Small-Diameter Blood Vessel Substitutes: Biomimetic Approaches to Improve Patency
Abstract
1. Introduction
- Thrombogenicity of the graft surface
- Intimal hyperplasia at the distal anastomosis
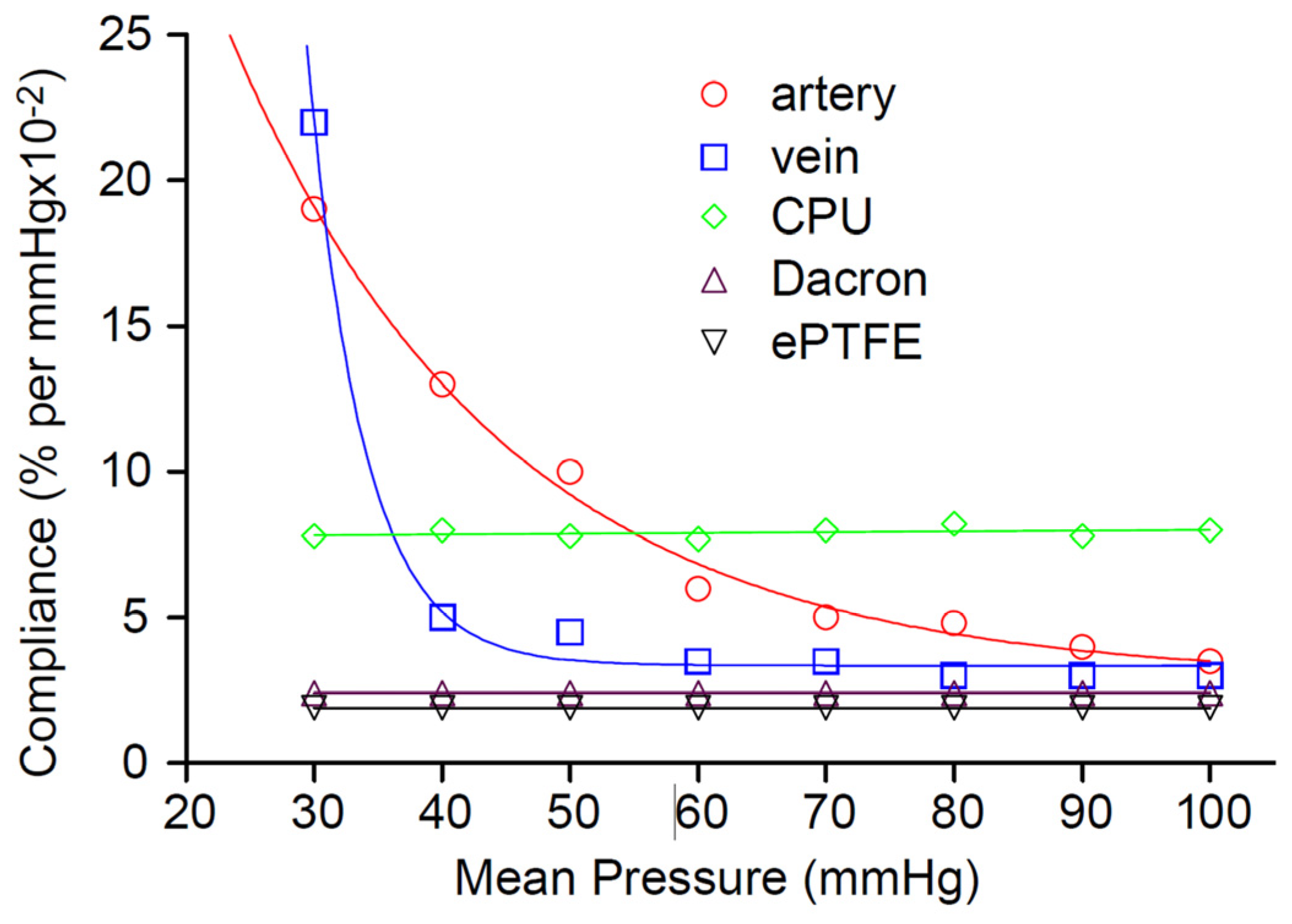
- Compliance mismatch between the native artery and the graft
2. Approaches to Improve Graft Patency
2.1. Surface Modification
2.2. Tissue-Engineered Vascular Grafts (TEVGs)
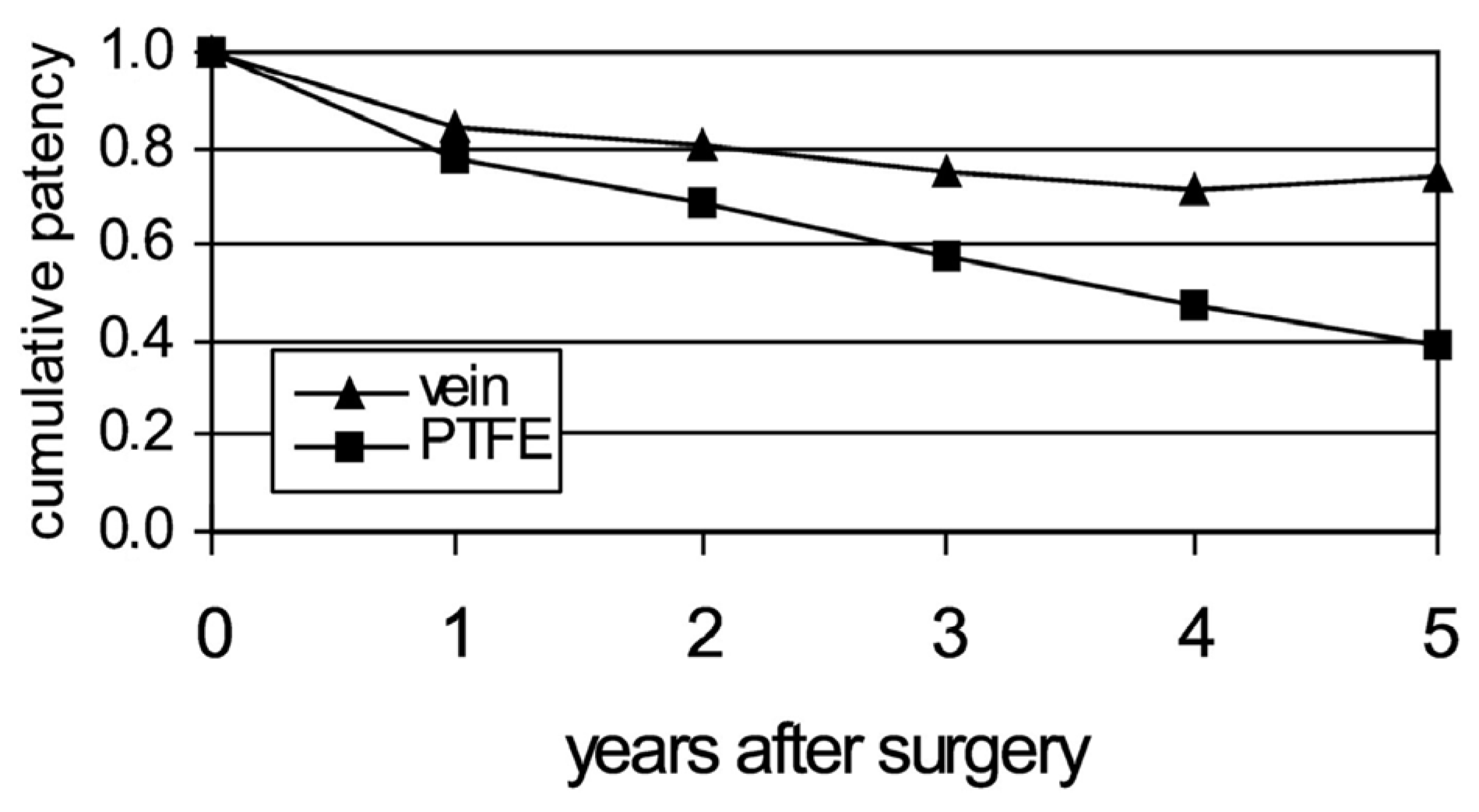
- Although thrombogenicity may be reduced compared to PTFE grafts, the patency rates are still as low as that of PTFE grafts in small-diameter arteries.
- Therefore, the other two causes of patency loss of restenosis, namely NIH caused by anastomoses and by compliance mismatch, may be responsible for the poor performance of decellularized vessels used as vascular grafts.
- Cellularization may resolve some of these issues leading to better patency for such grafts. Cellularization may be performed in situ after implantation by decorating the lumen surface of the graft with RGD motifs or integrin-binding domains. This mandates cellular access with pores in the graft that are sufficiently large, but not large enough to cause blood leakage. That has been very hard to fabricate and control, hence endothelization in situ has been poor [2,6,16,17,18,19,20].
- The presentation of a favorable substrate made of collagen and elastin that enables cell seeding and biochemical modification of the attached cell phenotype to the desired phenotypes (endothelial/smooth muscle cells etc.).
- The mechanical characteristics of the substrate are closer to those of native vessels, although a truly biomimetic matrix will have design features similar to that found in the native artery, as will be explained in later sections [6].
- In spite of the promise afforded by a tissue-engineered blood vessel substitute, a TEVG product is yet to be approved, although one or two are already in clinical use.
- The stability of the adhered cell constructs, and the long time involved in static culturing and cell penetration into the matrix, are both stumbling blocks that hinder the development of an off-the-shelf graft available for emergency procedures.
- Regulatory aspects of the product (this is a device/biologic combination product) are complex and may require several design verification tests of the product.
- Cell choice/selection remains a heavily researched aspect of TEVGs: stem cells vs precursor cells vs a combination of endothelial cells and SMCs: autologous vs allogeneic vs xenogeneic.
- Regardless of the choice of cells, even a fully cellularized matrix vessel remains far from the ideal substitute upon implantation; almost none approaches the highly non-linear compliance behavior of native arteries.
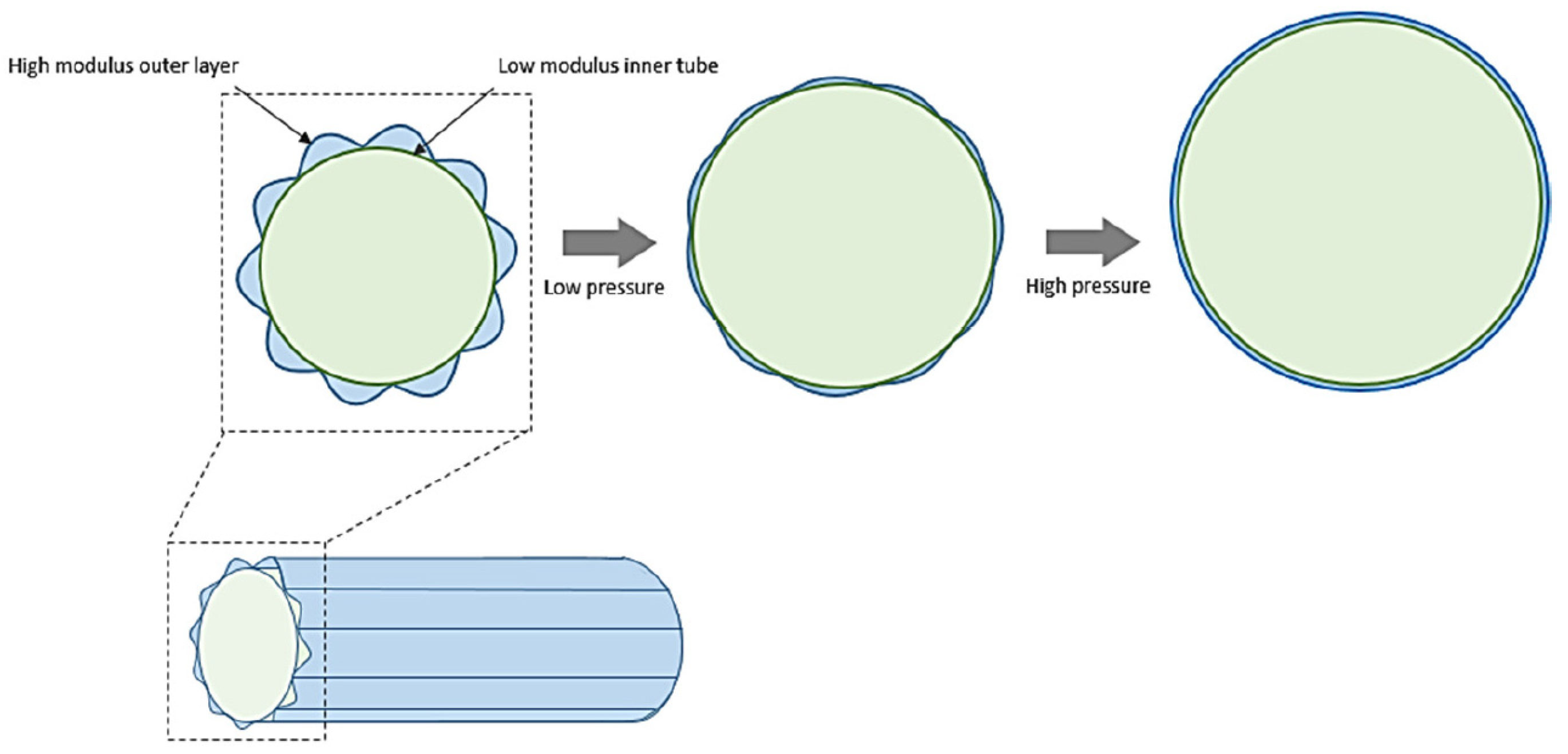
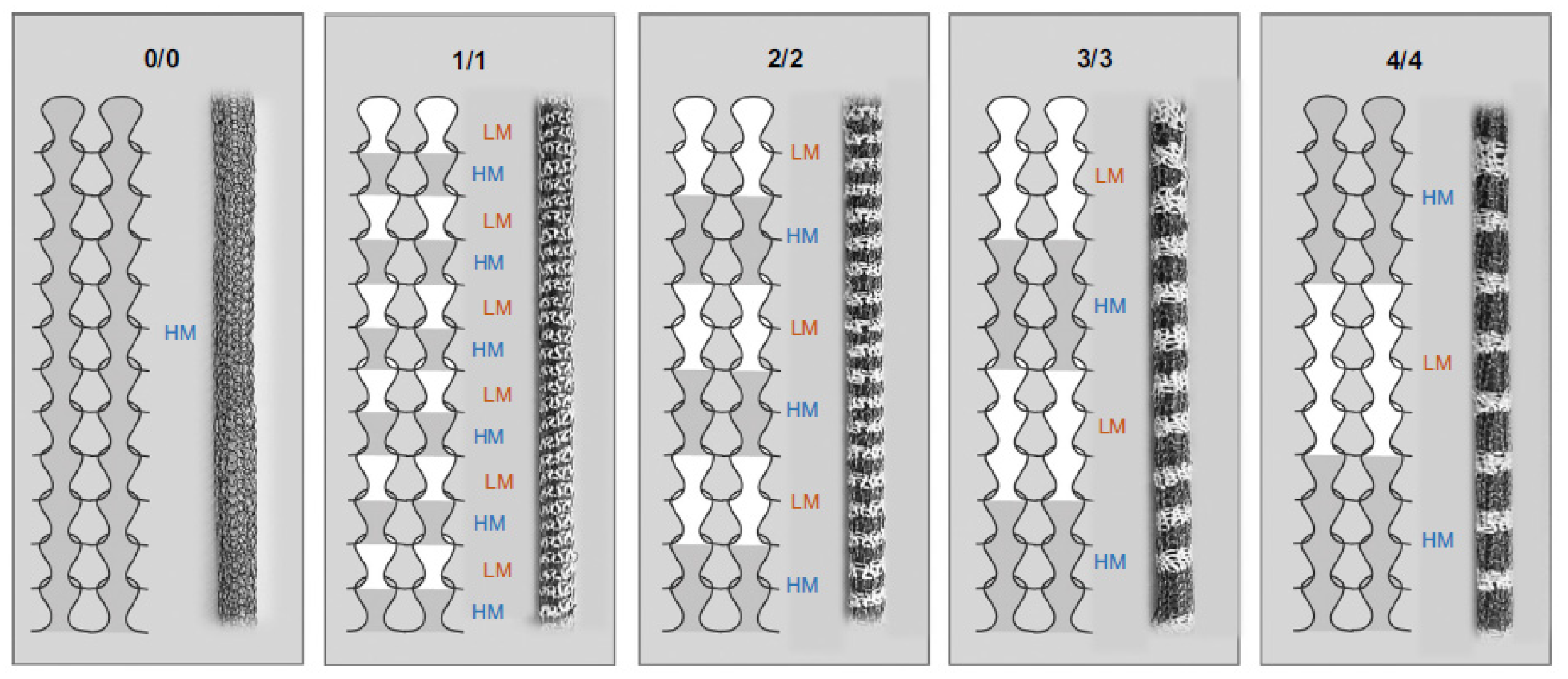
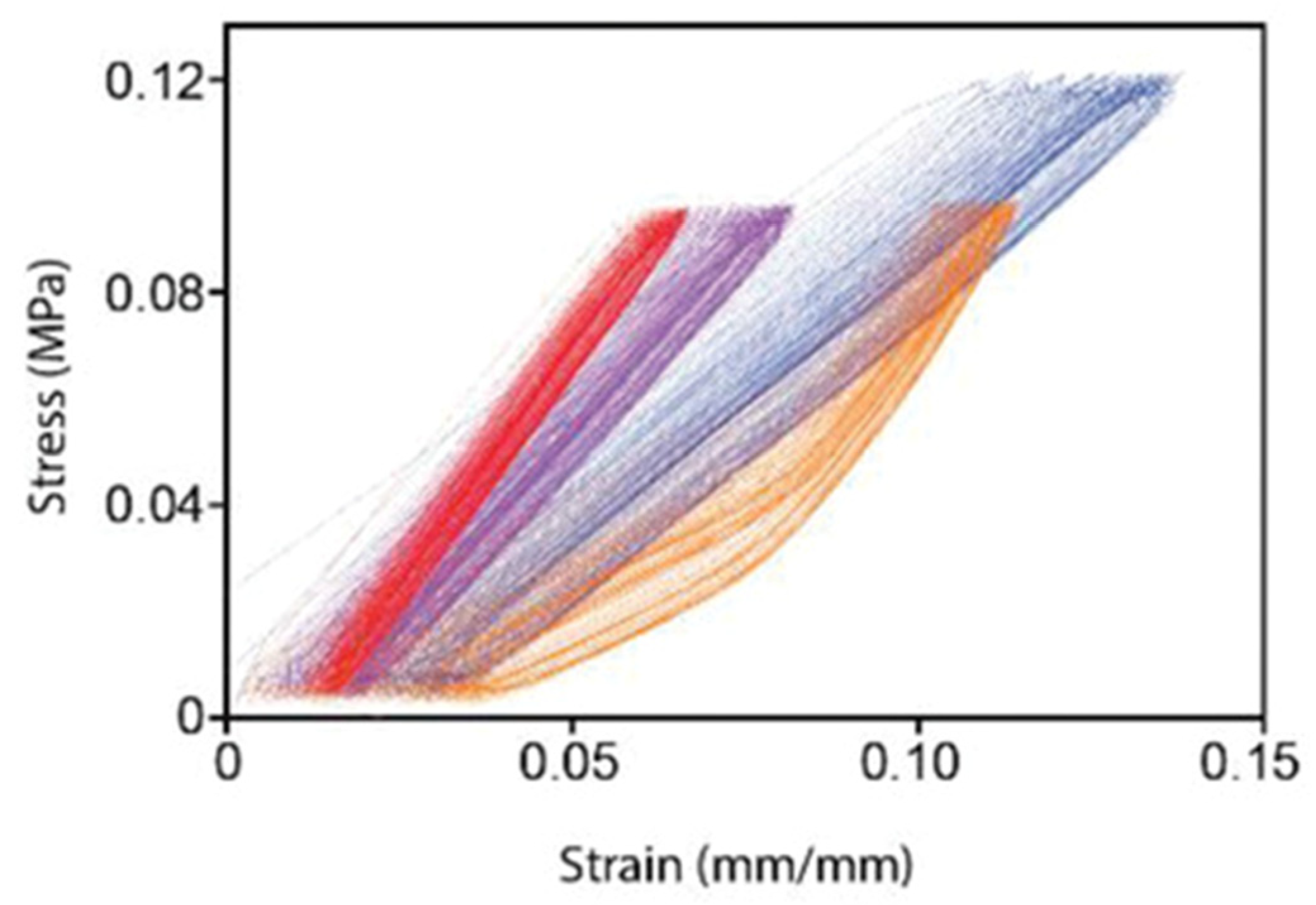
2.3. Design Strategies to Reduce Compliance Mismatch
2.4. Fabrication Techniques
- Automation
- Flexibility in shapes
- Ability to fabricate complex shapes
- Combination of multiple materials
- Rapid prototyping
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klinkert, P.; Post, P.; Breslau, P.; Van Bockel, J. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef]
- Chlupáč, J.; Filova, E.; Bačáková, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58 (Suppl. 2), S119–S139. [Google Scholar] [CrossRef]
- Conklin, B.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.-S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Dashwood, M.R.; Tsui, J.C. ‘No-touch’saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: A journey from bedside to bench. Vasc. Pharmacol. 2013, 58, 240–250. [Google Scholar] [CrossRef]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of vascular graft studies in large animal models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef]
- Venkatraman, S.; Yun, X.; Yingying, H.; Mondal, D.; Lin, L.K. Bioactive coatings for implanted devices. In Biological and Biomedical Coatings Handbook: Applications; CRC Press: Boca Raton, FL, USA, 2016; p. 471. [Google Scholar]
- Product Value—GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface. Available online: https://www.goremedical.com/value-based-solutions/product-value-summaries/gore-viabahn-endoprosthesis (accessed on 20 December 2023).
- Badv, M.; Alonso-Cantu, C.; Shakeri, A.; Hosseinidoust, Z.; Weitz, J.I.; Didar, T.F. Biofunctional lubricant-infused vascular grafts functionalized with silanized bio-inks suppress thrombin generation and promote endothelialization. ACS Biomater. Sci. Eng. 2019, 5, 6485–6496. [Google Scholar] [CrossRef]
- Bibevski, S.; Ruzmetov, M.; Fortuna, R.S.; Turrentine, M.W.; Brown, J.W.; Ohye, R.G. Performance of SynerGraft decellularized pulmonary allografts compared with standard cryopreserved allografts: Results from multiinstitutional data. Ann. Thorac. Surg. 2017, 103, 869–874. [Google Scholar] [CrossRef]
- Morisson, B.; Araújo, A.L.d.; Harduin, L.d.O.; Porcari, E.F.; Fiorelli, R.K.A.; Fiorelli, S.K.A.; Serafim, J.M.B.; Oliveira, J.C.P.d. A pilot study comparing bovine mesenteric artery and expanded polytetrafluoroethylene grafts as non-autogenous hemodialysis options. J. Vasc. Bras. 2018, 17, 303–309. [Google Scholar] [CrossRef]
- Nemes, A.; Acsády, G.; Fraefel, W.; Lichti, H.; Monos, E.; Oertli, R.; Somogyi, E.; Sótonyi, P. Application of a vascular graft material (Solcograft®-P) in experimental surgery. Biomaterials 1985, 6, 303–311. [Google Scholar] [CrossRef]
- Chemla, E.; Morsy, M. Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access. J. Br. Surg. 2009, 96, 34–39. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Irvine, S.A.; Yun, X.; Venkatraman, S. Anti-platelet and tissue engineering approaches to biomaterial blood compatibilization: How well have these been translated into the clinic? Drug Deliv. Transl. Res. 2012, 2, 384–397. [Google Scholar] [CrossRef]
- Behr, J.M. Engineering Compliant, Small Diameter Vascular Prosthesis for Bypass Grafting. Ph.D. Thesis, Nanyang Technological University, School of Material Science and Engineering, Singapore, 2018. [Google Scholar]
- Cutiongco, M.F.; Kukumberg, M.; Peneyra, J.L.; Yeo, M.S.; Yao, J.Y.; Rufaihah, A.J.; Le Visage, C.; Ho, J.P.; Yim, E.K. Submillimeter diameter poly (vinyl alcohol) vascular graft patency in rabbit model. Front. Bioeng. Biotechnol. 2016, 4, 44. [Google Scholar] [CrossRef]
- Knight, D.K.; Gillies, E.R.; Mequanint, K. Vascular grafting strategies in coronary intervention. Front. Mater. 2014, 1, 4. [Google Scholar] [CrossRef]
- Abbott, W.M.; Megerman, J.; Hasson, J.E.; L’Italien, G.; Warnock, D.F. Effect of compliance mismatch on vascular graft patency. J. Vasc. Surg. 1987, 5, 376–382. [Google Scholar] [CrossRef]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436.e2. [Google Scholar] [CrossRef]
- Shin’oka, T.; Matsumura, G.; Hibino, N.; Naito, Y.; Watanabe, M.; Konuma, T.; Sakamoto, T.; Nagatsu, M.; Kurosawa, H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005, 129, 1330–1338. [Google Scholar] [CrossRef]
- Sarkar, S.; Salacinski, H.; Hamilton, G.; Seifalian, A. The mechanical properties of infrainguinal vascular bypass grafts: Their role in influencing patency. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 627–636. [Google Scholar] [CrossRef]
- Tai, N.; Salacinski, H.; Edwards, A.; Hamilton, G.; Seifalian, A. Compliance properties of conduits used in vascular reconstruction. Br. J. Surg. 2000, 87, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A.; Gasser, T.C.; Ogden, R.W. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. Phys. Sci. Solids 2000, 61, 1–48. [Google Scholar]
- Schmidli, J.; Savolainen, H.; Heller, G.; Widmer, M.; Then-Schlagau, U.; Baumgartner, I.; Carrel, T. Bovine mesenteric vein graft (ProCol) in critical limb ischaemia with tissue loss and infection. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 251–253. [Google Scholar] [CrossRef]
- Wu, W.; Allen, R.A.; Wang, Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 2012, 18, 1148–1153. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Millam, R.; Yeoman, M.; Zilla, P. Adventitial Fabric Reinforced Porous Prosthetic Graft. U.S. Patent 10/201,498, 20 March 2003. [Google Scholar]
- Yeoman, M.; Reddy, B.; Bowles, H.; Zilla, P.; Bezuidenhout, D.; Franz, T. The use of finite element methods and genetic algorithms in search of an optimal fabric reinforced porous graft system. Ann. Biomed. Eng. 2009, 37, 2266–2287. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, M.S.; Reddy, B.D.; Bezuidenhout, D.; Bowles, H.C.; Zilla, P.; Franz, T. Development of a Fabric-Reinforced Porous Graft for Vascular Tissue Engineering Using Finite Element Methods and Genetic Algorithms. In Cardiovascular and Cardiac Therapeutic Devices; Springer: Berlin/Heidelberg, Germany, 2013; pp. 29–61. [Google Scholar]
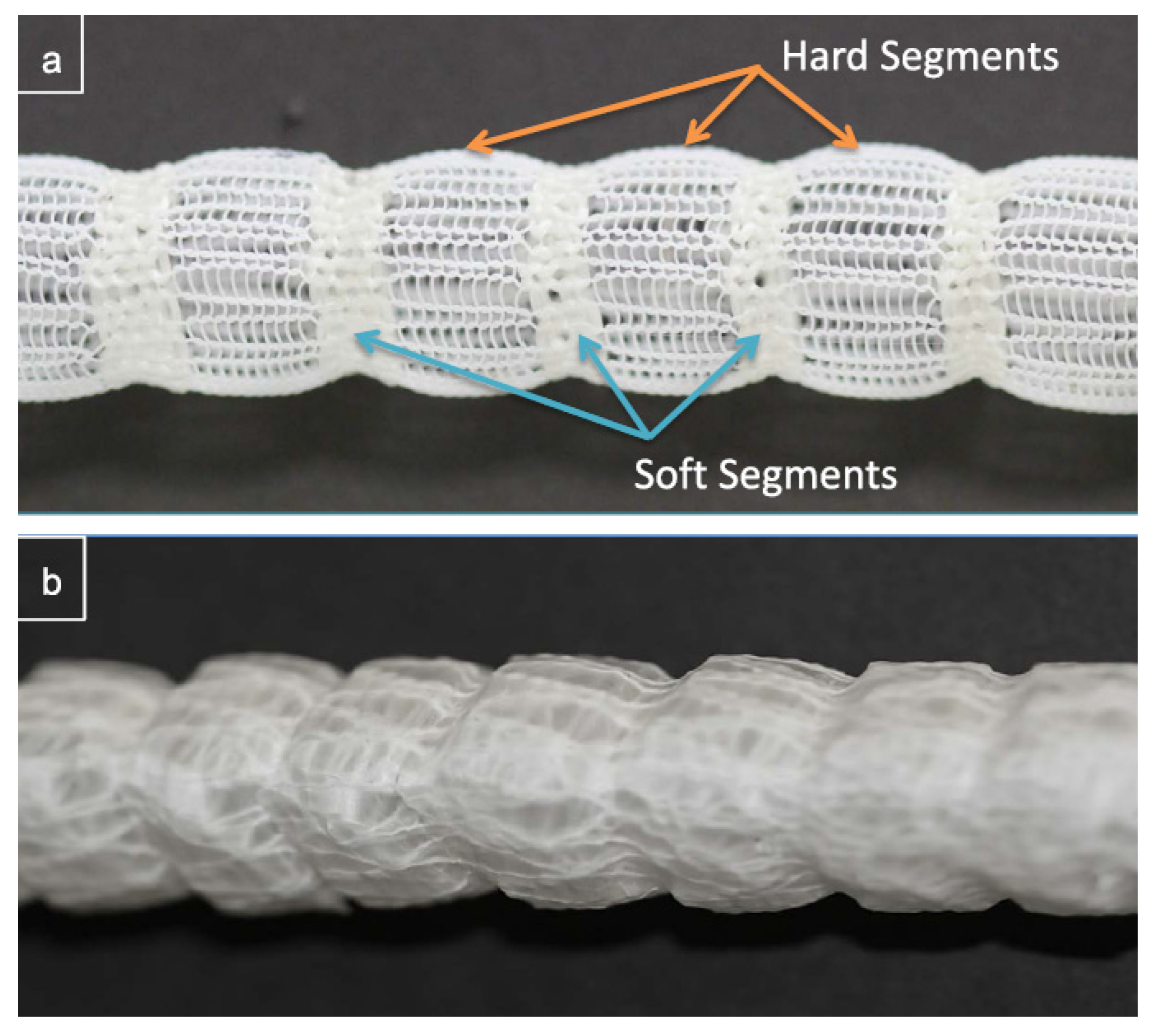
- Chen, Y.; Ding, X.; Li, Y.; King, M.W.; Gao, J.; Zhao, X. A bilayer prototype woven vascular prosthesis with improved radial compliance. J. Text. Inst. 2012, 103, 106–111. [Google Scholar] [CrossRef]
- Singh, C.; Wong, C.S.; Wang, X. Medical textiles as vascular implants and their success to mimic natural arteries. J. Funct. Biomater. 2015, 6, 500–525. [Google Scholar] [CrossRef]
- Singh, C.; Wang, X. A biomimetic approach for designing stent-graft structures: Caterpillar cuticle as design model. J. Mech. Behav. Biomed. Mater. 2014, 30, 16–29. [Google Scholar] [CrossRef]
- Singh, C.; Wang, X. A new design concept for knitted external vein-graft support mesh. J. Mech. Behav. Biomed. Mater. 2015, 48, 125–133. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Ma, S.; Wang, L.; Liu, C.; Xu, W.; Shi, J.; Qiao, W.; Yang, H. Small-diameter polyurethane vascular graft with high strength and excellent compliance. J. Mech. Behav. Biomed. Mater. 2021, 121, 104614. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Colino, A.; Wolf, F.; Rütten, S.; Schmitz-Rode, T.; Rodríguez-Cabello, J.C.; Jockenhoevel, S.; Mela, P. Small caliber compliant vascular grafts based on elastin-like recombinamers for in situ tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.; McAllister, T.N.; Dusserre, N.; Garrido, S.A.; Iyican, C.; Marini, A.; Fiorillo, A.; Avila, H.; Wystrychowski, W.; Zagalski, K. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009, 30, 1542–1550. [Google Scholar] [CrossRef]
- Behr, J.M.; Irvine, S.A.; Thwin, C.S.; Shah, A.H.; Bae, M.C.K.; Zussman, E.; Venkatraman, S. Matching Static and Dynamic Compliance of Small-Diameter Arteries, with Poly (lactide-co-caprolactone) Copolymers: In Vitro and In Vivo Studies. Macromol. Biosci. 2020, 20, 1900234. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mithieux, S.M.; Vindin, H.; Wang, Y.; Zhang, M.; Liu, L.; Zbinden, J.; Blum, K.M.; Yi, T.; Matsuzaki, Y. Rapid regeneration of a neoartery with elastic lamellae. Adv. Mater. 2022, 34, 2205614. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, X.; Meng, X.; Guo, X.; Jiang, Y.; Xu, Y.; Li, Q.; Shen, C. Controllable fiber orientation and nonlinear elasticity of electrospun nanofibrous small diameter tubular scaffolds for vascular tissue engineering. Biomed. Mater. 2019, 14, 035006. [Google Scholar] [CrossRef]
- Xie, Y.; Guan, Y.; Kim, S.-H.; King, M.W. The mechanical performance of weft-knitted/electrospun bilayer small diameter vascular prostheses. J. Mech. Behav. Biomed. Mater. 2016, 61, 410–418. [Google Scholar] [CrossRef]
- Maleckis, K.; Kamenskiy, A.; Lichter, E.Z.; Oberley-Deegan, R.; Dzenis, Y.; MacTaggart, J. Mechanically tuned vascular graft demonstrates rapid endothelialization and integration into the porcine iliac artery wall. Acta Biomater. 2021, 125, 126–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Guex, A.; Liu, S.; Müller, E.; Malini, R.I.; Zhao, H.; Rottmar, M.; Maniura-Weber, K.; Rossi, R. A compliant and biomimetic three-layered vascular graft for small blood vessels. Biofabrication 2017, 9, 025010. [Google Scholar] [CrossRef]
- Guan, G.; Yu, C.; Xing, M.; Wu, Y.; Hu, X.; Wang, H.; Wang, L. Hydrogel small-diameter vascular graft reinforced with a braided fiber strut with improved mechanical properties. Polymers 2019, 11, 810. [Google Scholar] [CrossRef]
- Elomaa, L.; Yang, Y.P. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng. Part B Rev. 2017, 23, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Strouse, R.; Hor, K.; Pepper, V.; Tipton, A.; Kelly, J.; Shinoka, T.; Breuer, C. Toward a patient-specific tissue engineered vascular graft. J. Tissue Eng. 2018, 9, 2041731418764709. [Google Scholar] [CrossRef] [PubMed]
- Emechebe, G.A.; Obiweluozor, F.O.; Jeong, I.S.; Park, J.-K.; Park, C.H.; Kim, C.S. Merging 3D printing with electrospun biodegradable small-caliber vascular grafts immobilized with VEGF. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102306. [Google Scholar] [CrossRef]
- Freeman, S.; Ramos, R.; Chando, P.A.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater. 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Weekes, A.; Bartnikowski, N.; Pinto, N.; Jenkins, J.; Meinert, C.; Klein, T.J. Biofabrication of small diameter tissue-engineered vascular grafts. Acta Biomater. 2022, 138, 92–111. [Google Scholar] [CrossRef]
- Tardalkar, K.R.; Chaudhari, L.R.; Damle, M.N.; Kawale, A.A.; Bhamare, N.C.; Kshersagar, J.R.; Kulkarni, T.S.; Joshi, M.G. Extracellular-matrix Composite Bioink for 3D bioprinting and molding of small diameter vascular grafts. Bioprinting 2023, 34, e00300. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Yu, D.; Shao, Y.; Wang, Z.; Liu, X.; Wang, W.; Chang, L.; Ma, T.; Mok, H. 3D-bioprinted vascular scaffold with tunable mechanical properties for simulating and promoting neo-vascularization. Smart Mater. Med. 2022, 3, 199–208. [Google Scholar] [CrossRef]
- ISO 7198:2016; Cardiovascular Implants and Extracorporeal Systems–Vascular Prostheses–Tubular Vascular Grafts and Vascular Patches. International Organization for Standardization: Geneva, Switzerland, 2016.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behr, J.-M.; Wong, Y.S.; Venkatraman, S. Small-Diameter Blood Vessel Substitutes: Biomimetic Approaches to Improve Patency. Biomimetics 2024, 9, 97. https://doi.org/10.3390/biomimetics9020097
Behr J-M, Wong YS, Venkatraman S. Small-Diameter Blood Vessel Substitutes: Biomimetic Approaches to Improve Patency. Biomimetics. 2024; 9(2):97. https://doi.org/10.3390/biomimetics9020097
Chicago/Turabian StyleBehr, Jean-Marc, Yee Shan Wong, and Subbu Venkatraman. 2024. "Small-Diameter Blood Vessel Substitutes: Biomimetic Approaches to Improve Patency" Biomimetics 9, no. 2: 97. https://doi.org/10.3390/biomimetics9020097
APA StyleBehr, J.-M., Wong, Y. S., & Venkatraman, S. (2024). Small-Diameter Blood Vessel Substitutes: Biomimetic Approaches to Improve Patency. Biomimetics, 9(2), 97. https://doi.org/10.3390/biomimetics9020097





