Current Research Status of Respiratory Motion for Thorax and Abdominal Treatment: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
- The paper introduced respiratory compensation and prediction in thoracic and abdominal surgery, including image-guided radiotherapy, thoracocentesis, or respiratory monitoring.
- The paper was available to the authors and was a scientific article written in English.
- The device or method considered was used to solve respiratory motion interference during treatment.
- The device or method was originally intended for use on parts of the body other than the thorax and abdomen.
- The study only evaluated system performance or clinical trials, with a lack of information in terms of design.
3. Results
3.1. Direct-Tracking Methodology
3.1.1. Contact Methods
3.1.2. Non-Contact Methods
3.2. Respiratory Prediction Method Based on Indirect Model
3.2.1. X-ray Imaging
3.2.2. MRI
3.2.3. Ultrasound Imaging
3.2.4. Others
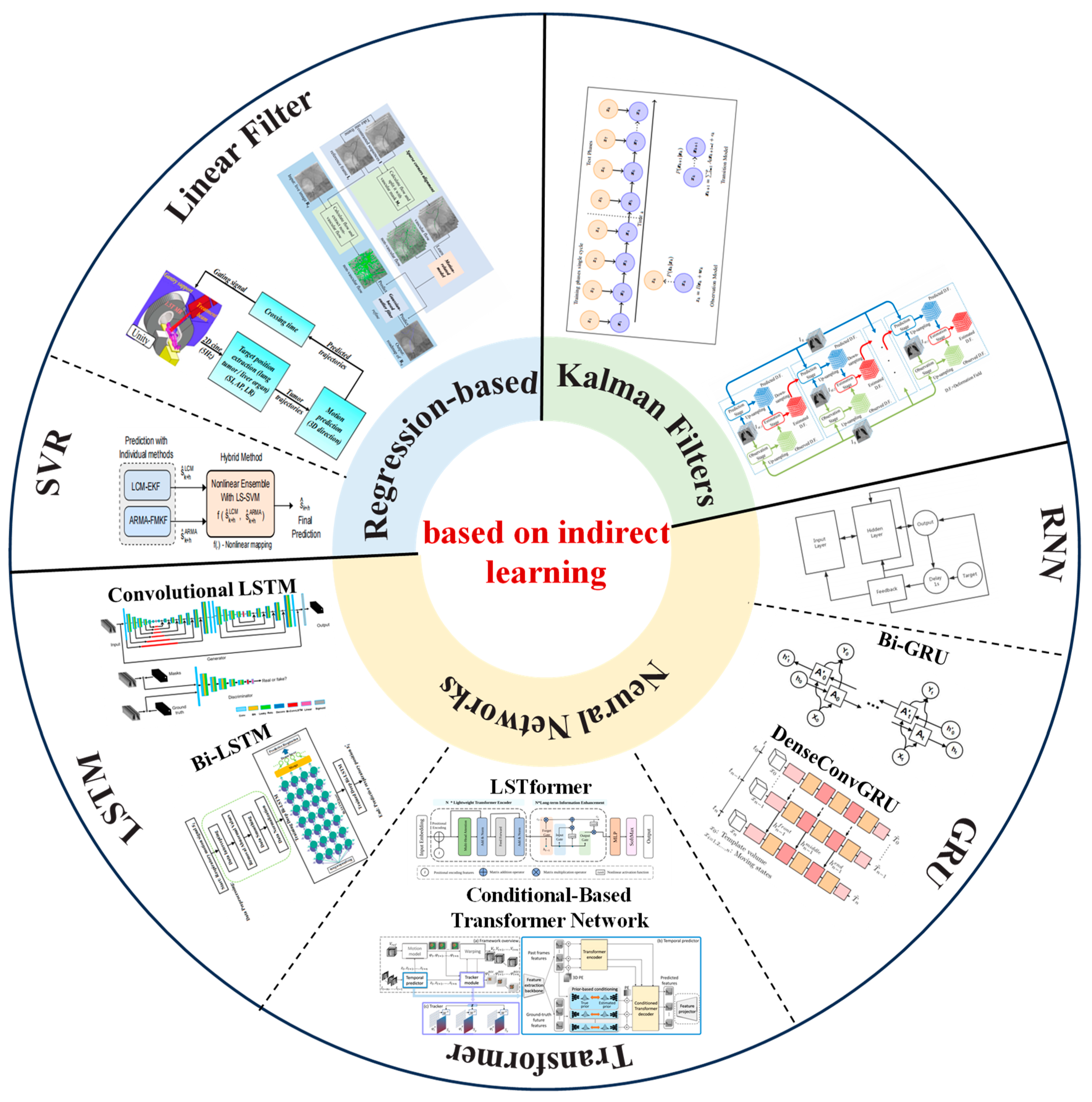
3.3. Respiratory Prediction Method Based on Indirect Learning
3.3.1. Regression-Based Methods
3.3.2. Kalman Filters
4. Discussion
- (1).
- Respiratory movement tracking without markers:
- (2).
- Guidance technology combined with ultrasonic imaging:
- (3).
- Combined deep learning and respiration prediction model construction:
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Dhont, J.; Vandemeulebroucke, J.; Burghelea, M.; Poels, K.; Depuydt, T.; Van Den Begin, R.; Jaudet, C.; Collen, C.; Engels, B.; Reynders, T.; et al. The long- and short-term variability of breathing induced tumor motion in lung and liver over the course of a radiotherapy treatment. Radiother. Oncol. 2018, 126, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.V.; Thomas, R.; Hardcastle, N.; Pham, D.; Kron, T.; Foroudi, F.; Ball, D.; te Marvelde, L.; Bressel, M.; Siva, S. Predictors of respiratory-induced lung tumour motion measured on four-dimensional computed tomography. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gunderman, A.L.; Musa, M.; Gunderman, B.O.; Banovac, F.; Cleary, K.; Yang, X.; Chen, Y. Autonomous Respiratory Motion Compensated Robot for CT-Guided Abdominal Radiofrequency Ablations. IEEE Trans. Med. Robot. Bionics 2023, 5, 206–217. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, L.; Cai, L.; Zhang, W.; Yang, L.; Yang, R. An Uncalibrated and Accurate Robotic Puncture Method Under Respiratory Motion. IEEE Sens. J. 2022, 22, 17266–17274. [Google Scholar] [CrossRef]
- Unberath, M.; Taubmann, O.; Aichert, A.; Achenbach, S.; Maier, A. Prior-Free Respiratory Motion Estimation in Rotational Angiography. IEEE Trans. Med. Imaging 2018, 37, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fan, L.; Wang, T.; Hu, L.; Zhai, G. A Respiratory Monitoring System in Surgical Environment Based on Fiducial Markers Tracking. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 12–14 October 2020; pp. 1–5. [Google Scholar]
- Durichen, R.; Wissel, T.; Ernst, F.; Pimentel, M.A.; Clifton, D.A.; Schweikard, A. A unified approach for respiratory motion prediction and correlation with multi-task Gaussian Processes. In Proceedings of the 2014 IEEE International Workshop on Machine Learning for Signal Processing (MLSP), Reims, France, 21–24 September 2014; pp. 1–6. [Google Scholar]
- Li, B.; Li, P.; Sun, R.; Yu, S.; Liu, H.; Sun, L.; Liu, Y. Respiratory Motion Estimation of Tumor Using Point Clouds of Skin Surface. IEEE Trans. Instrum. Meas. 2023, 72, 4010713. [Google Scholar] [CrossRef]
- Shiinoki, T.; Fujii, F.; Fujimoto, K.; Yuasa, Y.; Sera, T. A novel dynamic robotic moving phantom system for patient-specific quality assurance in real-time tumor-tracking radiotherapy. J. Appl. Clin. Med. Phys. 2020, 21, 16–28. [Google Scholar] [CrossRef]
- Pace, D.F.; Aylward, S.R.; Niethammer, M. A locally adaptive regularization based on anisotropic diffusion for deformable image registration of sliding organs. IEEE Trans. Med. Imaging 2013, 32, 2114–2126. [Google Scholar] [CrossRef]
- Sotiras, A.; Davatzikos, C.; Paragios, N. Deformable medical image registration: A survey. IEEE Trans. Med. Imaging 2013, 32, 1153–1190. [Google Scholar] [CrossRef]
- Li, X.; Dawant, B.M.; Welch, E.B.; Chakravarthy, A.B.; Freehardt, D.; Mayer, I.; Kelley, M.; Meszoely, I.; Gore, J.C.; Yankeelov, T.E. A nonrigid registration algorithm for longitudinal breast MR images and the analysis of breast tumor response. Magn. Reson. Imaging 2009, 27, 1170–1258. [Google Scholar] [CrossRef]
- Clatz, O.; Delingette, H.; Talos, I.F.; Golby, A.J.; Kikinis, R.; Jolesz, F.A.; Ayache, N.; Warfield, S.K. Robust nonrigid registration to capture brain shift from intraoperative MRI. IEEE Trans. Med. Imaging 2005, 24, 1417–1427. [Google Scholar] [CrossRef]
- Riblett, M.J.; Christensen, G.E.; Weiss, E.; Hugo, G.D. Data-driven respiratory motion compensation for four-dimensional cone-beam computed tomography (4D-CBCT) using groupwise deformable registration. Med. Phys. 2018, 45, 4471–4482. [Google Scholar] [CrossRef]
- Hamy, V.; Dikaios, N.; Punwani, S.; Melbourne, A.; Latifoltojar, A.; Makanyanga, J.; Chouhan, M.; Helbren, E.; Menys, A.; Taylor, S.; et al. Respiratory motion correction in dynamic MRI using robust data decomposition registration—Application to DCE-MRI. Med. Image Anal. 2014, 18, 301–313. [Google Scholar] [CrossRef]
- Wan, P.; Chen, F.; Shao, W.; Liu, C.; Zhang, Y.; Wen, B.; Kong, W.; Zhang, D. Irregular Respiratory Motion Compensation for Liver Contrast-Enhanced Ultrasound via Transport-Based Motion Estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Wilbert, J.; Baier, K.; Flentje, M.; Guckenberger, M. Feasibility study for markerless tracking of lung tumors in stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 618–627. [Google Scholar] [CrossRef]
- Andreychenko, A.; Raaijmakers, A.J.E.; Sbrizzi, A.; Crijns, S.P.M.; Lagendijk, J.J.W.; Luijten, P.R.; Van Den Berg, C.A.T. Thermal noise variance of a receive radiofrequency coil as a respiratory motion sensor. Magn. Reson. Med. 2017, 77, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Navest, R.J.; Andreychenko, A.; Lagendijk, J.J.; van den Berg, C.A. Prospective Respiration Detection in Magnetic Resonance Imaging by a Non-Interfering Noise Navigator. IEEE Trans. Med. Imaging 2018, 37, 1751–1760. [Google Scholar] [CrossRef]
- Wijenayake, U.; Park, S.-Y. PCA based analysis of external respiratory motion using an RGB-D camera. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar]
- Musa, M.J.; Sharma, K.; Cleary, K.; Chen, Y. Respiratory Compensated Robot for Liver Cancer Treatment: Design, Fabrication, and Benchtop Characterization. IEEE/ASME Trans. Mechatron. 2022, 27, 268–279. [Google Scholar] [CrossRef]
- Kok, E.N.D.; Eppenga, R.; Kuhlmann, K.F.D.; Groen, H.C.; van Veen, R.; van Dieren, J.M. Accurate surgical navigation with real-time tumor tracking in cancer surgery. npj Precis. Oncol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Addabbo, T.; Cataldo, G.; Cevenini, G.; Fort, A.; Franchi, F.; Moretti, R.; Mugnaini, M.; Scolletta, S.; Vignoli, V. A Measurement System to Estimate the Pleural Pressure from the CVP for Respiratory System Monitoring. IEEE Trans. Instrum. Meas. 2019, 68, 2469–2478. [Google Scholar] [CrossRef]
- Ladjal, H.; Beuve, M.; Giraud, P.; Shariat, B. Towards Non-Invasive Lung Tumor Tracking Based on Patient Specific Model of Respiratory System. IEEE Trans. Biomed. Eng. 2021, 68, 2730–2740. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Formica, D.; Presti, D.L.; Caponero, M.A.; Di Tomaso, G.; Giurazza, F.; Muto, M.; Schena, E. Design and Feasibility Assessment of a Magnetic Resonance-Compatible Smart Textile Based on Fiber Bragg Grating Sensors for Respiratory Monitoring. IEEE Sens. J. 2016, 16, 8103–8110. [Google Scholar] [CrossRef]
- Shi, C.; Tang, Z.; Zhang, H.; Liu, Y. Development of an FBG-based wearable sensor for simultaneous respiration and heartbeat measurement. IEEE Trans. Instrum. Meas. 2023, 72, 1–9. [Google Scholar]
- Gu, C.; Li, R.; Zhang, H.; Fung, A.Y.; Torres, C.; Jiang, S.B.; Li, C. Accurate respiration measurement using DC-coupled continuous-wave radar sensor for motion-adaptive cancer radiotherapy. IEEE Trans. Biomed. Eng. 2012, 59, 3117–3123. [Google Scholar] [PubMed]
- Lim, S.H.; Golkar, E.; Abd, A.A. Rahni, Respiratory motion tracking using the kinect camera. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 797–800. [Google Scholar]
- Fielding, A.L.; Pandey, A.K.; Jonmohamadi, Y.; Via, R.; Weber, D.C.; Lomax, A.J.; Fattori, G. Preliminary Study of the Intel RealSense D415 Camera for Monitoring Respiratory Like Motion of an Irregular Surface. IEEE Sens. J. 2021, 21, 14443–14453. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, H.; Yang, L.; Lao, Y.; Lin, Q.; Yang, R. A Novel Respiratory Follow-Up Robotic System for Thoracic-Abdominal Puncture. IEEE Trans. Ind. Electron. 2021, 68, 2368–2378. [Google Scholar] [CrossRef]
- Vijayan, S.; Klein, S.; Hofstad, E.F.; Lindseth, F.; Ystgaard, B.; Langø, T. Motion tracking in the liver: Validation of a method based on 4D ultrasound using a nonrigid registration technique. Med. Phys. 2014, 41, 082903. [Google Scholar] [CrossRef] [PubMed]
- Royer, L.; Marchal, M.; Le Bras, A.; Dardenne, G.; Krupa, A. Real-time tracking of deformable target in 3D ultrasound images. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 2430–2435. [Google Scholar]
- Schlosser, J.; Gong, R.H.; Bruder, R.; Schweikard, A.; Jang, S.; Henrie, J.; Kamaya, A.; Koong, A.; Chang, D.T.; Hristov, D. Robotic intrafractional US guidance for liver SABR: System design, beam avoidance, and clinical imaging. Med. Phys. 2016, 43, 5951. [Google Scholar] [CrossRef]
- Thomas, G.P.L.; Khokhlova, T.D.; Khokhlova, V.A. Partial Respiratory Motion Compensation for Abdominal Extracorporeal Boiling Histotripsy Treatments with a Robotic Arm. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2861–2870. [Google Scholar] [CrossRef]
- Seppenwoolde, Y.; Berbeco, R.I.; Nishioka, S.; Shirato, H.; Heijmen, B. Accuracy of tumor motion compensation algorithm from a robotic respiratory tracking system: A simulation study. Med. Phys. 2007, 34, 2774–2784. [Google Scholar] [CrossRef]
- Willoughby, T.R.; Kupelian, P.A.; Pouliot, J.; Shinohara, K.; Aubin, M.; Roach, M., III; Skrumeda, L.L.; Balter, J.M.; Litzenberg, D.W.; Hadley, S.W.; et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Yin, F.F.; Tenn, S.E.; Medin, P.M.; Solberg, T.D. Use of the BrainLAB ExacTrac X-ray 6D system in image-guided radiotherapy. Med. Dosim. 2008, 33, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Kresl, J.J.; Luketich, J.D.; Papiez, L.; Schulz, R.A.; Timmerman, R.D. (Eds.) Robotic Radiosurgery. Treating Tumors that Move with Respiration; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Wijenayake, U.; Park, S.-Y. Real-Time External Respiratory Motion Measuring Technique Using an RGB-D Camera and Principal Component Analysis. Sensors 2017, 17, 1840. [Google Scholar] [CrossRef]
- Vedam, S.S.; Kini, V.R.; Keall, P.J.; Ramakrishnan, V.; Mostafavi, H.; Mohan, R. Quantifying the predictability of diaphragm motion during respiration with a noninvasive external marker. Med. Phys. 2003, 30, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Goligher, E.C.; McAuley, D.F.; Rubenfeld, G.D.; Brochard, L.J.; Gattinoni, L.; Slutsky, A.S.; Fan, E. Mechanical Ventilation in Adults with Acute Respiratory Distress Syndrome. Summary of the Experimental Evidence for the Clinical Practice Guideline. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S4), S261–S270. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Bates, J.H. Variable Ventilation as a Diagnostic Tool for the Injured Lung. IEEE Trans. Biomed. Eng. 2015, 62, 2106–2113. [Google Scholar] [CrossRef]
- Lu, C.; Philips, N.; Chen, L. Monitoring respiratory mechanics. ERS Pract. Handb. Invasive Mech. Vent. 2019, 111. [Google Scholar]
- Vicario, F.; Albanese, A.; Karamolegkos, N.; Wang, D.; Seiver, A.; Chbat, N.W. Noninvasive Estimation of Respiratory Mechanics in Spontaneously Breathing Ventilated Patients: A Constrained Optimization Approach. IEEE Trans. Biomed. Eng. 2016, 63, 775–787. [Google Scholar] [CrossRef]
- De Tommasi, F.; Massaroni, C.; Carnevale, A.; Presti, D.L.; De Vita, E.; Iadicicco, A.; Faiella, E.; Grasso, R.F.; Longo, U.G.; Campopiano, S.; et al. Fiber Bragg Grating Sensors for Temperature Monitoring During Thermal Ablation Procedure: Experimental Assessment of Artefact Caused by Respiratory Movements. IEEE Sens. J. 2021, 21, 13342–13349. [Google Scholar] [CrossRef]
- Laurent, F.; Latrabe, V.; Vergier, B.; Montaudon, M.; Vernejoux, J.M.; Dubrez, J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: Results with an automated 20-gauge coaxial cutting needle. Clin. Radiol. 2000, 55, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, C.; Li, R.; Jiang, S.B. Radar motion sensing for accurate tumor tracking in radiation therapy. In Proceedings of the WAMICON 2011 Conference Proceedings, Clearwater Beach, FL, USA, 18–19 April 2011; pp. 1–6. [Google Scholar]
- Hou, P.; Sun, R.; Yu, S.; Kuang, S.; Sun, L. Correlation between Thoracic-abdominal Surface and Tumor Motion based on 3D Point Cloud: A Preliminary Study. In Proceedings of the 2018 IEEE International Conference on Information and Automation (ICIA), Wuyishan, China, 11–13 August 2018; p. 796-081. [Google Scholar]
- Liu, M.; Wei, X.; Ding, Y.; Cheng, C.; Yin, W.; Chen, J.; Wang, K.; Gu, W. Application of Optical Laser 3D Surface imaging system (Sentinel) in breast cancer radiotherapy. Sci. Rep. 2020, 10, 7550. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.B.; Brandner, E.; Selvaraj, R.; Chen, H.; Huq, M.S.; Heron, D.E. A review on the clinical implementation of respiratory-gated radiation therapy. Biomed. Imaging Interv. J. 2007, 3, e40. [Google Scholar] [CrossRef] [PubMed]
- Underberg, R.W.; Lagerwaard, F.J.; Slotman, B.J.; Cuijpers, J.P.; Senan, S. Benefit of respiration-gated stereotactic radiotherapy for stage I lung cancer: An analysis of 4DCT datasets. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 554–560. [Google Scholar] [CrossRef]
- McNair, H.A.; Brock, J.; Symonds-Tayler, J.R.N.; Ashley, S.; Eagle, S.; Evans, P.M.; Kavanagh, A.; Panakis, N.; Brada, M. Feasibility of the use of the Active Breathing Co ordinator (ABC) in patients receiving radical radiotherapy for non-small cell lung cancer (NSCLC). Radiother. Oncol. 2009, 93, 424–429. [Google Scholar] [CrossRef]
- Kitamura, K.; Shirato, H.; Seppenwoolde, Y.; Onimaru, R.; Oda, M.; Fujita, K.; Shimizu, S.; Shinohara, N.; Harabayashi, T.; Miyasaka, K. Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int. J. Radiat. Oncol.*Biol.*Phys. 2002, 53, 1117–1123. [Google Scholar] [CrossRef]
- Sakakibara-Konishi, J.; Oizumi, S.; Kinoshita, I.; Shinagawa, N.; Kikuchi, J.; Kato, M.; Inoue, T.; Katoh, N.; Onimaru, R.; Shirato, H.; et al. Phase I study of concurrent real-time tumor-tracking thoracic radiation therapy with paclitaxel and carboplatin in locally advanced non-small cell lung cancer. Lung Cancer 2011, 74, 248–252. [Google Scholar] [CrossRef]
- Dietrich, L.; Tücking, T.; Nill, S.; Oelfke, U. Compensation for respiratory motion by gated radiotherapy: An experimental study. Phys. Med. Biol. 2005, 50, 2405–2414. [Google Scholar] [CrossRef]
- Steidl, P.; Richter, D.; Schuy, C.; Schubert, E.; Haberer, T.; Durante, M.; Bert, C. A breathing thorax phantom with independently programmable 6D tumour motion for dosimetric measurements in radiation therapy. Phys. Med. Biol. 2012, 57, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Biederer, J.; Heller, M. Artificial thorax for MR imaging studies in porcine heart-lung preparations. Radiology 2003, 226, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Henry, R.W.; Bouley, D.M.; Bennett, N.R.; Fahrig, R. Characterization of a novel anthropomorphic plastinated lung phantom. Med. Phys. 2008, 35, 5934–5943. [Google Scholar] [CrossRef]
- Segars, W.P.; Mahesh, M.; Beck, T.J.; Frey, E.C.; Tsui, B.M. Realistic CT simulation using the 4D XCAT phantom. Med. Phys. 2008, 35, 3800–3808. [Google Scholar] [CrossRef]
- Segars, W.P.; Sturgeon, G.; Mendonca, S.; Grimes, J.; Tsui, B.M. 4D XCAT phantom for multimodality imaging research. Med. Phys. 2010, 37, 4902–4915. [Google Scholar] [CrossRef]
- Lee, C.; Lodwick, D.; Hasenauer, D.; Williams, J.L.; Lee, C.; Bolch, W.E. Hybrid computational phantoms of the male and female newborn patient: NURBS-based whole-body models. Phys. Med. Biol. 2007, 52, 3309–3333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Teng, C.; Kumar, S.; Li, X.; Min, R. Wearable Cardiorespiratory Sensor for Real-Time Monitoring with Smartphone Integration. IEEE Trans. Instrum. Meas. 2024, 73, 7000710. [Google Scholar] [CrossRef]
- Lamare, F.; Cresson, T.; Savean, J.; Le Rest, C.C.; Reader, A.J.; Visvikis, D. Respiratory motion correction for PET oncology applications using affine transformation of list mode data. Phys. Med. Biol. 2007, 52, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Mexner, V.; Wolthaus, J.W.; van Herk, M.; Damen, E.M.; Sonke, J.J. Effects of respiration-induced density variations on dose distributions in radiotherapy of lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1266–1275. [Google Scholar] [CrossRef]
- Mexner, V.; Wolthaus, J.W.; van Herk, M.; Damen, E.M.; Sonke, J.J. Dosimetric impact of motion in free-breathing and gated lung radiotherapy: A 4D Monte Carlo study of intrafraction and interfraction effects. Med. Phys. 2008, 35, 356–366. [Google Scholar]
- Cai, J.; Zhang, Y.; Vergalasova, I.; Zhang, F.; Segars, W.P.; Yin, F.F. An Integrated Simulation System Based on Digital Human Phantom for 4D Radiation Therapy of Lung Cancer. J. Cancer Ther. 2014, 05, 749–758. [Google Scholar] [CrossRef]
- Al-Mayah, A.; Moseley, J.; Velec, M.; Brock, K. Toward efficient biomechanical-based deformable image registration of lungs for image-guided radiotherapy. Phys. Med. Biol. 2011, 56, 4701–4713. [Google Scholar] [CrossRef]
- Bukovsky, I.; Homma, N.; Ichiji, K.; Cejnek, M.; Slama, M.; Benes, P.M.; Bila, J. A fast neural network approach to predict lung tumor motion during respiration for radiation therapy applications. BioMed Res. Int. 2015, 2015, 489679. [Google Scholar] [CrossRef]
- Fischer, P.; Pohl, T.; Faranesh, A.; Maier, A.; Hornegger, J. Unsupervised Learning for Robust Respiratory Signal Estimation from X-ray Fluoroscopy. IEEE Trans. Med. Imaging 2017, 36, 865–877. [Google Scholar] [CrossRef]
- Leni, P.E.; Laurent, R.; Salomon, M.; Gschwind, R.; Makovicka, L.; Henriet, J. Development of a 4D numerical chest phantom with customizable breathing. Phys. Med. 2016, 32, 795–800. [Google Scholar] [CrossRef]
- Qi, W.; Yang, Y.; Song, C.; Wernick, M.N.; Pretorius, P.H.; King, M.A. 4-D Reconstruction with Respiratory Correction for Gated Myocardial Perfusion SPECT. IEEE Trans. Med. Imaging 2017, 36, 1626–1635. [Google Scholar] [CrossRef]
- Chan, C.; Onofrey, J.; Jian, Y.; Germino, M.; Papademetris, X.; Carson, R.E.; Liu, C. Non-Rigid Event-by-Event Continuous Respiratory Motion Compensated List-Mode Reconstruction for PET. IEEE Trans. Med. Imaging 2018, 37, 504–515. [Google Scholar] [CrossRef]
- Rühaak, J.; Polzin, T.; Heldmann, S.; Simpson, I.J.; Handels, H.; Modersitzki, J.; Heinrich, M.P. Estimation of Large Motion in Lung CT by Integrating Regularized Keypoint Correspondences into Dense Deformable Registration. IEEE Trans. Med. Imaging 2017, 36, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiang, Z.; Lian, Z.; Xiao, L.; Zhang, J.; Wei, Z. Prediction of Lung Motion from Four-Dimensional Computer Tomography (4DCT) Images Using Bayesian Registration and Trajectory Modelling. IEEE Access 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Bao, X.; Gao, W.; Xiao, D.; Wang, J.; Jia, F. Bayesian model-based liver respiration motion prediction and evaluation using single-cycle and double-cycle 4D CT images. In Proceedings of the 2019 International Conference on Medical Imaging Physics and Engineering (ICMIPE), Shenzhen, China, 22–24 November 2019; pp. 1–6. [Google Scholar]
- Gong, L.; Duan, L.; Dai, Y.; He, Q.; Zuo, S.; Fu, T.; Yang, X.; Zheng, J. Locally Adaptive Total p-Variation Regularization for Non-Rigid Image Registration with Sliding Motion. IEEE Trans. Biomed. Eng. 2020, 67, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Emond, E.C.; Bousse, A.; Brusaferri, L.; Hutton, B.F.; Thielemans, K. Improved PET/CT Respiratory Motion Compensation by Incorporating Changes in Lung Density. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 594–602. [Google Scholar] [CrossRef]
- Chen, B.; Weber, N.; Odille, F.; Large-Dessale, C.; Delmas, A.; Bonnemains, L.; Felblinger, J. Design and Validation of a Novel MR-Compatible Sensor for Respiratory Motion Modeling and Correction. IEEE Trans. Biomed. Eng. 2017, 64, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, W.; Yang, C.; Wang, P. An Expanded Multi-scale Monte Carlo Simulation Method for Personalized Radiobiological Effect Estimation in Radiotherapy: A feasibility study. Sci. Rep. 2017, 7, 45019. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Faranesh, A.; Pohl, T.; Maier, A.; Rogers, T.; Ratnayaka, K.; Lederman, R.; Hornegger, J. An MR-Based Model for Cardio-Respiratory Motion Compensation of Overlays in X-ray Fluoroscopy. IEEE Trans. Med. Imaging 2018, 37, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Scannell, C.M.; Villa, A.D.; Lee, J.; Breeuwer, M.; Chiribiri, A. Robust Non-Rigid Motion Compensation of Free-Breathing Myocardial Perfusion MRI Data. IEEE Trans. Med. Imaging 2019, 38, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Huttinga, N.R.; Van den Berg, C.A.; Luijten, P.R.; Sbrizzi, A. MR-MOTUS: Model-based non-rigid motion estimation for MR-guided radiotherapy using a reference image and minimal k-space data. Phys. Med. Biol. 2020, 65, 015004. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Aigner, C.S.; Kolbitsch, C.; Mayer, J.; Ludwig, J.; Schmidt, S.; Schaeffter, T.; Schmitter, S. 3D Free-breathing multichannel absolute B1+ Mapping in the human body at 7T. Magn. Reason. Med. 2021, 85, 2552–2567. [Google Scholar] [CrossRef] [PubMed]
- Tretbar, S.H.; Hewener, H.J.; Speicher, D.; Barthscherer, T.; Bongers, A.; Jenne, J.W.; Günther, M. MR-compatible ultrasound research platform for motion tracking to reduce motion induced artifacts in MR imaging. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 553–556. [Google Scholar]
- von Siebenthal, M.; Székely, G.; Lomax, A.; Cattin, P.C. Inter-subject modelling of liver deformation during radiation therapy. Med. Image Comput. Comput. Assist. Interv. 2007, 10 Pt 1, 659–666. [Google Scholar]
- Arnold, P.; Preiswerk, F.; Fasel, B.; Salomir, R.; Scheffler, K.; Cattin, P.C. 3D organ motion prediction for MR-guided high intensity focused ultrasound. Med. Image Comput. Comput. Assist. Interv. 2011, 14 Pt 2, 623–630. [Google Scholar]
- Zhang, J.; Liu, T.; Wang, Y.; Jiang, W.; Yang, K.; Jin, H.; Zhu, Y. Self-Adaptive Ultrasound Scanning System for Imaging Human Spine. IEEE Trans. Ind. Electron. 2022, 69, 570–581. [Google Scholar] [CrossRef]
- Ha, I.Y.; Wilms, M.; Handels, H.; Heinrich, M.P. Model-Based Sparse-to-Dense Image Registration for Realtime Respiratory Motion Estimation in Image-Guided Interventions. IEEE Trans. Biomed. Eng. 2019, 66, 302–310. [Google Scholar] [CrossRef]
- Bengs, M.; Sprenger, J.; Gerlach, S.; Neidhardt, M.; Schlaefer, A. Real-Time Motion Analysis with 4D Deep Learning for Ultrasound-Guided Radiotherapy. IEEE Trans. Biomed. Eng. 2023, 70, 2690–2699. [Google Scholar] [CrossRef]
- Yu, S.; Hou, P.; Sun, R.; Kuang, S.; Zhang, F.; Zhou, M.; Guo, J.; Sun, L. Correlated Skin Surface and Tumor Motion Modeling for Treatment Planning in Robotic Radiosurgery. Front. Neurorobot. 2020, 14, 582385. [Google Scholar] [CrossRef]
- Wang, J.; Sun, R.; Yu, S.; Zhang, F.; Lining, S. An Improved Correlation Model for Respiration Tracking in Robotic Radiosurgery Using Essential Skin Surface Motion. IEEE Robot. Autom. Lett. 2021, 6, 7885–7892. [Google Scholar] [CrossRef]
- Shi, Y.; Deng, X.; Tong, Y.; Li, R.; Zhang, Y.; Ren, L.; Si, W. Synergistic Digital Twin and Holographic Augmented-Reality-Guided Percutaneous Puncture of Respiratory Liver Tumor. IEEE Trans. Hum.-Mach. Syst. 2022, 52, 1364–1374. [Google Scholar] [CrossRef]
- Shimizu, S.; Shirato, H.; Kitamura, K.; Shinohara, N.; Harabayashi, T.; Tsukamoto, T.; Koyanagi, T.; Miyasaka, K. Use of an implanted marker and real-time tracking of the marker for the positioning of prostate and bladder cancers. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.C.; Jiang, S.B.; Shimizu, S.; Shirato, H. Prediction of respiratory tumour motion for real-time image-guided radiotherapy. Phys. Med. Biol. 2004, 49, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, J.; Liu, J.; Sun, R.; Kuang, S.; Sun, L. Rapid Prediction of Respiratory Motion Based on Bidirectional Gated Recurrent Unit Network. IEEE Access 2020, 8, 49424–49435. [Google Scholar] [CrossRef]
- Ernst, F.; Schweikard, A. Forecasting respiratory motion with accurate online support vector regression (SVRpred). Int J Comput Assist Radiol Surg 2009, 4, 439–447. [Google Scholar] [CrossRef]
- Tatinati, S.; Veluvolu, K.C.; Hong, S.M.; Nazarpour, K. Real-time prediction of respiratory motion traces for radiotherapy with ensemble learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 4204–4207. [Google Scholar]
- Kashibe, N.; Fujii, F.; Shiinoki, T.; Shibuya, K. Construction of a respiratory-induced lung tumor motion model using phase oscillator. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 699–704. [Google Scholar]
- Li, Y.; Li, Z.; Zhu, J.; Li, B.; Shu, H.; Ge, D. Online prediction for respiratory movement compensation: A patient-specific gating control for MRI-guided radiotherapy. Radiat. Oncol. 2023, 18, 149. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Z.; Li, M.; Cao, T.; Ghaffari, M.; Song, J. Optical Flow-Based Vascular Respiratory Motion Compensation. IEEE Robot. Autom. Lett. 2023, 8, 6987–6994. [Google Scholar] [CrossRef]
- Smith, R.L.; Abd Rahni, A.A.; Jones, J. A Kalman-Based Approach with EM Optimization for Respiratory Motion Modeling in Medical Imaging. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 410–420. [Google Scholar] [CrossRef]
- Xue, P.; Fu, Y.; Ji, H.; Cui, W.; Dong, E. Lung Respiratory Motion Estimation Based on Fast Kalman Filtering and 4D CT Image Registration. IEEE J. Biomed. Health Inform. 2021, 25, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Frueh, M.; Schilling, A.; Gatidis, S.; Kuestner, T. Real Time Landmark Detection for within- and Cross Subject Tracking with Minimal Human Supervision. IEEE Access 2022, 10, 81192–81202. [Google Scholar] [CrossRef]
- Sun, W.Z.; Jiang, M.Y.; Ren, L.; Dang, J.; You, T.; Yin, F.F. Respiratory signal prediction based on adaptive boosting and multi-layer perceptron neural network. Phys. Med. Biol. 2017, 62, 6822–6835. [Google Scholar] [CrossRef]
- Kai, J.; Fujii, F.; Shiinoki, T. Prediction of Lung Tumor Motion Based on Recurrent Neural Network. In Proceedings of the 2018 IEEE International Conference on Mechatronics and Automation (ICMA), Changchun, China, 5–8 August 2018; pp. 1093–1099. [Google Scholar]
- Zhao, H.; Deng, L.; Xie, Y. A Training Strategy for Enhancing the Accuracy of Real-Time Tumor Tracking Based on Deep Bi-LSTM Learning. In Proceedings of the 2019 International Conference on Medical Imaging Physics and Engineering (ICMIPE), Shenzhen, China, 22–24 November 2019; pp. 1–4. [Google Scholar]
- Wang, R.; Liang, X.; Zhu, X.; Xie, Y. A Feasibility of Respiration Prediction Based on Deep Bi-LSTM for Real-Time Tumor Tracking. IEEE Access 2018, 6, 51262–51268. [Google Scholar] [CrossRef]
- Romaguera, L.V.; Mezheritsky, T.; Mansour, R.; Tanguay, W.; Kadoury, S. Predictive online 3D target tracking with population-based generative networks for image-guided radiotherapy. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Peng, H.; Liang, X.; Xie, Y.; Xia, Z.; Xiong, J. LSTformer: Long Short-Term Transformer for Real Time Respiratory Prediction. IEEE J. Biomed. Health Inform. 2022, 26, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Jiang, Z.; Chang, Y.; Ren, L. Real-time Markerless Tracking of Lung Tumors based on 2-D Fluoroscopy Imaging using Convolutional LSTM. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, L.V.; Alley, S.; Carrier, J.F.; Kadoury, S. Conditional-Based Transformer Network with Learnable Queries for 4D Deformation Forecasting and Tracking. IEEE Trans. Med. Imaging 2023, 42, 1603–1618. [Google Scholar] [CrossRef]
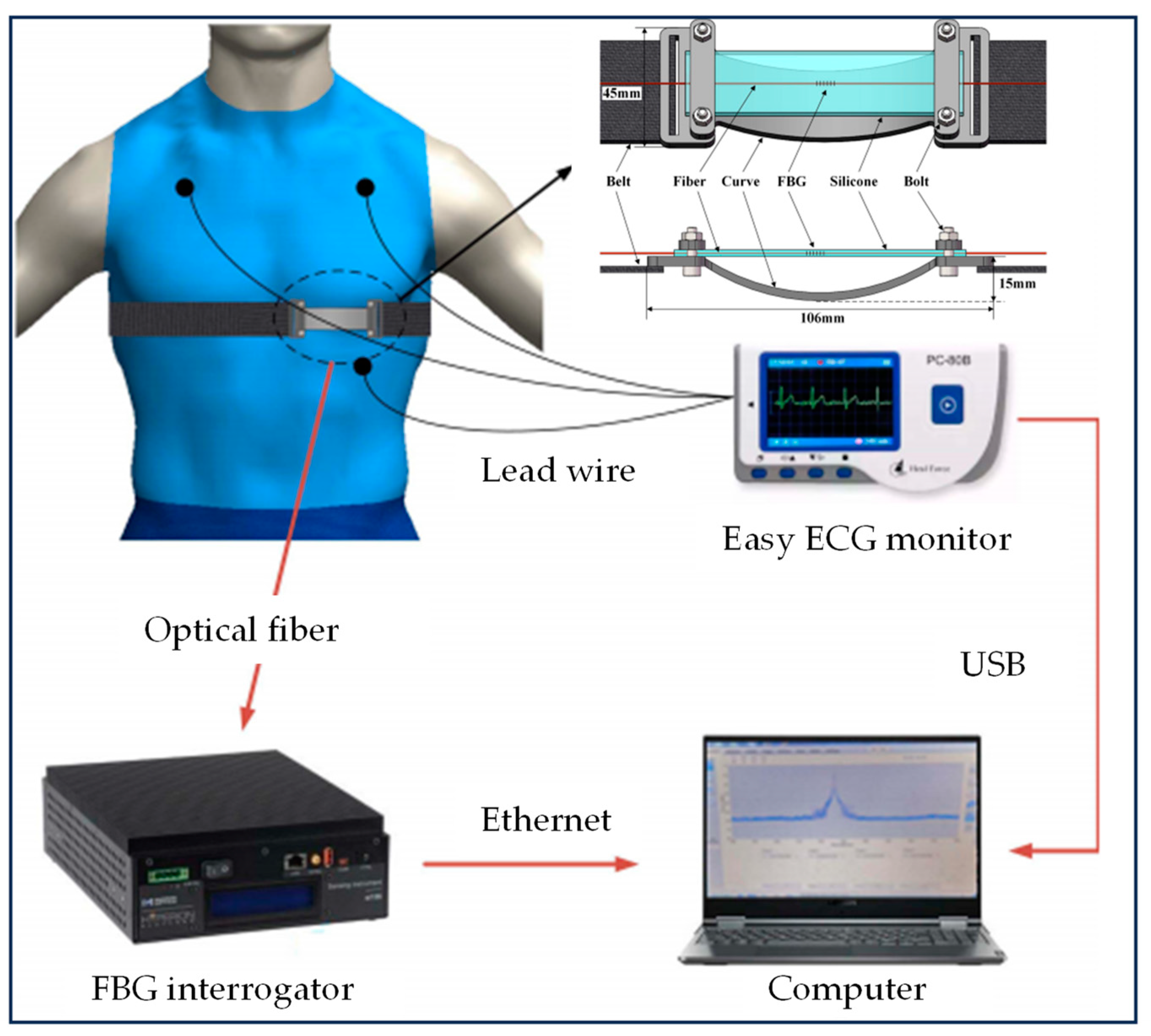
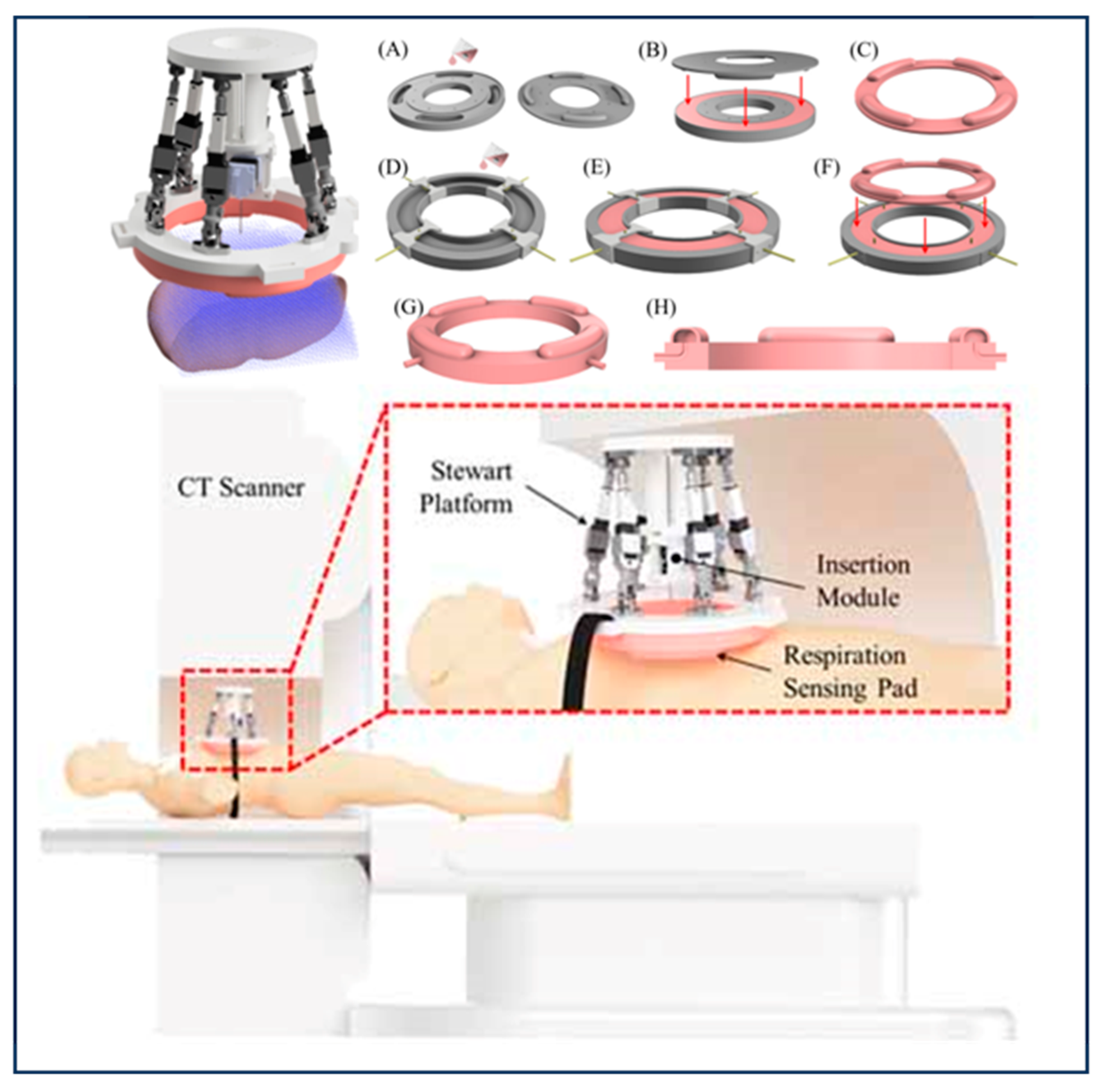
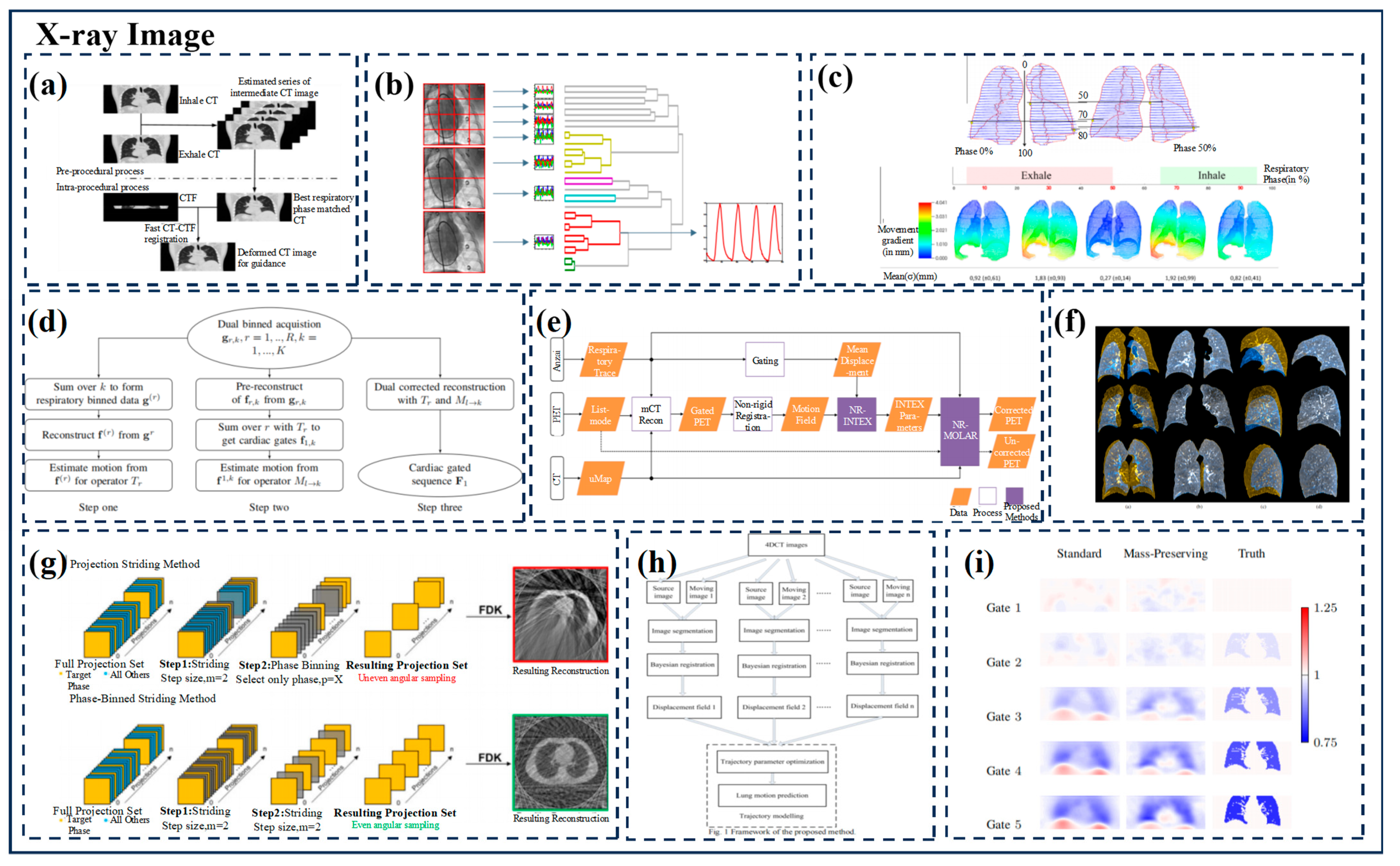
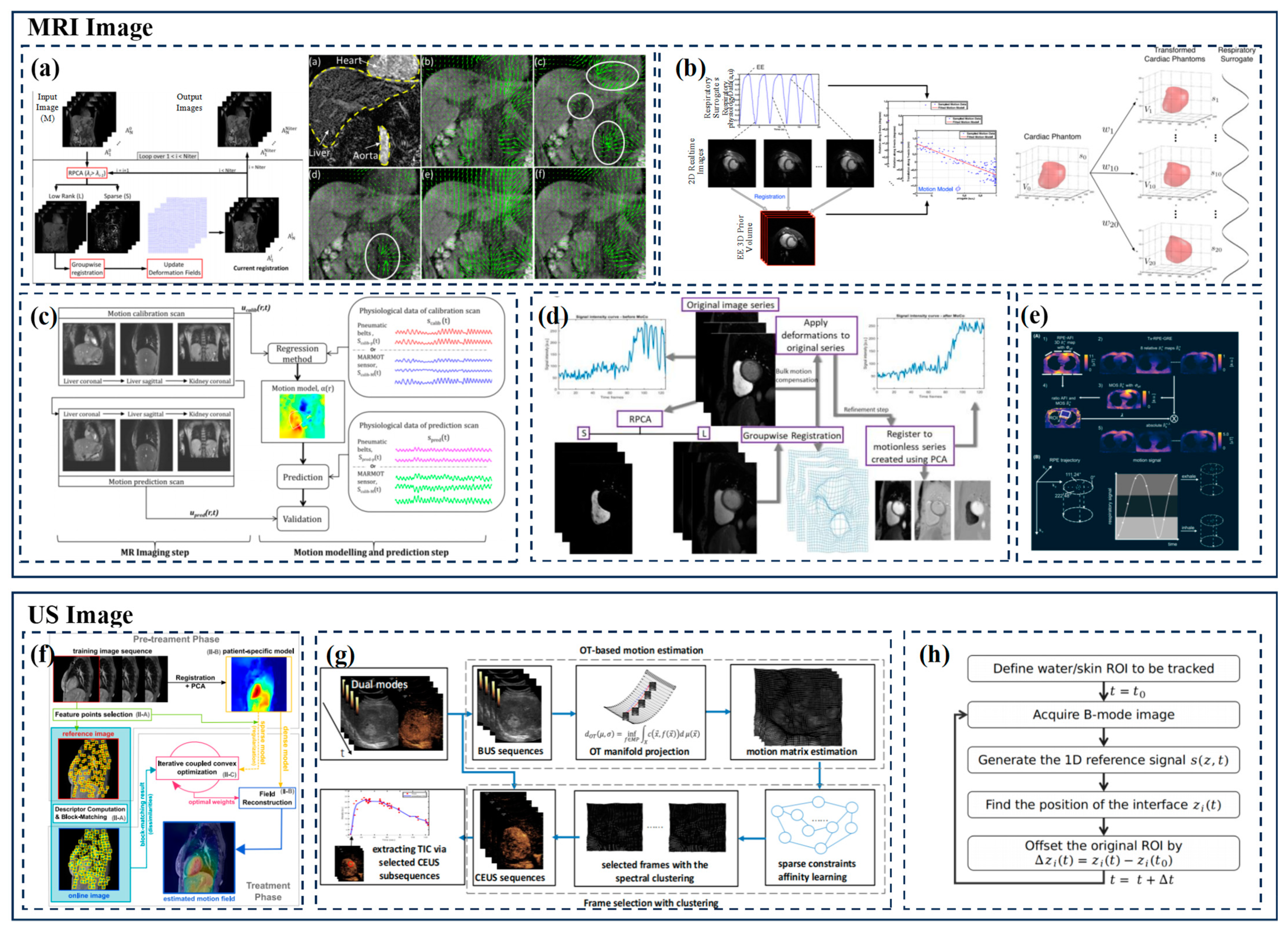
| Group | Tracking Strategy | Representative Works | Characteristics |
|---|---|---|---|
| Contact | Noise sensor: noise variance based on RF coil | A. Andreychenko et al. [20], J. M. Navest et al. [21] | (1) No need for careful positioning or any additional hardware; (2) Combined with Kalman filtering, respiratory signals can be extracted and predicted without delay; (3) Can measure breathing passively, independent of MR signal; (4) Some limitations in temporal resolution and spatial resolution. |
| RGB-D camera with markers | U. W and S. P. et al. [22], Y. Yu et al. [8], M. Musa et al. [23] | (1) The system setup is very simple, very flexible, and portable; (2) Will not interfere with the patient‘s breathing, non-invasive benchmark marking, shortens the treatment time, and high safety; (3) The surface positioning accuracy is high, which can reach the millimeter level; (4) The performance is easily disturbed by factors such as light, background, and occlusion; (5) The camera needs the right position and angle. | |
| Electromagnetic sensor | Esther N. D. Kok et al. [24] | (1) Real-time and accurate tumor location information and key anatomic information can be obtained, which may reduce the occurrence of positive resection margins and improve the patient prognosis; (2) High tracking accuracy for targets in vivo; (3) Susceptible to electromagnetic interference, not suitable for MR. | |
| Pressure sensor | T. Addabbo et al. [25], H. L. et al. [26], Anthony L. et al. [5] | (1) Other invasive devices can be avoided; (2) It has the potential to be applied in 4D dose calculation to remove respiratory motion artifacts in positron emission tomography (PET) or γ scintillation image reconstruction; (3) The measurement accuracy is relatively high; (4) Accuracy is affected by its installation location; (5) Some patients may not be able to adapt to the pressure of the sensor; (6) Prolonged use may cause performance degradation or damage. | |
| Fiber Bragg grating sensors | C. M. et al. [27], C. Shi et al. [28] | (1) Comfortable and easy to wear, will not cause discomfort to the wearer; (2) Can be used in an MR environment; (3) No image artifacts are generated; (4) It has high sensitivity and enables simultaneous and accurate measurement of respiratory and cardiac activity; (5) Installation and maintenance are complicated; (6) High cost compared with some other sensors; (7) Sensitive to environmental conditions. | |
| Non-contact | DC coupled CW radar sensor | C. Gu et al. [29] | (1) Non-contact and non-invasive; (2) Can accurately measure the movement, where the measurement accuracy can reach sub-millimeter level; (3) It has great potential in adaptive radiotherapy; (4) Relatively complex system; (5) High cost compared with some other sensors. |
| RGB-D camera without markers | Shi H. Lim, P. Hou et al. [30], Andrew L. Fielding et al. [31], L. Zheng et al. [32] | (1) The system setting is very simple, very flexible, and portable; (2) Will not interfere with the patient’s breathing, non-contact and non-invasive, shortens the treatment time, and high safety factor; (3) The accuracy of surface positioning is higher, but may be lower than that of a system with markers. | |
| Directly image-guided | S. Vijayan et al. [33], L. R. et al. [34], J. S. et al. [35], Gilles P.L. et al. [36] | (1) The system has high detection accuracy and good applicability and can track the internal target movement in real time; (2) May cause unnecessary radiation to patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Z.; Chu, Y.; Peng, R.; Peng, H.; Yang, H.; Guo, K.; Zhang, J. Current Research Status of Respiratory Motion for Thorax and Abdominal Treatment: A Systematic Review. Biomimetics 2024, 9, 170. https://doi.org/10.3390/biomimetics9030170
Wu Y, Wang Z, Chu Y, Peng R, Peng H, Yang H, Guo K, Zhang J. Current Research Status of Respiratory Motion for Thorax and Abdominal Treatment: A Systematic Review. Biomimetics. 2024; 9(3):170. https://doi.org/10.3390/biomimetics9030170
Chicago/Turabian StyleWu, Yuwen, Zhisen Wang, Yuyi Chu, Renyuan Peng, Haoran Peng, Hongbo Yang, Kai Guo, and Juzhong Zhang. 2024. "Current Research Status of Respiratory Motion for Thorax and Abdominal Treatment: A Systematic Review" Biomimetics 9, no. 3: 170. https://doi.org/10.3390/biomimetics9030170
APA StyleWu, Y., Wang, Z., Chu, Y., Peng, R., Peng, H., Yang, H., Guo, K., & Zhang, J. (2024). Current Research Status of Respiratory Motion for Thorax and Abdominal Treatment: A Systematic Review. Biomimetics, 9(3), 170. https://doi.org/10.3390/biomimetics9030170








