigues, J.M.M.; Marto, J.; Mano, J.F. Biomimetic Adhesive Micropatterned Hydrogel Patches for Drug Release. Adv. Healthc. Mater. 2023, 12, e2301513. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Progress. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nanomicro Lett. 2017, 9, 23. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yu, B.; Lv, Y.; Huang, Y.; Guo, J.; Li, L.; Chen, M.; Zheng, Y.; Liu, M.; Guo, S.; et al. Biomimetic Asymmetric Composite Dressing by Electrospinning with Aligned Nanofibrous and Micropatterned Structures for Severe Burn Wound Healing. ACS Appl. Mater. Interfaces 2022, 12, 32799–32812. [Google Scholar] [CrossRef]
- Tian, M.; Shuai, J.; Bishop, B.A.; Zhang, W.; Chen, J.; Wang, X. Plant cellulose-based biomimetic artificial Small-Diameter vascular materials enabled by gradient Dual-Network entanglement. Chem. Eng. J. 2023, 476, 146751. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Song, R.; Fang, C.; Wang, Z.; Wang, C.; Huang, Y. Freestanding silver/polypyrrole composite film for multifunctional sensor with biomimetic micropattern for physiological signals monitoring. Chem. Eng. J. 2021, 404, 126940. [Google Scholar] [CrossRef]
- Becker, J.; Speck, O.; Göppert, T.; Speck, T.; Müller, C. Learning from Self-Sealing Deformations of Plant Leaves: The Biomimetic Multilayer Actuator. Adv. Intell. Syst. 2022, 4, 2200215. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Ki, M.R.; Abd El-Hafeez, A.A.; Son, R.G.; Pack, S.P. Tailored Functionalized Protein Nanocarriers for Cancer Therapy: Recent Developments and Prospects. Pharmaceutics 2023, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C. Nanomedicine 2.0. Acc. Chem. Res. 2017, 50, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Gu, Z. Bioinspired and Biomimetic Nanomedicines. Acc. Chem. Res. 2019, 52, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.S.; Bose, R.J.; McCarthy, J.R.; Mat Azmi, I.D.; Madheswaran, T. Biomimetic bacterial and viral-based nanovesicles for drug delivery, theranostics, and vaccine applications. Drug Discov. Today 2021, 26, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Mougenot, M.F.; Pereira, V.S.; Costa, A.L.R.; Lancellotti, M.; Porcionatto, M.A.; da Silveira, J.C.; de la Torre, L.G. Biomimetic Nanovesicles-Sources, Design, Production Methods, and Applications. Pharmaceutics 2022, 14, 2008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Guo, J.; Yan, C.; He, Y.; Chen, J.; Zhang, M.; Xiang, K.; Xiang, X.; Zhang, C.; Wang, Y.; et al. Biomimetic hybrid nanovesicles improve infected diabetic wound via enhanced targeted delivery. J. Control Release 2024, 365, 193–207. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, M.; Wang, X.; Wu, F.; Yao, C.; Shen, J.; Zhou, N.; Sun, B. An Immunomodulatory Biomimetic Single-Atomic Nanozyme for Biofilm Wound Healing Management. Small 2023, 19, e2302587. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Jin, H.; Gao, H.; Liu, S.; Shi, W.; Sun, W.; Liu, Y.; Zhang, H. Biomimetic dual-nanozymes with catalytic cascade reactions against diabetic wound infection. J. Colloid. Interface Sci. 2023, 651, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, e1805368. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Son, R.G.; Park, K.S.; Pack, S.P. Oriented multivalent silaffin-affinity immobilization of recombinant lipase on diatom surface: Reliable loading and high performance of biocatalyst. Colloids Surf. B Biointerfaces 2022, 219, 112830. [Google Scholar] [CrossRef]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured biosilica of diatoms: From water world to biomedical applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple Routes to Smart Nanostructured Materials from Diatom Microalgae: A Chemical Perspective. Adv. Mater. 2018, 30, e1704289. [Google Scholar] [CrossRef]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Ghobara, M.; El-Sheekh, M.; Hamed, A.F.; Abdelhamid, M.A.; Pack, S.P. Diatom Nanostructured Biosilica. In Value-Added Products from Algae: Phycochemical Production and Applications; Springer: Cham, Switzerland, 2023; pp. 461–492. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic Diatom Biosilica and Its Potential for Biomedical Applications and Prospects: A Review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Pan, G.; Li, F.; He, S.; Li, W.; Wu, Q.; He, J.; Ruan, R.; Xiao, Z.; Zhang, J.; Yang, H. Mussel-and barnacle cement proteins-inspired dual-bionic bioadhesive with repeatable wet-tissue adhesion, multimodal self-healing, and antibacterial capability for nonpressing hemostasis and promoted wound healing. Adv. Funct. Mater. 2022, 32, 2200908. [Google Scholar] [CrossRef]
- Sun, X.; Li, N.; Su, C.; Mu, Y.; Cong, X.; Cao, Z.; Wang, X.; Yang, X.; Chen, X.; Feng, C. Diatom-Inspired Bionic Hydrophilic Polysaccharide Adhesive for Rapid Sealing Hemostasis. ACS Nano 2023, 17, 19121–19135. [Google Scholar] [CrossRef]
- Ki, M.-R.; Park, K.S.; Abdelhamid, M.A.A.; Pack, S.P. Novel silicatein-like protein for biosilica production from Amphimedon queenslandica and its use in osteogenic composite fabrication. Korean J. Chem. Eng. 2023, 40, 419–428. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.A.; Shin, M.; Juang, L.J.; Kastrup, C.J.; Go, G.M.; Lee, H. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. ACS Nano 2020, 14, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fang, Y.; Liang, X.; Huang, C.; Liang, Y.; Yang, Z.; Yu, J.; Wang, J.; Zhao, G. Yeast cell templated porous hollow silica spheres for rapid hemostasis accompanied by antibacterial action. Biomater. Sci. 2023, 11, 3104–3113. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, S.J.; Wu, Z.; Ong, B.C.; Tan, K.W.; Dong, Z.; Tay, C.Y. Diatom-inspired 2D nitric oxide releasing anti-infective porous nanofrustules. J. Mater. Chem. B 2021, 9, 7229–7237. [Google Scholar] [CrossRef]
- Luo, H.; Shen, Y.; Liao, Z.; Yang, X.; Gao, B.; He, B. Spidroin Composite Biomimetic Multifunctional Skin with Meta-Structure. Adv. Mater. Technol. 2022, 7, 2101097. [Google Scholar] [CrossRef]
- Waseem, A.; Abdullah, A.; Bagal, I.V.; Ha, J.-S.; Lee, J.K.; Ryu, S.-W. Self-powered and flexible piezo-sensors based on conductivity-controlled GaN nanowire-arrays for mimicking rapid- and slow-adapting mechanoreceptors. NPJ Flex. Electron. 2022, 6, 58. [Google Scholar] [CrossRef]
- Corniani, G.; Saal, H.P. Tactile innervation densities across the whole body. J. Neurophysiol. 2020, 124, 1229–1240. [Google Scholar] [CrossRef]
- Johansson, R.S.; Vallbo, A.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300. [Google Scholar] [CrossRef]
- Kobayashi, S. Temperature receptors in cutaneous nerve endings are thermostat molecules that induce thermoregulatory behaviors against thermal load. Temperature 2015, 2, 346–351. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Yu, Z.; Wu, T.; Liang, L.; Liu, S. Recent progress in stretchable organic field-effect transistors: Key materials, fabrication and applications. New J. Chem. 2023, 47, 5086–5109. [Google Scholar] [CrossRef]
- Sanderson, K. Electronic skin: From flexibility to a sense of touch. Nature 2021, 591, 685–687. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Tee, B.C.; Bettinger, C.J.; Tok, J.B.; Bao, Z. Chemical and engineering approaches to enable organic field-effect transistors for electronic skin applications. Acc. Chem. Res. 2012, 45, 361–371. [Google Scholar] [CrossRef]
- Mudhulu, S.; Channegowda, M.; Balaji, S.; Khosla, A.; Sekhar, P. Trends in Graphene-Based E-Skin and Artificial Intelligence for Biomedical Applications—A Review. IEEE Sens. J. 2023, 23, 18963–18976. [Google Scholar] [CrossRef]
- Wong, T.H.; Yiu, C.K.; Zhou, J.; Song, Z.; Liu, Y.; Zhao, L.; Yao, K.; Park, W.; Yoo, W.; Song, E.; et al. Tattoo-like epidermal electronics as skin sensors for human machine interfaces. Soft Sci. 2021, 1, 10. [Google Scholar] [CrossRef]
- Yu, Y.; Nassar, J.; Xu, C.; Min, J.; Yang, Y.; Dai, A.; Doshi, R.; Huang, A.; Song, Y.; Gehlhar, R.; et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 2020, 5, eaaz7946. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, F.; Yuan, M.; Xie, D.; Shen, L.; Zheng, K.; Wang, Z.; Li, X.; Tao, L.Q. A Dual-Functional Graphene-Based Self-Alarm Health-Monitoring E-Skin. Adv. Funct. Mater. 2019, 29, 1904706. [Google Scholar] [CrossRef]
- Oh, H.; Lee, C.; Kim, N.; An, T.; Kim, G. Review: Sensors for Biosignal/Health Monitoring in Electronic Skin. Polymers 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.A.; Soliman, M.; Saleh, M.A.; Radwan, A.G. Biohybrid Soft Robots, E-Skin, and Bioimpedance Potential to Build Up Their Applications: A Review. IEEE Access 2020, 8, 184524–184539. [Google Scholar] [CrossRef]
- Byun, J.; Lee, Y.; Yoon, J.; Lee, B.; Oh, E.; Chung, S.; Lee, T.; Cho, K.-J.; Kim, J.; Hong, Y. Electronic skins for soft, compact, reversible assembly of wirelessly activated fully soft robots. Sci. Robot. 2018, 3, eaas9020. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.-C.; Dediu, V.; Laszlo, E.A.; Pistriţu, F.; Carp, M.; Iliescu, F.S.; Ionescu, O.N.; Iliescu, C. E-Skin: The Dawn of a New Era of On-Body Monitoring Systems. Micromachines 2021, 12, 1091. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, X.; Jian, J.; Wu, Q.; Wei, Y.; Shuai, H.; Hirtz, T.; Zhi, Y.; Deng, G.; Wang, Y.; et al. Substrate-Free Multilayer Graphene Electronic Skin for Intelligent Diagnosis. ACS Appl. Mater. Interfaces 2020, 12, 49945–49956. [Google Scholar] [CrossRef]
- Navaraj, W.; Smith, C.; Dahiya, R. Chapter 5—E-skin and wearable systems for health care. In Materials Today, Wearable Bioelectronics; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 133–178. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Choe, A.; Cho, S.; Kim, J.; Ko, H. Mimicking Human and Biological Skins for Multifunctional Skin Electronics. Adv. Funct. Mater. 2020, 30, 1904523. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Q.; Lee, C. Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials 2022, 12, 1366. [Google Scholar] [CrossRef]
- Heng, W.; Solomon, S.; Gao, W. Flexible Electronics and Devices as Human-Machine Interfaces for Medical Robotics. Adv. Mater. 2022, 34, e2107902. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Smart Bandage with Wireless Strain and Temperature Sensors and Batteryless NFC Tag. IEEE Internet Things J. 2021, 8, 5093–5100. [Google Scholar] [CrossRef]
- Liu, J.; Tian, G.; Yang, W.; Deng, W. Recent progress in flexible piezoelectric devices toward human-machine interactions. Soft Sci. 2022, 2, 22. [Google Scholar]
- Huang, X.; Liang, B.; Zheng, S.; Wu, F.; He, M.; Huang, S.; Yang, J.; Ouyang, Q.; Liu, F.; Liu, J.; et al. Microarrow sensor array with enhanced skin adhesion for transdermal continuous monitoring of glucose and reactive oxygen species. Bio-Des. Manuf. 2023, 7, 14–30. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Zheng, Y.; Zhao, S.; Wei, W.; Zhang, D.; Fu, X.; Jiang, K.; Shen, G.; Han, W. Highly-stable polymer-crosslinked 2D MXene-based flexible biocompatible electronic skins for in vivo biomonitoring. Nano Energy 2021, 84, 105921. [Google Scholar] [CrossRef]
- Liu, X.; Cui, B.; Wang, X.; Zheng, M.; Bai, Z.; Yue, O.; Fei, Y.; Jiang, H. Nature-Skin-Derived e-Skin as Versatile “Wound Therapy-Health Monitoring” Bioelectronic Skin-Scaffolds: Skin to Bio-e-Skin. Adv. Healthc. Mater. 2023, 12, e2202971. [Google Scholar] [CrossRef]
- Mirani, B.; Hadisi, Z.; Pagan, E.; Dabiri, S.M.H.; van Rijt, A.; Almutairi, L.; Noshadi, I.; Armstrong, D.G.; Akbari, M. Smart Dual-Sensor Wound Dressing for Monitoring Cutaneous Wounds. Adv. Healthc. Mater. 2023, 12, e2203233. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Zong, Y.; Zong, B.; Zha, R.; Zhang, Y.; Li, X.; Wang, Y.; Fang, H.; Wong, W.L.; Li, C. An Antibacterial and Anti-Oxidative Hydrogel Dressing for Promoting Diabetic Wound Healing and Real-Time Monitoring Wound pH Conditions with a NIR Fluorescent Imaging System. Adv. Healthc. Mater. 2023, 12, e2300431. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors—Enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Lee, J.H.; Kim, E.; Chan, K.Y.; Venkatesan, H.; Adegun, M.H.; Agbabiaka, O.G.; Shen, X.; Zheng, Q.; et al. Bioinspired Chromotropic Ionic Skin with In-Plane Strain/Temperature/Pressure Multimodal Sensing and Ultrahigh Stimuli Discriminability. Adv. Funct. Mater. 2022, 32, 2208362. [Google Scholar] [CrossRef]
- Sharma, N.; Nair, N.M.; Nagasarvari, G.; Ray, D.; Swaminathan, P. A review of silver nanowire-based composites for flexible electronic applications. Flex. Print. Electron. 2022, 7, 014009. [Google Scholar] [CrossRef]
- Zhang, H.; He, R.; Niu, Y.; Han, F.; Li, J.; Zhang, X.; Xu, F. Graphene-enabled wearable sensors for healthcare monitoring. Biosens. Bioelectron. 2022, 197, 113777. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Wang, B.; Facchetti, A. Mechanically Flexible Conductors for Stretchable and Wearable E-Skin and E-Textile Devices. Adv. Mater. 2019, 31, e1901408. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, R.; Ling, S.; Zhou, T.; Wu, Z.; Fu, M.; He, H.; Li, X.; Zhang, C. Self-Healable, Self-Adhesive and Degradable MXene-Based Multifunctional Hydrogel for Flexible Epidermal Sensors. ACS Appl. Mater. Interfaces 2024, 16, 7826–7837. [Google Scholar] [CrossRef]
- Wang, L.; Li, N.; Zhang, Y.; Di, P.; Li, M.; Lu, M.; Liu, K.; Li, Z.; Ren, J.; Zhang, L.; et al. Flexible multiresponse-actuated nacre-like MXene nanocomposite for wearable human-machine interfacing. Matter 2022, 5, 3417–3431. [Google Scholar] [CrossRef]
- Wu, C.S.; Wang, A.C.; Ding, W.B.; Guo, H.Y.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Kao, F.C.; Ho, H.H.; Chiu, P.Y.; Hsieh, M.-R.K.; Liao, J.C.; Lai, P.L.; Huang, Y.F.; Dong, M.Y.; Tsai, T.T.; Lin, Z.H. Self-assisted wound healing using piezoelectric and triboelectric nanogenerators. Sci. Technol. Adv. Mater. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Buyong, M.R.; Mohd Yunus, M.H. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13, 3790. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Liu, D.; Bi, S.; Wang, H.; Gu, J.; Wang, S. Polydopamine interface-modulated MXene-based conductive antibacterial hydrogels for on-skin health monitoring and diabetic wound healing. Compos. Part. A Appl. Sci. Manuf. 2024, 180, 108065. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Zhai, S.; Cheng, R.; Liu, D.; Gao, X.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Liu, Y.; Ji, K.; Li, K.; Wang, J.; Gu, Z. Biomimetic design strategies for biomedical applications. Matter 2024, 7, 826–854. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, S.; Li, B.; Yu, B.; Lee, H.; Cai, M.; Gorb, S.N.; Zhou, F.; Liu, W. Gecko’s Feet-Inspired Self-Peeling Switchable Dry/Wet Adhesive. Chem. Mater. 2021, 33, 2785–2795. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Zhao, M.; Ma, Y.; Zheng, T.; Shi, L. Stretchable, self-healing and adhesive sodium alginate-based composite hydrogels as wearable strain sensors for expansion–contraction motion monitoring. Soft Matter 2022, 18, 1644–1652. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Li, M.; Yuan, Y.; Wang, W.; Zhang, L.; Wan, P. Mussel-inspired self-healing adhesive MXene hydrogel for epidermal electronics. Device 2024, 2, 100253. [Google Scholar] [CrossRef]
- Kang, B.; Yan, X.; Zhao, Z.; Song, S. Dual-Sensing, Stretchable, Fatigue-Resistant, Adhesive, and Conductive Hydrogels Used as Flexible Sensors for Human Motion Monitoring. Langmuir 2022, 38, 7013–7023. [Google Scholar] [CrossRef]
- Wang, C.; Hwang, D.; Yu, Z.; Takei, K.; Park, J.; Chen, T.; Ma, B.; Javey, A. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Z.; Ma, D.; Qi, C.; Yang, D.; Huang, S. Smart colloidal photonic crystal sensors. Adv. Colloid. Interface Sci. 2024, 324, 103089. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’ Reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2015, 8, 14966–14974. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Gebeyehu, E.K.; Beyene, K.A.; Tadesse, M.G.; Liyew, E.Z.; Abdul Khalil, H.P.S. Self-Responsive Electrospun Nanofibers Wound Dressings: The Future of Wound Care. Adv. Mater. Sci. Eng. 2022, 2022, 2025170. [Google Scholar] [CrossRef]
- Ha, J.H.; Kim, J.Y.; Kim, D.; Ahn, J.; Jeong, Y.; Ko, J.; Hwang, S.; Jeon, S.; Jung, Y.; Gu, J.; et al. Multifunctional Micro/Nanofiber Based-Dressing Patch with Healing, Protection, and Monitoring Capabilities for Advanced Wound Care. Adv. Mater. Technol. 2023, 8, 2201765. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Xu, Z.; Lu, L.; Pan, Z.; Mao, Y. Electrospun Nanofiber-Based Bioinspired Artificial Skins for Healthcare Monitoring and Human-Machine Interaction. Biomimetics 2023, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Patil, T.V.; Dutta, S.D.; Lee, J.; Ganguly, K.; Randhawa, A.; Kim, H.; Lim, K.T. Extracellular Matrix-Bioinspired Anisotropic Topographical Cues of Electrospun Nanofibers: A Strategy of Wound Healing through Macrophage Polarization. Adv. Healthc. Mater. 2024, 13, e2304114. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A multimodal antimicrobial platform based on MXene for treatment of wound infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Chan, L.W.; Fleming, H.E.; Bhatia, S.N. Conditional Antimicrobial Peptide Therapeutics. ACS Nano 2022, 16, 15779–15791. [Google Scholar] [CrossRef]
- Wang, L.; Su, Q.; Liu, Y.; Yimamumaimaiti, T.; Hu, D.; Zhu, J.J.; Zhang, J.R. A self-powered and drug-free diabetic wound healing patch breaking hyperglycemia and low H2O2 limitations and precisely sterilizing driven by electricity. Chem. Sci. 2022, 13, 12136–12143. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, T.; Chen, Y.; Xiao, Y.; Wen, W.; Wang, S.; Wang, D.; Zhang, X.; Liang, J.; Xiong, H. Reactive Oxygen-Correlated Photothermal Imaging of Smart COF Nanoreactors for Monitoring Chemodynamic Sterilization and Promoting Wound Healing. Small 2024, e2310247. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Xu, C.; Wang, C.; Song, Y.; Min, J.; Tu, J.; Solomon, S.A.; Li, J.; Banks, J.L.; Armstrong, D.G.; et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 2023, 9, eadf7388. [Google Scholar] [CrossRef]
- Xu, L.; Ding, L.; Sun, Y.; Zhang, T.; Zhu, Y.; Yan, B.; Yang, M.; Ramakrishna, S.; Zhang, J.; Long, Y.Z. Stretchable, flexible and breathable polylactic acid/polyvinyl pyrrolidone bandage based on Kirigami for wounds monitoring and treatment. Int. J. Biol. Macromol. 2023, 237, 124204. [Google Scholar] [CrossRef]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef]
- Karperien, L. pH Sensitive Thread-Based Wound Dressing with Integrated Drug Delivery and Wireless Bluetooth Interface. Master’s Dissertation, Department of Mechanical Engineering, University of Victoria, Victoria, BC, Canada, 2019. Available online:http://hdl.handle.net/1828/11315 (accessed on 20 March 2024).
- Li, Y.; Qu, X.; Wang, Q.; Li, S.; Zhang, Q.; Zhang, X. Tannic acid and carboxymethyl chitosan-based multi-functional double-layered hydrogel with pH-stimulated response behavior for smart real-time infection monitoring and wound treatment. Int. J. Biol. Macromol. 2024, 261, 129042. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, R.; Han, F.; Zheng, Z.; Liu, Y.; Zhou, X.; Li, Q.; Zhai, X.; Wu, J.; Pan, X.; et al. A soft intelligent dressing with pH and temperature sensors for early detection of wound infection. RSC Adv. 2022, 12, 3243–3252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, X.; Zhang, J.; Yue, O.; Zhang, J.; Bai, Z.; Jiang, H.; Wu, J.; Wen, L.; Liu, X. Nature-skin-derived multi-responsive e-skin as on-demand drug-delivery system facilitated melanoma postoperative therapy. J. Mater. Sci. Technol. 2024, 188, 155–168. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, D.; Ge, C.; Gao, C.; Wei, Y.; Chen, Z.; Su, Z.; Liu, K.; Xu, W.; Fang, J. Bifunctional Smart Textiles with Simultaneous Motion Monitoring and Thermotherapy for Human Joint Injuries. Adv. Sci. 2024, 11, e2305312. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ding, Q.; Qi, M.; Zhang, W.; Hou, Y.; Cao, R.; Li, C.; Xu, L.; Wang, L.; Kim, J.S. Integrated Bilayer Microneedle Dressing and Triboelectric Nanogenerator for Intelligent Management of Infected Wounds. Adv. Funct. Mater. 2024, 2316820. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, W.; Tao, Y.; Sun, H.; Meng, X.; Cao, L.; Zhang, S.; Liu, W.; Akhtar, M.L.; Li, Y.; et al. Wireless and Closed-Loop Smart Dressing for Exudate Management and On-Demand Treatment of Chronic Wounds. Adv. Mater. 2023, 35, e2304005. [Google Scholar] [CrossRef]
- Kang, K.; Ye, S.; Jeong, C.; Jeong, J.; Ye, Y.S.; Jeong, J.Y.; Kim, Y.J.; Lim, S.; Kim, T.H.; Kim, K.Y.; et al. Bionic artificial skin with a fully implantable wireless tactile sensory system for wound healing and restoring skin tactile function. Nat. Commun. 2024, 15, 10. [Google Scholar] [CrossRef]
- Duan, S.; Shi, Q.; Hong, J.; Zhu, D.; Lin, Y.; Li, Y.; Lei, W.; Lee, C.; Wu, J. Water-Modulated Biomimetic Hyper-Attribute-Gel Electronic Skin for Robotics and Skin-Attachable Wearables. ACS Nano 2023, 17, 1355–1371. [Google Scholar] [CrossRef]
- Yuan, R.; Yang, N.; Li, W.; Liu, Z.; Feng, F.; Zhang, Q.; Ge, L. LBL Noninvasively Peelable Biointerfacial Adhesives for Cutaneo-Inspired pH/Tactility Artificial Receptors. Adv. Healthc. Mater. 2023, 12, e2202296. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xu, N.; Wu, X.; Liu, M.; Liu, Y.; Zhao, J.; Zhang, T.; Zhao, J.; Zhou, Y.; Xie, Q.; et al. Enhanced heterogeneous interface to construct intelligent conductive hydrogel gas sensor for individualized treatment of infected wounds. Int. J. Biol. Macromol. 2024, 258, 128520. [Google Scholar] [CrossRef]
- Dwivedi, P.; Singh, K.; Chaudhary, K.; Mangal, R. Biomimetic Polymer Adhesives. ACS Appl. Polym. Mater. 2022, 4, 4588–4608. [Google Scholar] [CrossRef]
- Mazzolai, B.; Beccai, L.; Mattoli, V. Plants as model in biomimetics and biorobotics: New perspectives. Front. Bioeng. Biotechnol. 2014, 2, 2. [Google Scholar] [CrossRef]
- He, R.; Wang, K.; Ren, J.; Zhang, W.; Wang, Z.; Deng, K.; Shi, Y.; Luo, Y.; Yuan, Y.; Xu, T. Efficacy of a synthetic biomimetic skin substitute of PLLA/gelatin nanofiber membrane in facilitating chronic cutaneous wound healing. Mater. Technol. 2020, 35, 872–880. [Google Scholar] [CrossRef]
- Chantre, C.O.; Hoerstrup, S.P.; Parker, K.K. Engineering biomimetic and instructive materials for wound healing and regeneration. Curr. Opin. Biomed. Eng. 2019, 10, 97–106. [Google Scholar] [CrossRef]
- Ha, J.F.; Longnecker, N. Doctor-patient communication: A review. Ochsner J. 2010, 10, 38–43. [Google Scholar] [PubMed]
- Sharma, S.; Kumari, B.; Ali, A.; Yadav, R.K.; Sharma, A.K.; Sharma, K.K.; Hajela, K.; Singh, G.K. Mobile technology: A tool for healthcare and a boon in pandemic. J. Fam. Med. Prim. Care 2022, 11, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Lewis, D.; Whittaker, M. mHealth Technologies in Developing Countries: A Feasibility Assessment and a Proposed Framework; University of Queensland: Herston, QLD, Australia, 2013; pp. 1–47. [Google Scholar]
- Jaworek-Korjakowska, J.; Kleczek, P. eSkin: Study on the Smartphone Application for Early Detection of Malignant Melanoma. Wirel. Commun. Mob. Comput. 2018, 2018, 5767360. [Google Scholar] [CrossRef]
- Jahn, A.S.; Navarini, A.A.; Cerminara, S.E.; Kostner, L.; Huber, S.M.; Kunz, M.; Maul, J.T.; Dummer, R.; Sommer, S.; Neuner, A.D.; et al. Over-Detection of Melanoma-Suspect Lesions by a CE-Certified Smartphone App: Performance in Comparison to Dermatologists, 2D and 3D Convolutional Neural Networks in a Prospective Data Set of 1204 Pigmented Skin Lesions Involving Patients’ Perception. Cancers 2022, 14, 3829. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.S.; An, H.G.; Oh, B.H.; Yang, S. Artificial Intelligence in Cutaneous Oncology. Front. Med. 2020, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Ouyang, W.; Jeong, H.; Kim, J.T.; Tzaveils, A.; Mirzazadeh, A.; Wu, C.; Lee, J.Y.; Keller, M.; Mummidisetty, C.K.; et al. Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2026610118. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Orchanian-Cheff, A.; Wu, R. Evaluating the Validity and Utility of Wearable Technology for Continuously Monitoring Patients in a Hospital Setting: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e17411. [Google Scholar] [CrossRef]
- Shao, B.; Lu, M.-H.; Wu, T.-C.; Peng, W.-C.; Ko, T.-Y.; Hsiao, Y.-C.; Chen, J.-Y.; Sun, B.; Liu, R.; Lai, Y.-C. Large-area, untethered, metamorphic, and omnidirectionally stretchable multiplexing self-powered triboelectric skins. Nat. Commun. 2024, 15, 1238. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, Y.; Sun, F.; Xie, Z.; Zhang, M.; Wang, Y.; Liu, D.; Cheng, Z.; Mao, Y.; Zhao, C. Recent Advances in Self-Powered Electronic Skin Based on Triboelectric Nanogenerators. Energies 2024, 17, 638. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, T.; Li, D.; Ji, S.; Chen, Z.; Shao, W.; Liu, H.; Ren, T.L. Breathable Electronic Skins for Daily Physiological Signal Monitoring. Nanomicro Lett. 2022, 14, 161. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, N. Perspectives in translating microfluidic devices from laboratory prototyping into scale-up production. Biomicrofluidics 2022, 16, 021301. [Google Scholar] [CrossRef]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef] [PubMed]
- Muehlematter, U.J.; Daniore, P.; Vokinger, K.N. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): A comparative analysis. Lancet Digit. Health 2021, 3, e195–e203. [Google Scholar] [CrossRef]
- Ravizza, A.; De Maria, C.; Di Pietro, L.; Sternini, F.; Audenino, A.L.; Bignardi, C. Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing. Front. Bioeng. Biotechnol. 2019, 7, 313. [Google Scholar] [CrossRef]
- Peng, Z.; Xue, H.; Liu, X.; Wang, S.; Liu, G.; Jia, X.; Zhu, Z.; Orvy, M.J.; Yang, Y.; Wang, Y.; et al. Tough, adhesive biomimetic hyaluronic acid methacryloyl hydrogels for effective wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1222088. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, H.; Tang, G.; Wang, H.; Shen, J.; Sun, Y.; Wang, S.; Kong, W.; Chai, Y.; Liu, X.; et al. Biomimetic hydrogel derived from decellularized dermal matrix facilitates skin wounds healing. Mater. Today Bio 2023, 21, 100725. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.P.; Sabu, C.; Nivitha, K.P.; Sankar, R.; Ameena Shirin, V.K.; Henna, T.K.; Raphey, V.R.; Gangadharappa, H.V.; Kotta, S.; Pramod, K. Bioinspired and biomimetic micro- and nanostructures in biomedicine. J. Control Release 2022, 343, 724–754. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Zhang, X.; Gu, Z. Bioinspired sequential-responsive supramolecular dendritic systems with programmed tumor targeting for site-specific drug delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1868–1869. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Xu, X.; Liu, G.; Liu, H.; Qiao, Y.; Chen, J.; Cao, S.; Cha, Q.; Wang, T. Self-Healing Materials-Based Electronic Skin: Mechanism, Development and Applications. Gels 2022, 8, 356. [Google Scholar] [CrossRef]
- Shi, C.; Zou, Z.; Lei, Z.; Zhu, P.; Zhang, W.; Xiao, J. Heterogeneous integration of rigid, soft, and liquid materials for self-healable, recyclable, and reconfigurable wearable electronics. Sci. Adv. 2020, 6, eabd0202. [Google Scholar] [CrossRef]
- Cao, H.L.; Cai, S.Q. Recent advances in electronic skins: Material progress and applications. Front. Bioeng. Biotechnol. 2022, 10, 1083579. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.B.; Root, S.E.; Michalek, L.; Wu, S.; Lai, J.C.; Khatib, M.; Oyakhire, S.T.; Zhao, R.; Qin, J.; Bao, Z. Autonomous alignment and healing in multilayer soft electronics using immiscible dynamic polymers. Science 2023, 380, 935–941. [Google Scholar] [CrossRef]
- Lim, K.; Seo, H.; Chung, W.G.; Song, H.; Oh, M.; Ryu, S.Y.; Kim, Y.; Park, J.-U. Material and structural considerations for high-performance electrodes for wearable skin devices. Commun. Mater. 2024, 5, 49. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Wei, Y.; Wang, Y.; Feng, Z.; Cheng, L.; Huo, Z.; Lei, Y.; Sun, Q. Recent Progress in Self-Powered Wireless Sensors and Systems Based on TENG. Sensors 2023, 23, 1329. [Google Scholar] [CrossRef]
- Hang, C.-Z.; Zhao, X.-F.; Xi, S.-Y.; Shang, Y.-H.; Yuan, K.-P.; Yang, F.; Wang, Q.-G.; Wang, J.-C.; Zhang, D.W.; Lu, H.-L. Highly stretchable and self-healing strain sensors for motion detection in wireless human-machine interface. Nano Energy 2020, 76, 105064. [Google Scholar] [CrossRef]
- Xi, Y.; Tan, P.; Li, Z.; Fan, Y. Self-powered wearable IoT sensors as human-machine interfaces. Soft Sci. 2023, 3, 26. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Zhong, D.; Zhang, Z.; Choudhury, S.; Lai, J.C.; Gong, H.; Niu, S.; Yan, X.; Zheng, Y.; et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 2023, 380, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liang, Q.; Zhao, Z.-H.; Wu, Y.; Yang, J.; Han, J.; Cao, Y.; Wang, Y.; Li, C.-H.; Zhong, A.; et al. Self-powered sensors for flexible electronic skins capable of self-healing under multiple extreme environments. Nano Energy 2024, 121, 109239. [Google Scholar] [CrossRef]
- Xu, C.; Solomon, S.A.; Gao, W. Artificial Intelligence-Powered Electronic Skin. Nat. Mach. Intell. 2023, 5, 1344–1355. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, S.; Ki, M.-R.; Abdelhamid, M.A.A.; Pack, S.-P. Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin. Biomimetics 2024, 9, 278. https://doi.org/10.3390/biomimetics9050278
Youn S, Ki M-R, Abdelhamid MAA, Pack S-P. Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin. Biomimetics. 2024; 9(5):278. https://doi.org/10.3390/biomimetics9050278
Chicago/Turabian StyleYoun, Sol, Mi-Ran Ki, Mohamed A. A. Abdelhamid, and Seung-Pil Pack. 2024. "Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin" Biomimetics 9, no. 5: 278. https://doi.org/10.3390/biomimetics9050278
APA StyleYoun, S., Ki, M.-R., Abdelhamid, M. A. A., & Pack, S.-P. (2024). Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin. Biomimetics, 9(5), 278. https://doi.org/10.3390/biomimetics9050278







