Lamellar Septa-like Structured Carbonate Apatite Scaffolds with Layer-by-Layer Fracture Behavior for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CAp-CB Scaffolds
2.2. Characterization of CAp-CB Scaffolds
2.2.1. Structural Analysis
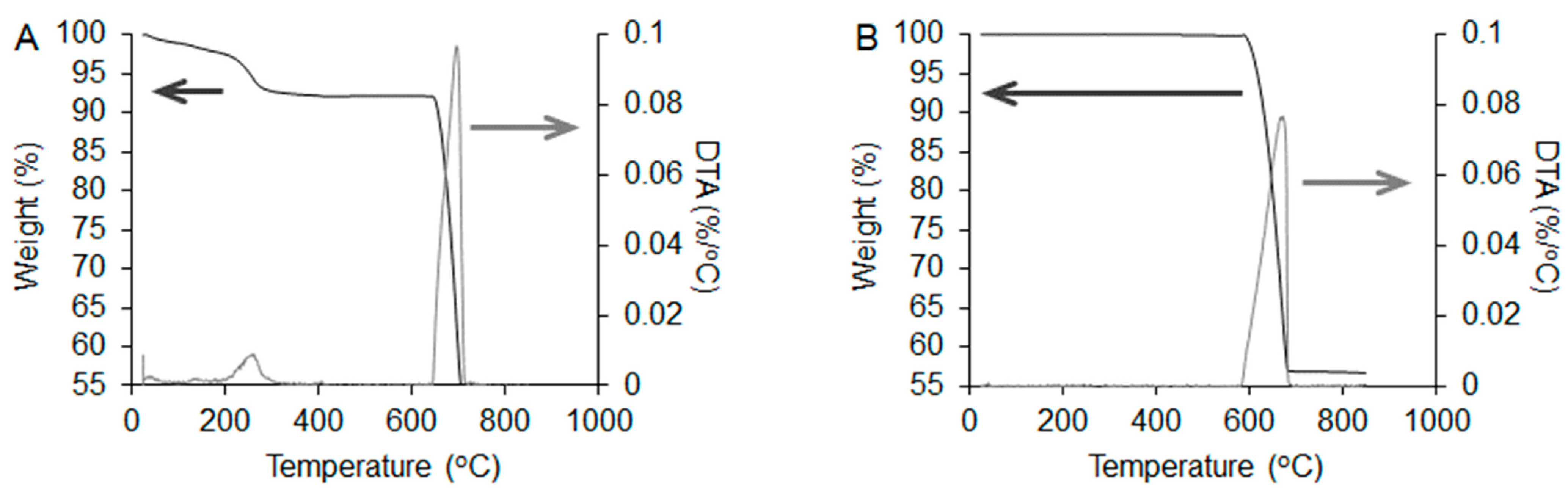
2.2.2. Organic Residue Evaluation
2.2.3. Composition Analysis
2.2.4. Carbonate Content Analysis
2.2.5. Bulk Porosity
2.2.6. Pore Size Distribution and Volume Analysis
2.2.7. Compressive Strength Test
2.3. Animal Experiments
2.4. µ-CT and Histological Evaluation
2.5. Statistical Analysis
3. Results and Discussion
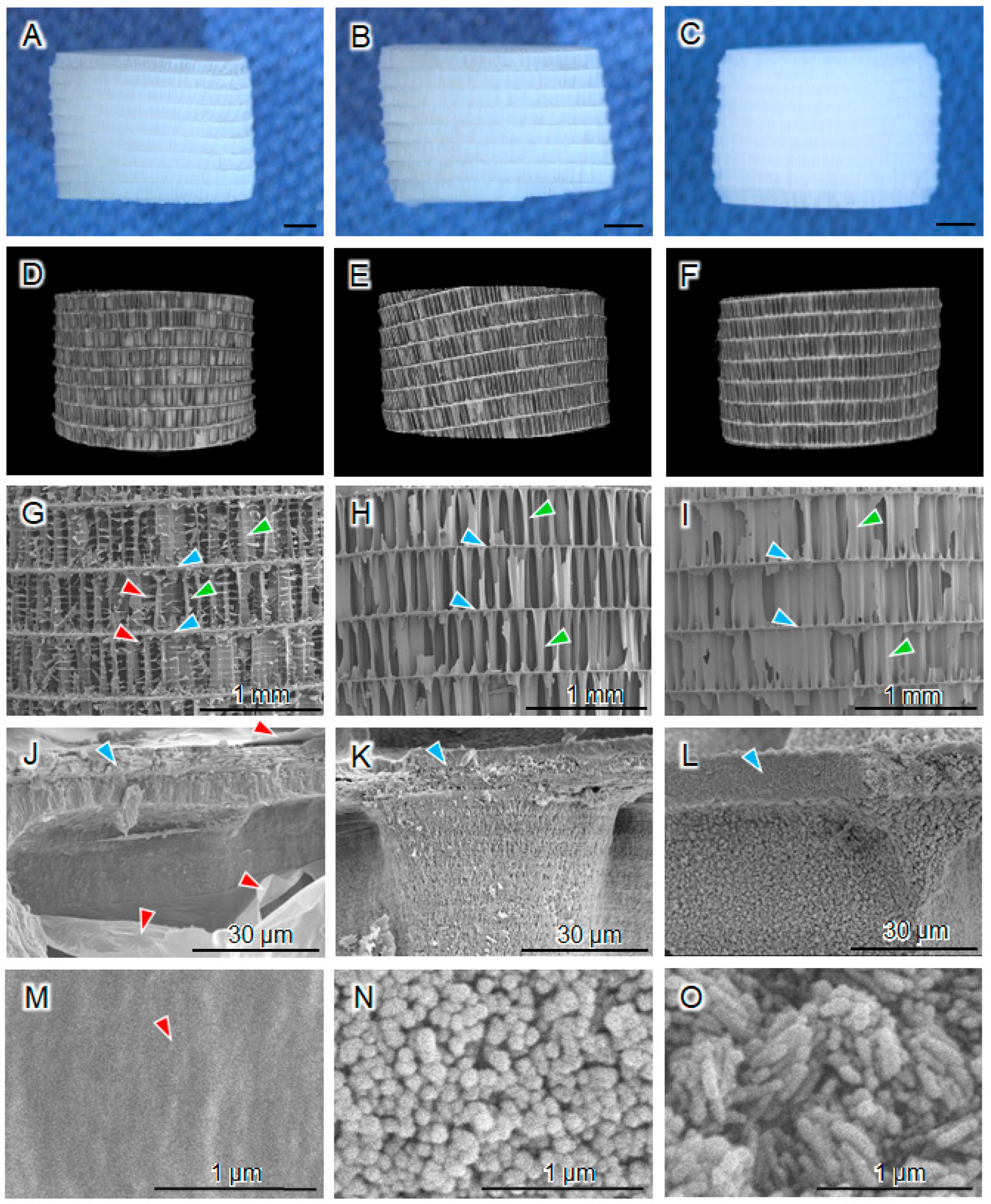
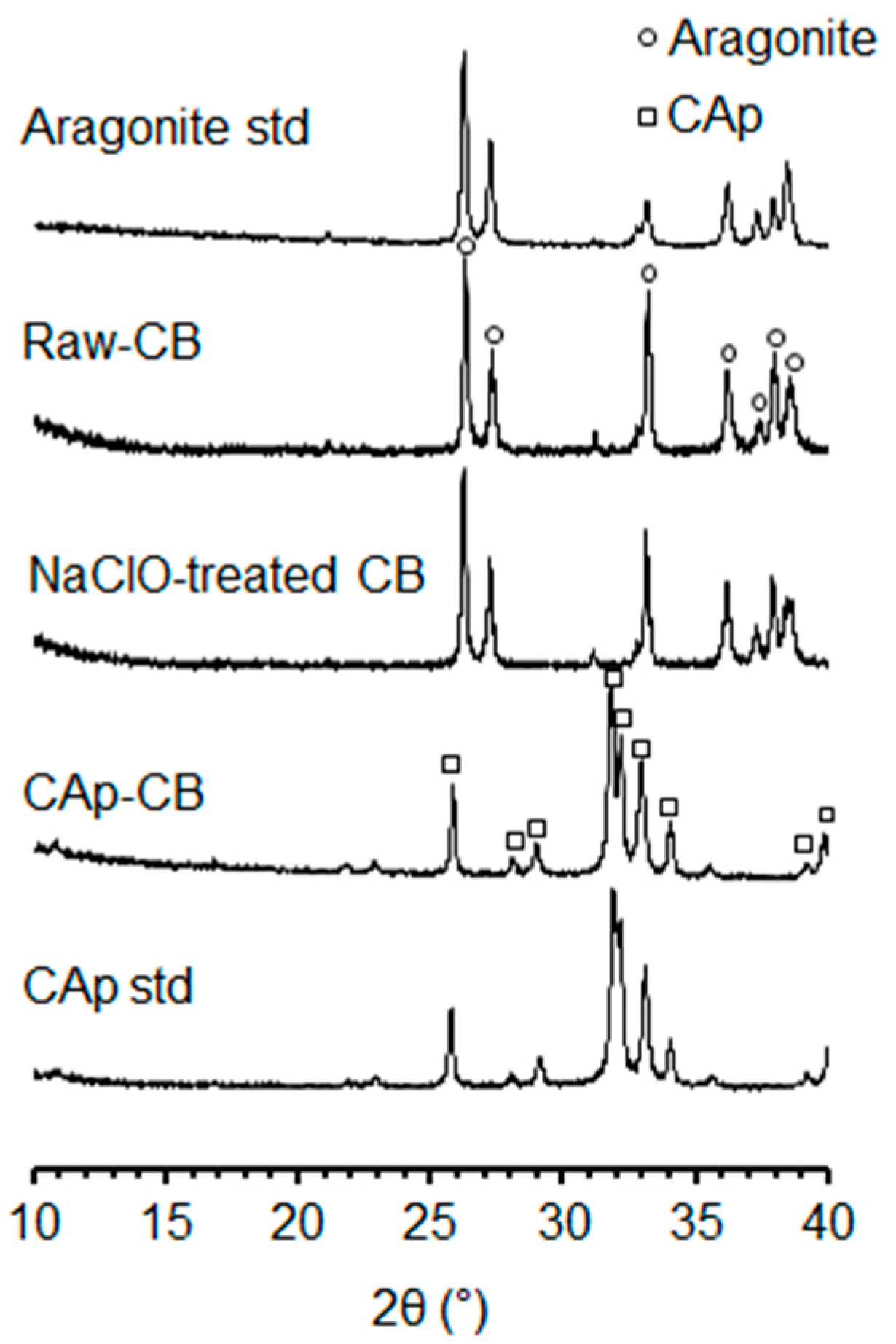
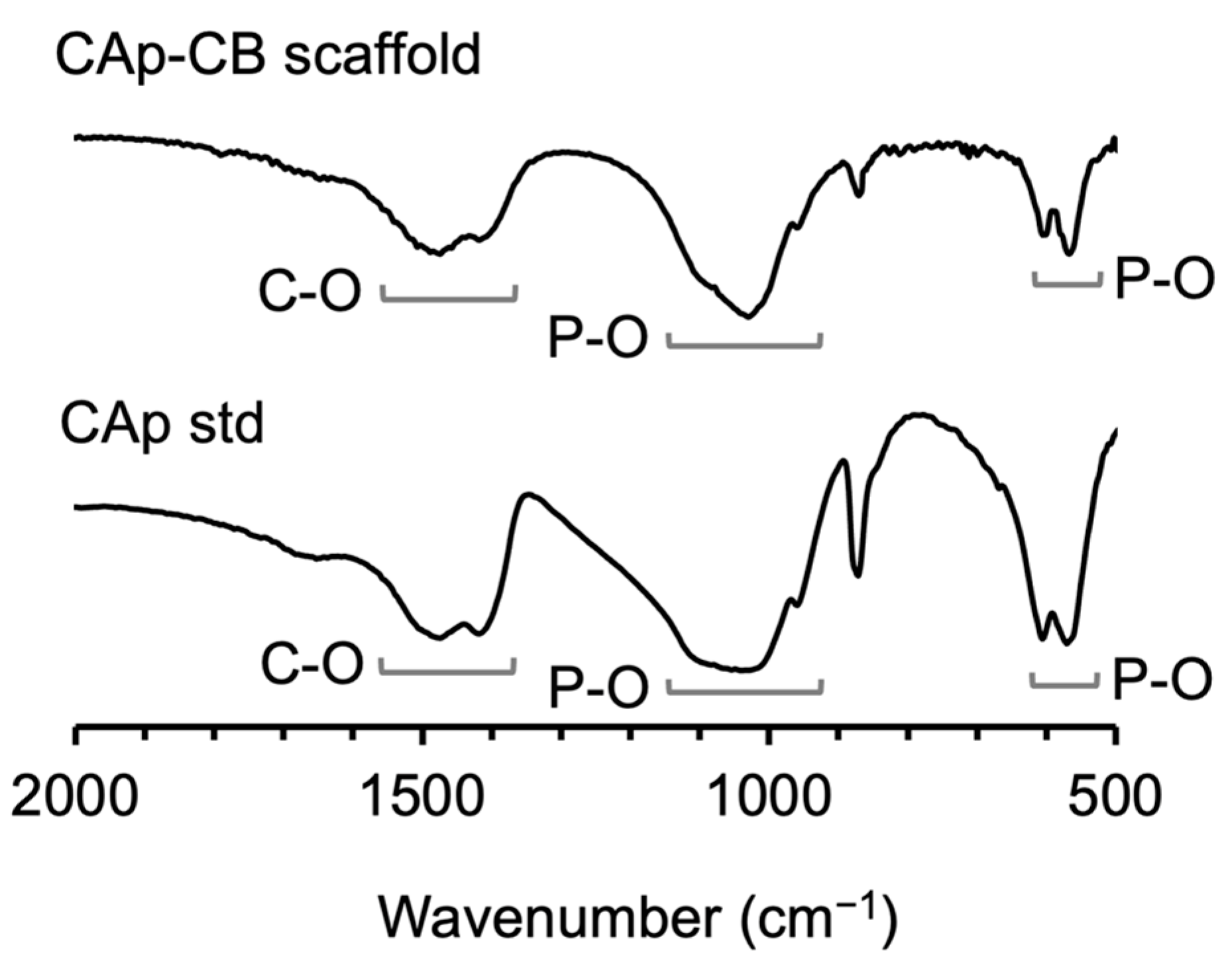
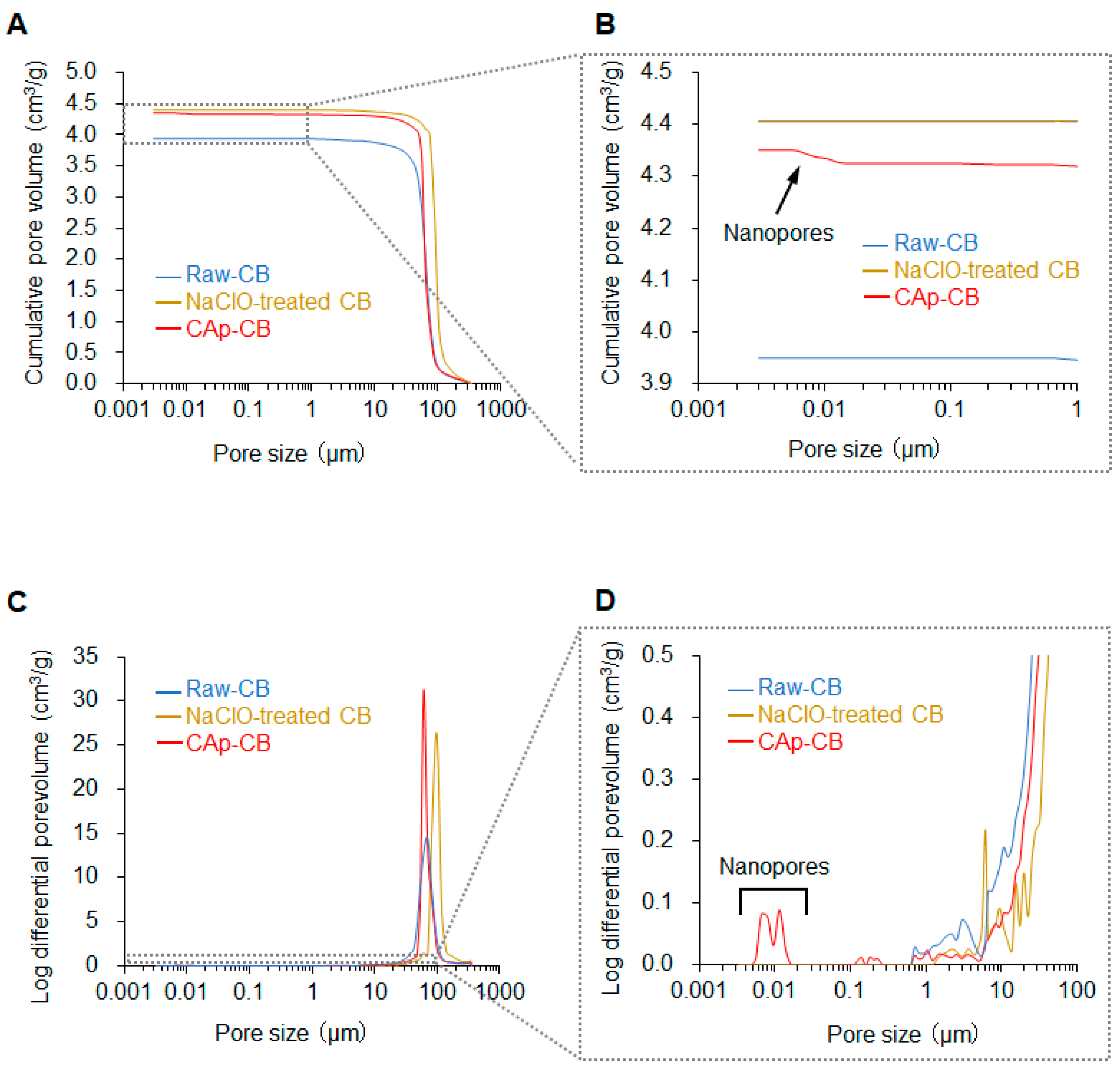
3.1. Structural and Physicochemical Properties of CAp-CB Scaffolds
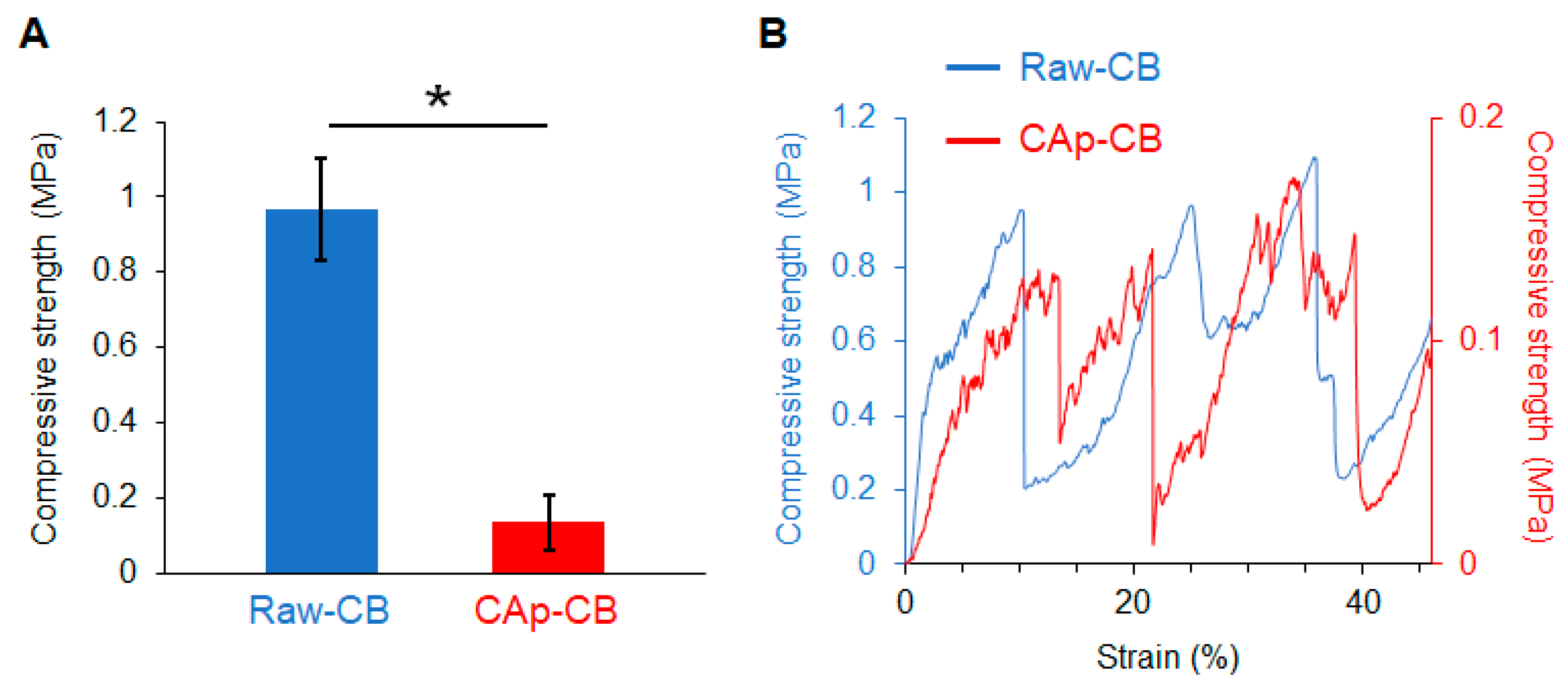
3.2. Mechanical Properties of CAp-CB Scaffolds
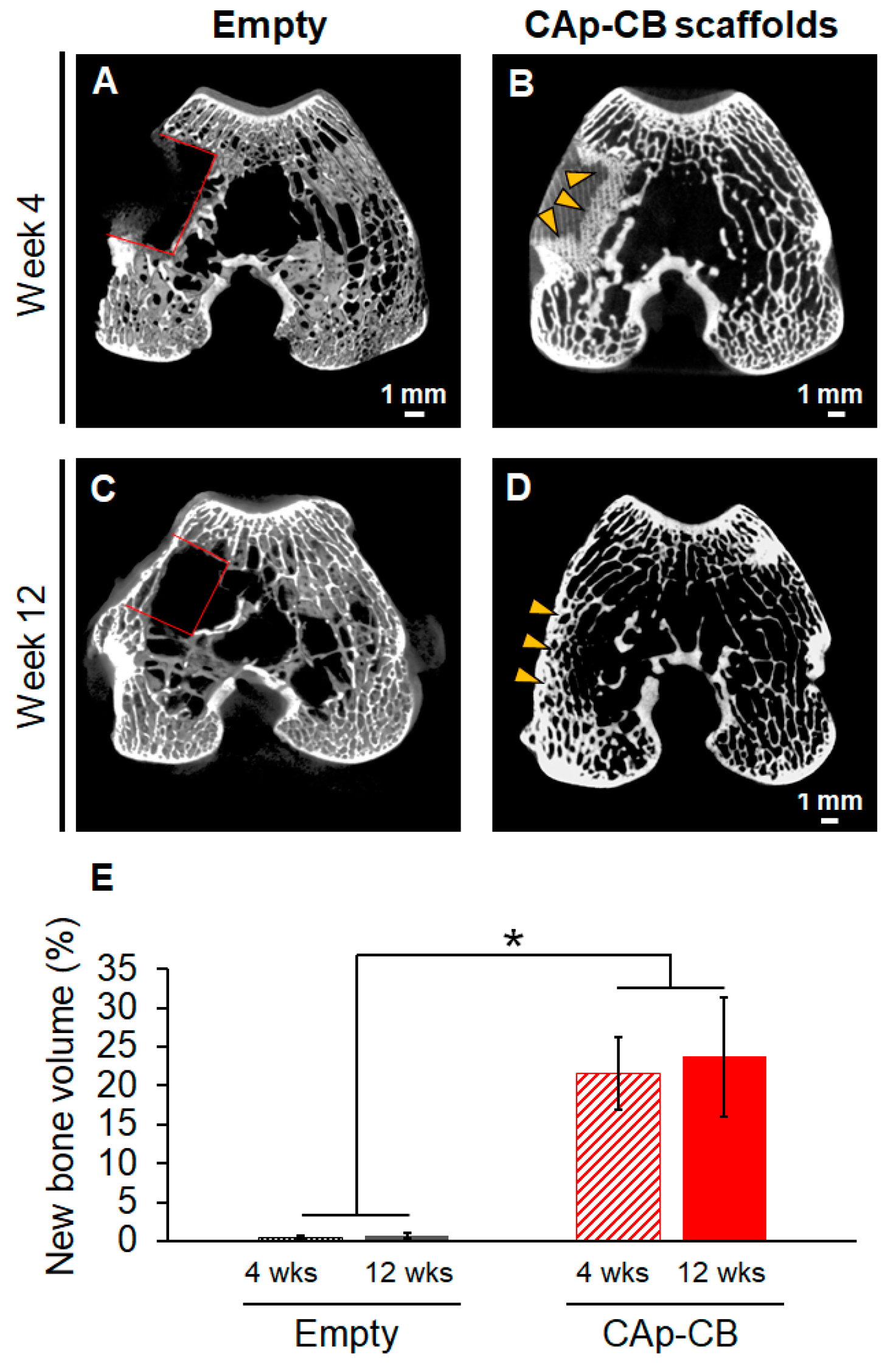
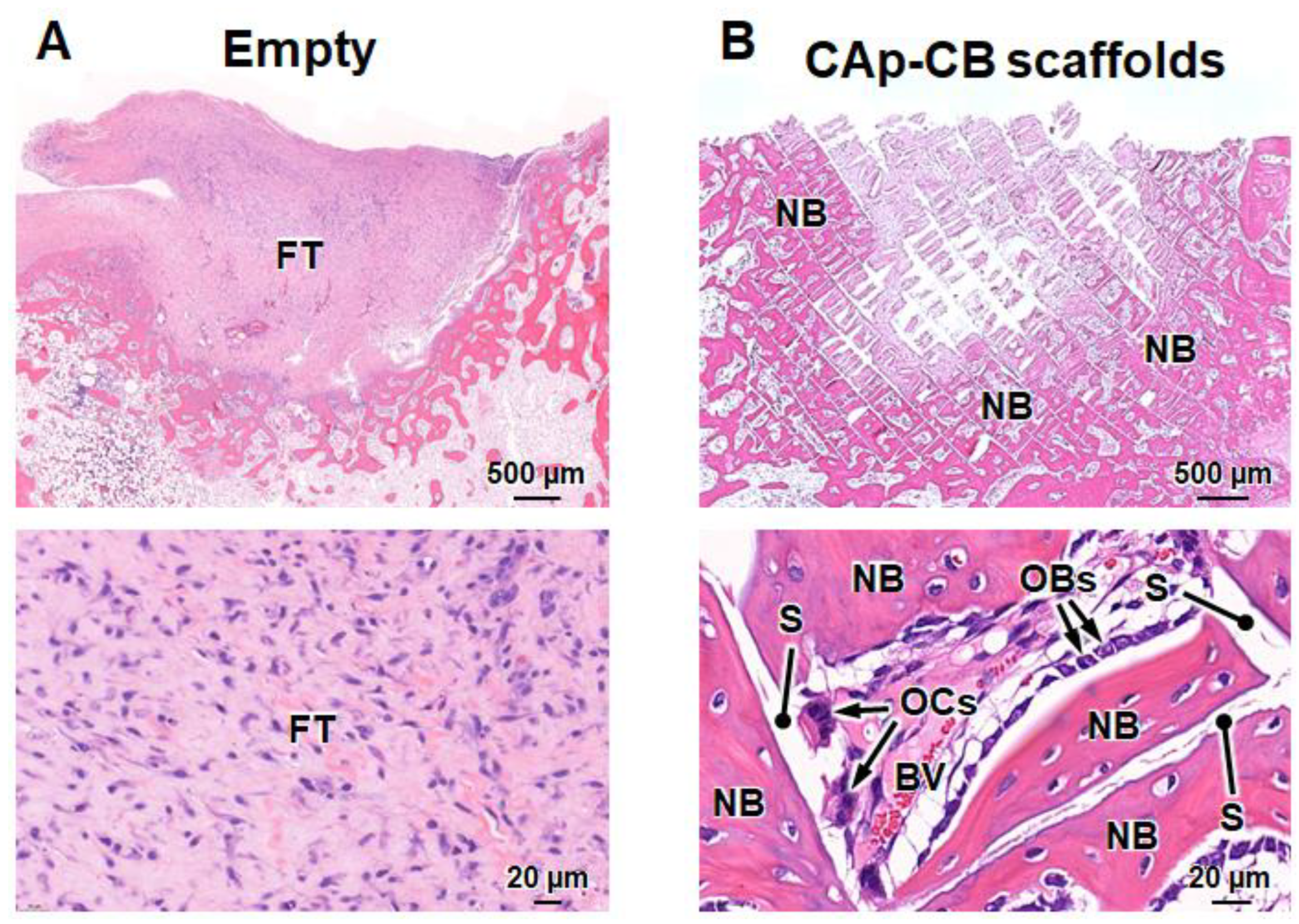
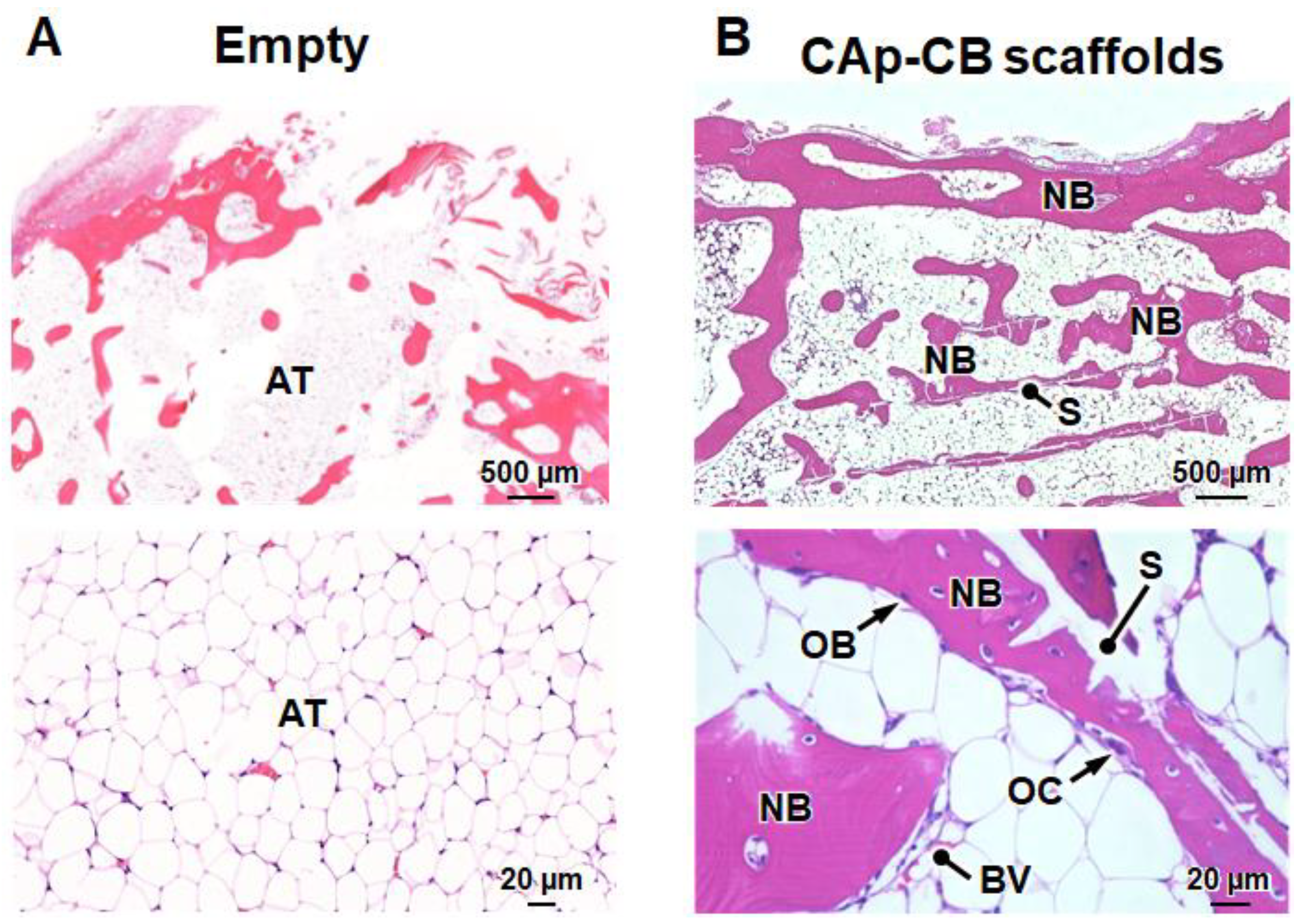
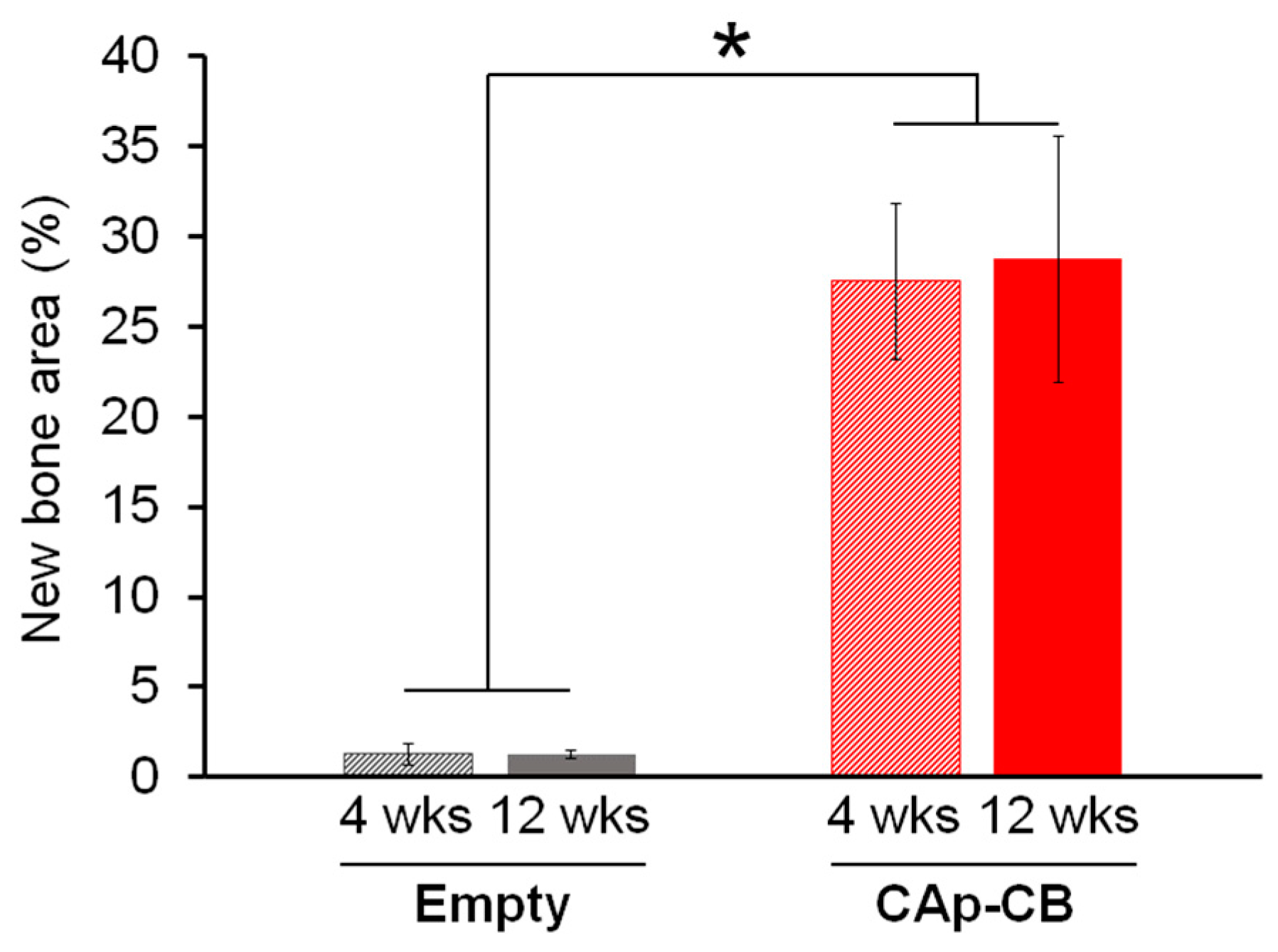
3.3. In Vivo Evaluations of Bone Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial bone grafts claimed as an alternative to autografts: Current trends for clinical applications in orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef] [PubMed]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef] [PubMed]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Jt. Surg. 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.G.; Abrams, G.D.; Sherman, S.L.; Safran, M.R.; Murray, I.R. Autologous Bone Grafting. Oper. Tech. Sports Med. 2020, 28, 150780. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef]
- Guastaldi, F.P.S.; Matheus, H.R.; de Faloni, A.P.S.; de Almeida-Filho, E.; Cominotte, M.A.; Moretti, L.A.C.; Verzola, M.H.A.; Marcantonio, E.; de Almeida, J.M.; Guastaldi, A.C.; et al. A new multiphase calcium phosphate graft material improves bone healing—An in vitro and in vivo analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2686–2704. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Haba, Y.; Köckerling, M.; Schick, C.; Bader, R. Determination of Bone Density and Bone Composition Using Thermoaravimetric Analysis. In Proceedings of the 12th International Conference on Measurement, Smolenice, Slovakia, 27–29 May 2019; pp. 178–181. [Google Scholar] [CrossRef]
- Bergamo, R.R.; de Carvalho, H.M.; Guerra-Junior, G.; de Moraes, A.M. Body Composition, Bone Mass and Bone Geometry in Adolescent Athletes. Med. Sci. Sports Exerc. 2019, 51, 681–682. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications. Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Samourides, A.; Browning, L.; Hearnden, V.; Chen, B. The effect of porous structure on the cell proliferation, tissue ingrowth and angiogenic properties of poly(glycerol sebacate urethane) scaffolds. Mater. Sci. Eng. C 2020, 108, 110384. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Vijayavenkataraman, S. Design of 3D printed scaffolds for bone tissue engineering: A review. Bioprinting 2021, 24, e00167. [Google Scholar] [CrossRef]
- Hayashi, K.; Kato, N.; Kato, M.; Ishikawa, K. Impacts of channel direction on bone tissue engineering in 3D-printed carbonate apatite scaffolds. Mater. Des. 2021, 204, 109686. [Google Scholar] [CrossRef]
- Tangsuksant, T.; Ummartyotin, S.; Pongprayoon, T.; Arpornmaeklong, P.; Apinyauppatham, K. Property and biological effects of the cuttlebone derived calcium phosphate particles, a potential bioactive bone substitute material. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1207–1223. [Google Scholar] [CrossRef]
- Mao, A.; Zhao, N.; Liang, Y.; Bai, H. Mechanically Efficient Cellular Materials Inspired by Cuttlebone. Adv. Mater. 2021, 33, 2007348. [Google Scholar] [CrossRef]
- Yang, T.; Jia, Z.; Chen, H.; Deng, Z.; Liu, W.; Chen, L.; Li, L. Mechanical design of the highly porous cuttlebone: A bioceramic hard buoyancy tank for cuttlefish. Proc. Natl. Acad. Sci. USA 2020, 117, 23450–23459. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Superiority of Triply Periodic Minimal Surface Gyroid Structure to Strut-Based Grid Structure in Both Strength and Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 34570–34577. [Google Scholar] [CrossRef]
- Hayashi, K.; Yanagisawa, T.; Shimabukuro, M.; Kishida, R.; Ishikawa, K. Granular honeycomb scaffolds composed of carbonate apatite for simultaneous intra- and inter-granular osteogenesis and angiogenesis. Mater. Today Bio 2022, 14, 100247. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A.; Ivanković, T.; Ivanišević, I.; Cvetnić, M.; Antunović, M.; Urlić, I.; Ivanković, H.; Ivanković, M. Multiphase zinc and magnesium mono-substituted calcium phosphates derived from cuttlefish bone: A multifunctional biomaterials. Ceram. Int. 2023, 49, 11005–11017. [Google Scholar] [CrossRef]
- Akpan, E.S.; Dauda, M.; Kuburi, L.S.; Obada, D.O.; Bansod, N.D.; Dodoo-Arhin, D. Hydroxyapatite ceramics prepared from two natural sources by direct thermal conversion: From material processing to mechanical measurements. In Mater Today Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 2291–2294. [Google Scholar] [CrossRef]
- Cozza, N.; Monte, F.; Bonani, W.; Aswath, P.; Motta, A.; Migliaresi, C. Bioactivity and mineralization of natural hydroxyapatite from cuttlefish bone and Bioglass® co-sintered bioceramics. J. Tissue Eng. Regen. Med. 2018, 12, e1131–e1142. [Google Scholar] [CrossRef] [PubMed]
- Henggu, K.U.; Ibrahim, B.; Suptijah, P. Hidroksiapatit dari cangkang sotong sebagai sediaan biomaterial perancah tulang. J. Pengolah. Has. Perikan. Indones. 2019, 22, 1–13. [Google Scholar]
- Aminatun, A.; Handayani, F.D.E.; Widiyanti, P.; Winarni, D.; Siswanto, S. In vivo approach on femur bone regeneration of white rat (Rattus norvegicus) with the use of hydroxyapatite from cuttlefish bone (Sepia spp.) as bone filler. Vet. World 2019, 12, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Valério, P.; Kannan, S.; Oktar, F.N.; Ferreira, J.M.F. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Tuan, W.H.; Lai, P.L. Biphasic ceramic bone graft with biphasic degradation rates. Mater. Sci. Eng. C 2021, 118, 111421. [Google Scholar] [CrossRef]
- Lett, J.A.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, D.; Zhou, Q.; Wu, Z.; Weng, S.; Boodhun, V.; Bai, B.; Shen, Z.; Tang, J.; Chen, L.; et al. The fast degradation of β-TCP ceramics facilitates healing of bone defects by the combination of BMP-2 and Teriparatide. Biomed. Pharmacother. 2019, 112, 108578. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Jun, Y.-K.; Hong, S.-H.; Kim, H.-E. Synthesis and Dissolution Behavior of b-TCP and HA/b-TCP Composite Powders. J. Eur. Ceram. Soc. 2003, 23, 1039–1045. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Granular Honeycombs Composed of Carbonate Apatite, Hydroxyapatite, and β-Tricalcium Phosphate as Bone Graft Substitutes: Effects of Composition on Bone Formation and Maturation. ACS Appl. Bio Mater. 2020, 3, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Mirthia, A.; Munting, E. Calcium phosphate cements: Action of setting regulators on the properties of the p-tricalcium phosphate-monocalcium phosphate cements. Biomaterials 1989, 10, 634–638. [Google Scholar]
- Čadež, V.; Škapin, S.D.; Leonardi, A.; Križaj, I.; Kazazić, S.; Salopek-Sondi, B.; Sondi, I. Formation and morphogenesis of a cuttlebone’s aragonite biomineral structures for the common cuttlefish (Sepia officinalis) on the nanoscale: Revisited. J. Colloid. Interface Sci. 2017, 508, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Gomez-Morales, J.; Iafisco, M.; Sarda, S.; Rimondini, L.; Bianchi, C. Calcium phosphate surface tailoring technologies for drug delivering and tissue engineering. Surf. Tailoring Inorg. Mater. Biomed. Appl. 2012, 2, 43–111. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Materials Science, Engineering and Technology; InTech: London, UK, 2012; pp. 123–147. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Hayashi, K.; Ishikawa, K. Effects of nanopores on the mechanical strength, osteoclastogenesis, and osteogenesis in honeycomb scaffolds. J. Mater. Chem. B 2020, 8, 8536–8545. [Google Scholar] [CrossRef]
- Hayashi, K.; Yanagisawa, T.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Gear-shaped carbonate apatite granules with a hexagonal macropore for rapid bone regeneration. Comput. Struct. Biotechnol. J. 2023, 21, 2514–2523. [Google Scholar] [CrossRef]
- Hayashi, K.; Yanagisawa, T.; Kishida, R.; Ishikawa, K. Effects of Scaffold Shape on Bone Regeneration: Tiny Shape Differences Affect the Entire System. ACS Nano 2022, 16, 11755–11768. [Google Scholar] [CrossRef]
- Heikkilä, J.T.; Aho, H.J.; Yli-urpo, A.; Happonen, R.P.; Aho, A.J. Bone formation in rabbit cancellous bone defects filled with bioactive glass granules. Acta Orthop. 1995, 66, 463–467. [Google Scholar] [CrossRef]
- Bagi, C.M.; Berryman, E.; Moalli, M.R. Comparative Bone Anatomy of Commonly Used Laboratory Animals: Implications for Drug Discovery. Comp. Med. 2011, 61, 76–85. [Google Scholar] [PubMed]
- Battistella, E.; Mele, S.; Foltran, I.; Lesci, I.G.; Roveri, N.; Sabatino, P.; Rimondini, L. Cuttlefish bone scaffold for tissue engineering: A novel hydrothermal transformation, chemical-physical, and biological characterization. J. Appl. Biomater. Funct. Mater. 2012, 10, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.J.; Yang, S.S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Transformable Carbonate Apatite Chains as a Novel Type of Bone Graft. Adv. Healthc. Mater. 2024, e2303245. [Google Scholar] [CrossRef] [PubMed]
- Hadagalli, K.; Panda, A.K.; Mandal, S.; Basu, B. Faster Biomineralization and Tailored Mechanical Properties of Marine-Resource-Derived Hydroxyapatite Scaffolds with Tunable Interconnected Porous Architecture. ACS Appl. Bio Mater. 2019, 2, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taleb Alashkar, A.N.; Hayashi, K.; Ishikawa, K. Lamellar Septa-like Structured Carbonate Apatite Scaffolds with Layer-by-Layer Fracture Behavior for Bone Regeneration. Biomimetics 2024, 9, 112. https://doi.org/10.3390/biomimetics9020112
Taleb Alashkar AN, Hayashi K, Ishikawa K. Lamellar Septa-like Structured Carbonate Apatite Scaffolds with Layer-by-Layer Fracture Behavior for Bone Regeneration. Biomimetics. 2024; 9(2):112. https://doi.org/10.3390/biomimetics9020112
Chicago/Turabian StyleTaleb Alashkar, Ahmad Nazir, Koichiro Hayashi, and Kunio Ishikawa. 2024. "Lamellar Septa-like Structured Carbonate Apatite Scaffolds with Layer-by-Layer Fracture Behavior for Bone Regeneration" Biomimetics 9, no. 2: 112. https://doi.org/10.3390/biomimetics9020112
APA StyleTaleb Alashkar, A. N., Hayashi, K., & Ishikawa, K. (2024). Lamellar Septa-like Structured Carbonate Apatite Scaffolds with Layer-by-Layer Fracture Behavior for Bone Regeneration. Biomimetics, 9(2), 112. https://doi.org/10.3390/biomimetics9020112





