Analysis of the Prognostic Factors That Influence the Outcome of Periapical Surgery, including Biomimetic Membranes for Tissue Regeneration: A Review
Abstract
1. Introduction
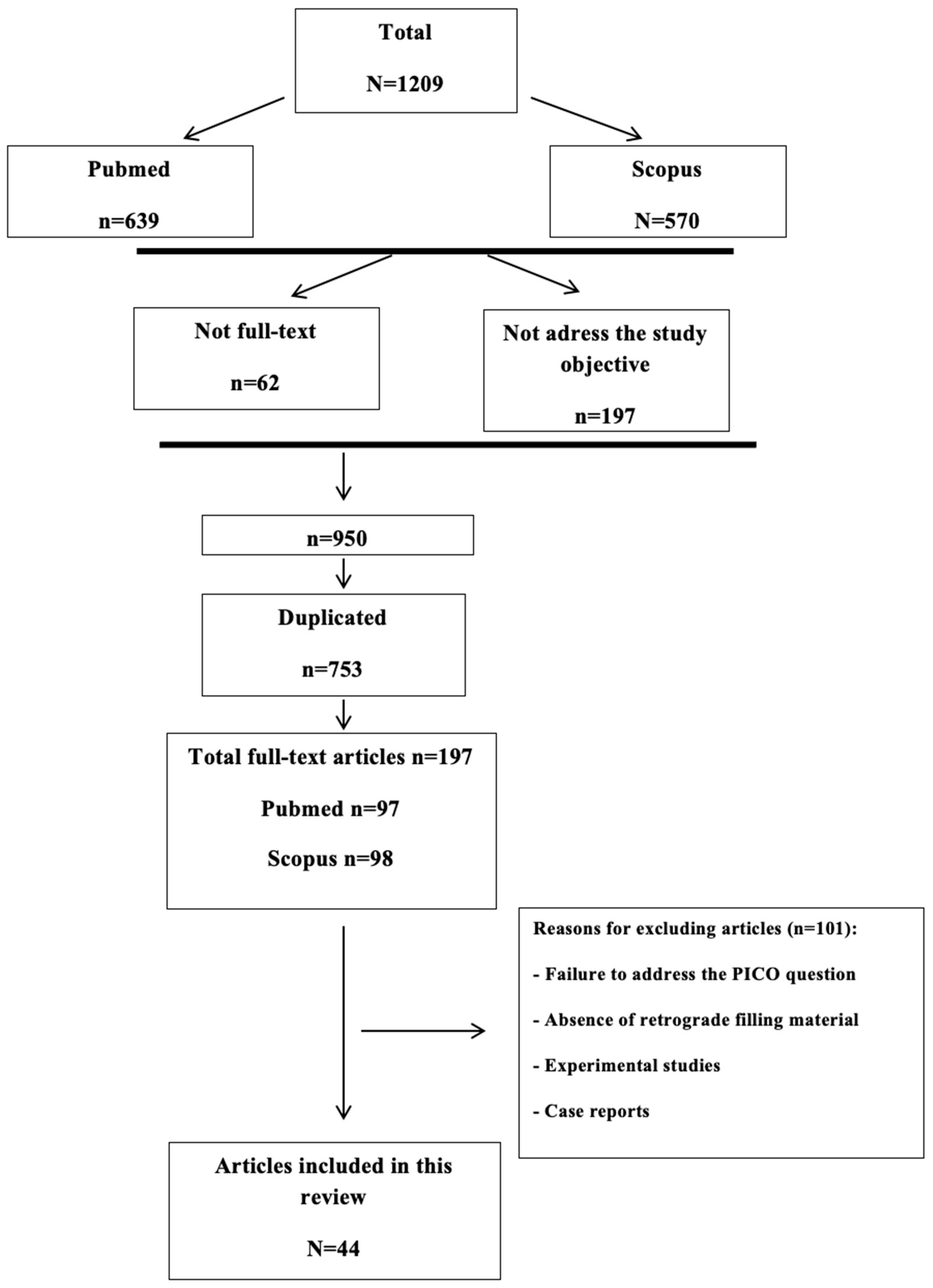
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Review and Screening of Articles
3. Results and Discussion
3.1. Preoperative Factors
3.1.1. Sex
3.1.2. Age
3.1.3. Periodontal Status
3.1.4. Type of Tooth
3.1.5. Preoperative Pain or Symptomatology
3.1.6. Endodontic Status
3.1.7. Presence of Root-Canal Post
3.1.8. Lesion Size
3.2. Intraoperative or Treatment-Related Factors
3.2.1. Type of Surgery: First Surgery versus Repeat Surgery
3.2.2. Retrograde Cavity Preparation
3.2.3. Retrograde Root-End Filling Material
3.2.4. Experience of the Surgeon
3.2.5. Guided Tissue Regeneration (GTR)
3.2.6. New Technologies
3.3. Post-Operative Factors
Crown-Sealing Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano-Giménez, M.; Sánchez-Torres, A.; Gay-Escoda, C. Prognostic Factors on Periapical Surgery: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e715–e722. [Google Scholar] [CrossRef]
- Carrillo, C.; Peñarrocha, M.; Bagán, J.V.; Vera, F. Relationship between Histological Diagnosis and Evolution of 70 Periapical Lesions at 12 Months, Treated by Periapical Surgery. J. Oral Maxillofac. Surg. 2008, 66, 1606–1609. [Google Scholar] [CrossRef]
- Sánchez-Torres, A.; Sánchez-Garcés, M.Á.; Gay-Escoda, C. Materials and Prognostic Factors of Bone Regeneration in Periapical Surgery: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e419–e425. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Martí, E.; García, B.; Gay, C. Relationship of Periapical Lesion Radiologic Size, Apical Resection, and Retrograde Filling with the Prognosis of Periapical Surgery. J. Oral Maxillofac. Surg. 2007, 65, 1526–1529. [Google Scholar] [CrossRef]
- Rahbaran, S.; Gilthorpe, M.S.; Harrison, S.D.; Gulabivala, K. Comparison of Clinical Outcome of Periapical Surgery in Endodontic and Oral Surgery Units of a Teaching Dental Hospital: A Retrospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 700–709. [Google Scholar] [CrossRef]
- Zuolo, M.L.; Ferreira, M.O.; Gutmann, J.L. Prognosis in Periradicular Surgery: A Clinical Prospective Study. Int. Endod. J. 2000, 33, 91–98. [Google Scholar] [CrossRef]
- Friedman, S. The Prognosis and Expected Outcome of Apical Surgery. Endod. Top. 2000, 33, 91–98. [Google Scholar] [CrossRef]
- Friedman, S. Prognosis of Initial Endodontic Theraphy. Endod. Top. 2002, 2, 59–88. [Google Scholar] [CrossRef]
- Lustmann, J.; Friedman, S.; Shaharabany, V. Relation of Pre- and Intraoperative Factors to Prognosis of Posterior Apical Surgery. J. Endod. 1991, 17, 239–241. [Google Scholar] [CrossRef]
- Jensen, S.S.; Nattestad, A.; Egdø, P.; Sewerin, I.; Munksgaard, E.C.; Schou, S. A Prospective, Randomized, Comparative Clinical Study of Resin Composite and Glass Ionomer Cement for Retrograde Root Filling. Clin. Oral. Investig. 2002, 6, 236–243. [Google Scholar] [CrossRef]
- Wang, Q.; Cheung, G.S.P.; Ng, R.P.Y. Survival of Surgical Endodontic Treatment Performed in a Dental Teaching Hospital: A Cohort Study. Int. Endod. J. 2004, 37, 764–775. [Google Scholar] [CrossRef]
- von Arx, T.; Jensen, S.S.; Hänni, S. Clinical and Radiographic Assessment of Various Predictors for Healing Outcome 1 Year after Periapical Surgery. J. Endod. 2007, 33, 123–128. [Google Scholar] [CrossRef]
- von Arx, T.; Peñarrocha, M.; Jensen, S. Prognostic Factors in Apical Surgery with Root-End Filling: A Meta-Analysis. J. Endod. 2010, 36, 957–973. [Google Scholar] [CrossRef]
- Tsesis, I.; Faivishevsky, V.; Kfir, A.; Rosen, E. Outcome of Surgical Endodontic Treatment Performed by a Modern Technique: A Meta-Analysis of Literature. J. Endod. 2009, 35, 1505–1511. [Google Scholar] [CrossRef]
- Song, M.; Jung, I.-Y.; Lee, S.-J.; Lee, C.-Y.; Kim, E. Prognostic Factors for Clinical Outcomes in Endodontic Microsurgery: A Retrospective Study. J. Endod. 2011, 37, 927–933. [Google Scholar] [CrossRef]
- Chong, B.S.; Pitt Ford, T.R. Postoperative Pain after Root-End Resection and Filling. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 762–766. [Google Scholar] [CrossRef]
- Maddalone, M.; Gagliani, M. Periapical Endodontic Surgery: A 3-Year Follow-up Study. Int. Endod. J. 2003, 36, 193–198. [Google Scholar] [CrossRef]
- von Arx, T.; Hänni, S.; Jensen, S.S. Clinical Results with Two Different Methods of Root-End Preparation and Filling in Apical Surgery: Mineral Trioxide Aggregate and Adhesive Resin Composite. J. Endod. 2010, 36, 1122–1129. [Google Scholar] [CrossRef]
- Wesson, C.M.; Gale, T.M. Molar Apicectomy with Amalgam Root-End Filling: Results of a Prospective Study in Two District General Hospitals. Br. Dent. J. 2003, 195, 707–714; discussion 698. [Google Scholar] [CrossRef]
- Platt, A.S.; Wannfors, K. The Effectiveness of Compomer as a Root-End Filling: A Clinical Investigation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 508–512. [Google Scholar] [CrossRef]
- Wang, N.; Knight, K.; Dao, T.; Friedman, S. Treatment Outcome in Endodontics-The Toronto Study. Phases I and II: Apical Surgery. J. Endod. 2004, 30, 751–761. [Google Scholar] [CrossRef]
- Gagliani, M.M.; Gorni, F.G.M.; Strohmenger, L. Periapical Resurgery versus Periapical Surgery: A 5-Year Longitudinal Comparison. Int. Endod. J. 2005, 38, 320–327. [Google Scholar] [CrossRef]
- Lindeboom, J.A.H.; Frenken, J.W.F.H.; Kroon, F.H.M.; van den Akker, H.P. A Comparative Prospective Randomized Clinical Study of MTA and IRM as Root-End Filling Materials in Single-Rooted Teeth in Endodontic Surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 495–500. [Google Scholar] [CrossRef]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Francetti, L.; Weinstein, R. Endodontic Surgery with Ultrasonic Retrotips: One-Year Follow-Up. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 380–387. [Google Scholar] [CrossRef]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Weinstein, R.L. Endodontic Reoperation Using an Endoscope and Microsurgical Instruments: One Year Follow-Up. Br. J. Oral Maxillofac. Surg. 2007, 45, 582–585. [Google Scholar] [CrossRef]
- De Lange, J.; Putters, T.; Baas, E.M.; van Ingen, J.M. Ultrasonic Root-End Preparation in Apical Surgery: A Prospective Randomized Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 841–845. [Google Scholar] [CrossRef]
- Wälivaara, D.-Å.; Abrahamsson, P.; Fogelin, M.; Isaksson, S. Super-EBA and IRM as Root-End Fillings in Periapical Surgery with Ultrasonic Preparation: A Prospective Randomized Clinical Study of 206 Consecutive Teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 258–263. [Google Scholar] [CrossRef]
- Taschieri, S.; Del Fabbro, M.; Testori, T.; Saita, M.; Weinstein, R. Efficacy of Guided Tissue Regeneration in the Management of Through-and-through Lesions Following Surgical Endodontics: A Preliminary Study. Int. J. Periodontics Restor. Dent. 2008, 28, 265–271. [Google Scholar]
- Kim, E.; Song, J.-S.; Jung, I.-Y.; Lee, S.-J.; Kim, S. Prospective Clinical Study Evaluating Endodontic Microsurgery Outcomes for Cases with Lesions of Endodontic Origin Compared with Cases with Lesions of Combined Periodontal-Endodontic Origin. J. Endod. 2008, 34, 546–551. [Google Scholar] [CrossRef]
- Saunders, W.P. A Prospective Clinical Study of Periradicular Surgery Using Mineral Trioxide Aggregate as a Root-End Filling. J. Endod. 2008, 34, 660–665. [Google Scholar] [CrossRef]
- Garcia, B.; Penarrocha, M.; Martí, E.; Martínez, J.M.; Gay-Escoda, C. Periapical Surgery in Maxillary Premolars and Molars: Analysis in Terms of the Distance between the Lesion and the Maxillary Sinus. J. Oral Maxillofac. Surg. 2008, 66, 1212–1217. [Google Scholar] [CrossRef]
- Christiansen, R.; Kirkevang, L.-L.; Hørsted-Bindslev, P.; Wenzel, A. Randomized Clinical Trial of Root-End Resection Followed by Root-End Filling with Mineral Trioxide Aggregate or Smoothing of the Orthograde Gutta-Percha Root Filling—1-Year Follow-Up. Int. Endod. J. 2009, 42, 105–114. [Google Scholar] [CrossRef]
- Barone, C.; Dao, T.T.; Basrani, B.B.; Wang, N.; Friedman, S. Treatment Outcome in Endodontics: The Toronto Study—Phases 3, 4, and 5: Apical Surgery. J. Endod. 2010, 36, 28–35. [Google Scholar] [CrossRef]
- Tsesis, I.; Shoshani, Y.; Givol, N.; Yahalom, R.; Fuss, Z.; Taicher, S. Comparison of Quality of Life after Surgical Endodontic Treatment Using Two Techniques: A Prospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 367–371. [Google Scholar] [CrossRef]
- Martí-Bowen, E.; Peñarrocha-Diago, M.; García-Mira, B. Periapical Surgery Using the Ultrasound Technique and Silver Amalgam Retrograde Filling. A Study of 71 Teeth with 100 Canals. Med. Oral Patol. Oral Cir. Bucal 2005, 10 (Suppl. S1), E67–E73. [Google Scholar]
- Rud, J.; Rud, V.; Munksgaard, E.C. Periapical Healing of Mandibular Molars after Root-End Sealing with Dentine-Bonded Composite. Int. Endod. J. 2001, 34, 285–292. [Google Scholar] [CrossRef]
- Tsesis, I.; Rosen, E.; Schwartz-Arad, D.; Fuss, Z. Retrospective Evaluation of Surgical Endodontic Treatment: Traditional versus Modern Technique. J. Endod. 2006, 32, 412–416. [Google Scholar] [CrossRef]
- Chong, B.S.; Pitt Ford, T.R.; Hudson, M.B. A Prospective Clinical Study of Mineral Trioxide Aggregate and IRM When Used as Root-End Filling Materials in Endodontic Surgery. Int. Endod. J. 2003, 36, 520–526. [Google Scholar] [CrossRef]
- Wälivaara, D.-A.; Abrahamsson, P.; Isaksson, S.; Blomqvist, J.-E.; Sämfors, K.-A. Prospective Study of Periapically Infected Teeth Treated with Periapical Surgery Including Ultrasonic Preparation and Retrograde Intermediate Restorative Material Root-End Fillings. J. Oral Maxillofac. Surg. 2007, 65, 931–935. [Google Scholar] [CrossRef]
- Wälivaara, D.-A.; Abrahamsson, P.; Sämfors, K.-A.; Isaksson, S. Periapical Surgery Using Ultrasonic Preparation and Thermoplasticized Gutta-Percha with AH Plus Sealer or IRM as Retrograde Root-End Fillings in 160 Consecutive Teeth: A Prospective Randomized Clinical Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 784–789. [Google Scholar] [CrossRef]
- Von Arx, T.; Frei, C.; Bornstein, M.M. Periradicular surgery with and without endoscopy: A prospective clinical comparative study. Schweiz. Monatsschr Zahnmed. 2003, 113, 860–865. [Google Scholar]
- Tobón, S.I.; Arismendi, J.A.; Marín, M.L.; Mesa, A.L.; Valencia, J.A. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int. Endod. J. 2002, 35, 635–641. [Google Scholar] [CrossRef]
- Dominiak, M.; Lysiak-Drwal, K.; Gedrange, T.; Zietek, M.; Gerber, H. Efficacy of healing process of bone defects after apicectomy: Results after 6 and 12 months. J. Physiol. Pharmacol. 2009, 60 (Suppl. S8), 51–55. [Google Scholar]
- Liu, T.J.; Zhou, J.N.; Guo, L.H. Impact of different regenerative techniques and materials on the healing outcome of endodontic surgery: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 536–555. [Google Scholar] [CrossRef]
- Zubizarreta-Macho, Á.; Tosin, R.; Tosin, F.; Velasco Bohórquez, P.; San Hipólito Marín, L.; Montiel-Company, J.M.; Mena-Álvarez, J.; Hernández Montero, S. Influence of Guided Tissue Regeneration Techniques on the Success Rate of Healing of Surgical Endodontic Treatment: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 18, 1062. [Google Scholar] [CrossRef]
- Marín-Botero, M.L.; Domínguez-Mejía, J.S.; Arismendi-Echavarría, J.A.; Mesa-Jaramillo, A.L.; Flórez-Moreno, G.A.; Tobón-Arroyave, S.I. Healing response of apicomarginal defects to two guided tissue regeneration techniques in periradicular surgery: A double-blind, randomized-clinical trial. Int. Endod. J. 2006, 39, 368–377. [Google Scholar] [CrossRef]
- Tsesis, I.; Rosen, E.; Tamse, A.; Taschieri, S.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment: A systematic review and meta-analysis. J. Endod. 2011, 37, 1039–1045. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Tsesis, I.; Bortolin, M.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: A retrospective study at 4-year follow-up. Oral Maxillofac. Surg. 2011, 15, 153–159. [Google Scholar] [CrossRef]
- Parmar, P.D.; Dhamija, R.; Tewari, S.; Sangwan, P.; Gupta, A.; Duhan, J.; Mittal, S. 2D and 3D radiographic outcome assessment of the effect of guided tissue regeneration using resorbable collagen membrane in the healing of through-and-through periapical lesions—A randomized controlled trial. Int. Endod. J. 2019, 52, 935–948. [Google Scholar] [CrossRef]
- Johri, S.; Verma, P.; Tikku, A.P.; Bains, R.; Kohli, N. Effect of amniotic membrane and platelet-rich fibrin membrane on bone healing post endodontic surgery: An ultrasonographic, randomized controlled study. J. Tissue Eng. Regen. Med. 2022, 16, 1208–1222. [Google Scholar] [CrossRef]
- Garg, M.; Srivastava, V.; Chauhan, R.; Pramanik, S.; Khanna, R. Application of platelet-rich fibrin and freeze-dried bone allograft following apicoectomy: A comparative assessment of radiographic healing. Indian. J. Dent. Res. 2023, 34, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Albagle, A.; Kohli, M.R.; Kratchman, S.I.; Lee, S.M.; Karabucak, B. Periapical healing following endodontic microsurgery with collagen-based bone-filling material: A randomized controlled clinical trial. Int. Endod. J. 2023, 56, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
| Zuolo 2000 [6] | Rahbaran 2001 [5] | Jensen 2002 [10] | Chong 2003 [16] | Maddalone 2003 [17] | von Arx 2003 [18] | Wesson 2003 [19] | Platt 2004 [20] | Wang 2004 [11] | Wang 2004 [21] | Gagliani 2005 [22] | Lindeboom 2005 [23] | Taschieri 2005 [24] | Taschieri 2006 [25] | Tsesis 2006 [14] | de Lange 2007 [26] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | RS | RS | RCT | RCT | PS | PS | PS | RCT | PS | RS | PS | RCT | RCT | RCT | RS | RCT | |
| Initial sample | 114 | 314 | 134 | 131 | 146 | 129 | 1007 | 34 | 155 | 238 | 185 | 100 | 50 | 80 | 110 | 399 | |
| Final sample | 102 | 176 | 122 | 108 | 120 | 115 | 790 | 34 | 90 | 194 | 164 | 100 | 46 | 71 | 71 | 290 | |
| Follow-up time (years) | 1–4 | 4 | 1 | 2 | 3 | 1 | 5 | 1 | 4–8 | 1 | 5 | 1 | 1 | 1 | 1–4 | 1 | |
| Success rate (%) | 91 ϕ | 37 θ 19 ғ | 73 c 37 з | 92 ɱ 87 ϕ | 92 θ | 88 c 75 c | 57 ғ | 89 ℧ 44 з | 74 θ | … | 78 θ | 92 ɱ 86 ϕ | 91 θ | 92 θ | 91 ϕ | 80 ϕ 70 ϕ | |
| Sex |  |  |  | … | … | … |  | … | … |  | … | … | … | … |  | … | |
| Age |  |  |  | … | … | … |  | … |  |  | … | … | … | … |  | … | |
| Periodontal status | … | … |  | … | … | … |  |  | … |  | … | … | … | … | … | … | |
| Type of teeth |  |  | … | … | … | … |  | … | … |  |  |  |  |  |  |  | |
| Lesion type | … | … | … | … | … | … | … |  | … | … | … | … | … | … | … | … | |
| Previous pain and symptoms | … |  |  | … | … | … | … | … | … |  | … | … | … | … |  | … | |
| Post-surgical pain and symptoms | … | … |  | … | … | … | … | … | … | … | … | … | … | … | … | … | |
| Endodontic status | … |  |  | … | … | … |  |  |  |  | … | … | … | … |  | … | |
| Post | … |  |  | … |  | … | … | … | … | … | … | … |  |  |  | … | |
| Lesion size | … |  |  | … | … | … | … | … |  |  | … | … | … | … |  | … | |
| Type of surgery | … |  | … | … | … | … | … | … |  |  |  | … | … | … | … | … | |
| Retrograde cavity preparation | … |  | … | … | … | … | … | … | … |  | … | … |  | … |  |  | |
| Filling material | … |  |  |  | … | … | … |  | … |  | … |  | … | … | … | … | |
| Surgeon’s experience | … |  | … | … | … | … | … | … | … |  | … | … | … | … | … | … | |
| New technologies | … | … | … | … | … |  | … | … | … | … | … | … | … |  |  | … | |
| Crown sealing | … |  | … | … | … | … | … | … |  |  | … | … | … | … |  | … | |
| Wälivaara 2007 [27] | von Arx 2007 [12] | Taschieri 2007 [28] | Peñarrocha 2007 [4] | Kim 2008 [29] | Saunders 2008 [30] | Taschieri 2008 [25] | García 2008 [31] | Christiansen 2009 [32] | Tsesis 2009 [14] | Wälivaara 2009 [27] | Barone 2010 [33] | von Arx 2010 [18] | von Arx 2010 [13] | Song 2011 [15] | Song 2011 [15] | Wälivaara 2011 [27] | |
| Study design | PS | PS | PS | PS | RCT | PS | RCT | PS | RCT | MET | RCT | PS | PS | MET | RS | PS | RCT |
| Initial sample Final sample | 56 55 | 194 191 | 30 27 | 363 363 | 263 188 | 321 276 | 34 31 | 97 92 | 42 36 | 11* | 160 147 | 261 134 | 353 339 | 38* | 907 491 | 54 42 | 206 194 |
| Follow-up time (years) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | …. | 1 | 4–8 | 1 | …. | 1 | 2 | 1 |
| Success rate (%) | 80 ϕ | 83 ɱ θ c | 93 θ | 74 ғ | 91 ɱ 77 θ | 88 ɱ | 88 θ 57 θ | 75 ғ | 96 ɱ 52 g | 91 | 85 ϕ 90 g | 74 ɱ F θ | 91 ɱ 79 c | … | … | 92 ɱ θ | 91 ϕ 82 θ |
| Sex | … |  | … | … | … | … | … | … | … |  | … | … |  |  |  | … | … |
| Age | … |  | … | … | … | … | … | … | … |  | … |  |  |  |  | … | … |
| Periodontal status | … | … | … | … |  | … | … | … |  | … | … | … | … | … | … | … | … |
| Type of teeth |  |  |  |  | … | … | … |  | … |  |  | … |  |  |  | … |  |
| Lesion type | … | … | … | … |  | … |  | … |  | … | … | … | … | … |  | … |  |
| Previous pain and symptoms | … |  | … | … | … | … | … | … |  | … | … | … | … |  |  | … | … |
| Post-surgical pain and symptoms | … |  | … | … | … | … | … |  | … | … | … | … | … | … | … | … | … |
| Endodontic status | … |  | … |  | … | … | … | … |  | … | … |  | … |  |  | … |  |
| Post | … |  |  | … | … |  | … | … | … | … | … | … |  |  |  | … | … |
| Lesion size | … |  | … |  |  | … | … |  |  | … | … |  | … |  | ... | … |  |
| Type of surgery | … |  | … | … | … |  | … | … | … | … | … |  |  | … |  |  | … |
| Retrograde cavity preparation | … | … | … |  | … | … | … | … |  | … | … |  | … | … | … | … | … |
| Filling material | … |  | … | … | … |  | … | … |  |  |  | … |  | … |  |  |  |
| Surgeon’s experience | … | … | … | … | … | … | … | … | … | … | … | … | … | … |  | … | … |
| New technologies | … | … |  | … | … |  |  | … | … |  | … | … | … |  | … |  | … |
| Crown sealing | … | … | … | … | … | … | … | … |  | … | … |  | … | … |  | … | … |
 : statistically significant (p < 0.05);
: statistically significant (p < 0.05);  : not statistically significant (p > 0.05);
: not statistically significant (p > 0.05);  : close to significant association. RCT: Randomized controlled clinical trial; PS: prospective study; RS: retrospective study; MET: meta-analysis (11* and 38* studies included). Retrograde root-end filling material used: ɱ: MTA; θ: Super-EBA; ϕ: IRM; c: composite; з: ionomer; ғ: amalgam; ℧: compomer; g: gutta-percha.
: close to significant association. RCT: Randomized controlled clinical trial; PS: prospective study; RS: retrospective study; MET: meta-analysis (11* and 38* studies included). Retrograde root-end filling material used: ɱ: MTA; θ: Super-EBA; ϕ: IRM; c: composite; з: ionomer; ғ: amalgam; ℧: compomer; g: gutta-percha.| Author | Study Design | Initial and Final Sample | Follow-Up Time (Months) | Type of Treatment | Guided Tissue Regeneration (GTR) versus Control |
|---|---|---|---|---|---|
| Tobón 2004 [42] | PS | 28 28 | 12 | 1. Conventional. 2. Non-absorbable membrane (Goretex). 3. Non-absorbable membrane (Goretex) + hydroxylapatite (Osteogen). |  (Only between groups 1 and 3) (Only between groups 1 and 3) |
| Marín-Botero 2006 [46] | RCT | 30 30 | 12 | 1. Periosteal graft. 2. Bioabsorbable membrane of poliglactin 910. |  |
| Dominiak 2009 [43] | PS | 106 106 | 6–12 | 1. Control group. 2. Resorbable collagen membrane. 3. Xenogenic bovine material. 4. Xenogenic bovine material + Platelet-rich plasma. |  |
| Tsesis 2011 [47] | MET | 11* | … | … |  |
| Taschieri 2011 [48] | RS | 40 33 | 48 | 1. Guided tissue regeneration + xenogenic bone graft. |  |
| Parmar 2019 [49] | RCT | 40 32 | 12 | 1. Control (without membrane). 2. Collagen membrane (Healiguide). |  |
| Liu 2020 [44] | MET | 11* | … | 1. Control (without membrane). 2. Non-absorbable membrane (e-PTFE). 3. Collagen membrane. 4. Collagen membrane + bovine-derived hydroxyapatite. 5. Autologous platelet concentrates. |  (Only between groups 1 and 4) (Only between groups 1 and 4) |
| Zubizarreta-Macho 2022 [45] | MET | 11* | … | 1. Control. 2. Bone graft. 3. Platelet-enriched plasma. 4. Membrane. 5. Membrane + bone graft. 6. Membrane + platelet-enriched plasma. |  |
| Johri 2022 [50] | RCT | 34 | 6 | 1. Amniotic membrane. 2. Platelet-rich fibrin (PRF). |  |
| Garg 2023 [51] | RCT | 19 | 12 | 1. Platelet-rich fibrin (PRF). 2. Mineralized freeze-dried bone allograft (FDBA). |  |
| Albagle 2023 [52] | RCT | 86 | 12 | 1. Control (without membrane). 2. Resorbable collagen-based bone-filling material. |  |
 : statistically significant (p < 0.05);
: statistically significant (p < 0.05);  : not statistically significant (p > 0.05); RCT: randomized controlled clinical trial; PS: prospective study; RS: retrospective study; MET: meta-analysis (11* studies analyzed in each meta-analysis).
: not statistically significant (p > 0.05); RCT: randomized controlled clinical trial; PS: prospective study; RS: retrospective study; MET: meta-analysis (11* studies analyzed in each meta-analysis). Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiz-Pardo-Pinos, A.J.; Manzano-Moreno, F.J.; Muñoz-Soto, E.; González-Rodríguez, M.P.; Romero-Olid, N.; Olmedo-Gaya, M.V. Analysis of the Prognostic Factors That Influence the Outcome of Periapical Surgery, including Biomimetic Membranes for Tissue Regeneration: A Review. Biomimetics 2024, 9, 258. https://doi.org/10.3390/biomimetics9050258
Saiz-Pardo-Pinos AJ, Manzano-Moreno FJ, Muñoz-Soto E, González-Rodríguez MP, Romero-Olid N, Olmedo-Gaya MV. Analysis of the Prognostic Factors That Influence the Outcome of Periapical Surgery, including Biomimetic Membranes for Tissue Regeneration: A Review. Biomimetics. 2024; 9(5):258. https://doi.org/10.3390/biomimetics9050258
Chicago/Turabian StyleSaiz-Pardo-Pinos, Antonio J., Francisco J. Manzano-Moreno, Esther Muñoz-Soto, María Paloma González-Rodríguez, Nuria Romero-Olid, and María Victoria Olmedo-Gaya. 2024. "Analysis of the Prognostic Factors That Influence the Outcome of Periapical Surgery, including Biomimetic Membranes for Tissue Regeneration: A Review" Biomimetics 9, no. 5: 258. https://doi.org/10.3390/biomimetics9050258
APA StyleSaiz-Pardo-Pinos, A. J., Manzano-Moreno, F. J., Muñoz-Soto, E., González-Rodríguez, M. P., Romero-Olid, N., & Olmedo-Gaya, M. V. (2024). Analysis of the Prognostic Factors That Influence the Outcome of Periapical Surgery, including Biomimetic Membranes for Tissue Regeneration: A Review. Biomimetics, 9(5), 258. https://doi.org/10.3390/biomimetics9050258






