Isolation and Characterization of Nanocellulose from Polypodiophyta Fern Using Chemo-Mechanical Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Isolation of Nanocellulose
2.3. Isolation of Nanocellulose
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. Energy-Dispersive X-ray Spectroscopy (EDS)
2.3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.4. Thermogravimetric Analysis (TGA)
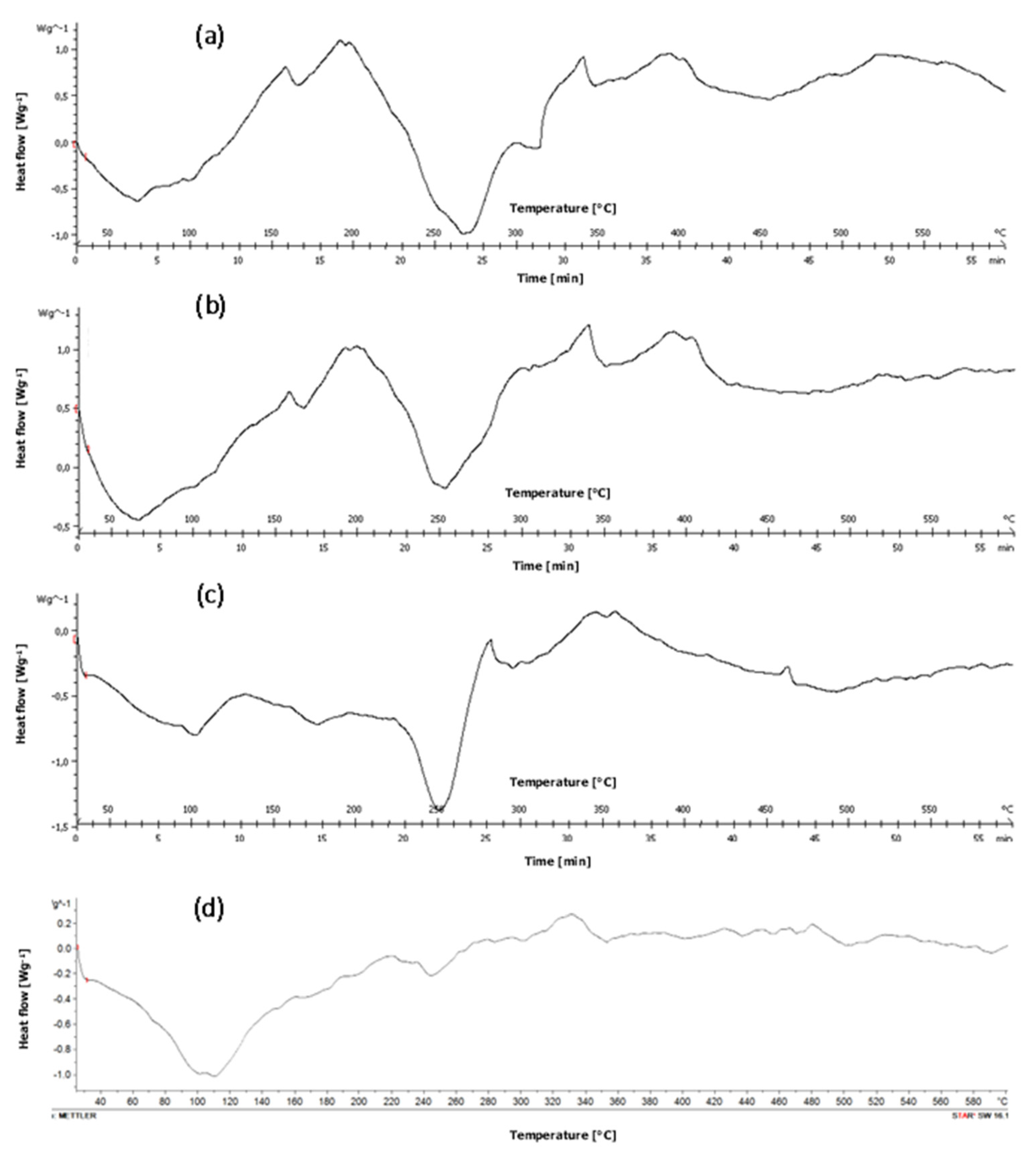
2.3.5. Differential Scanning Calorimetry (DSC)
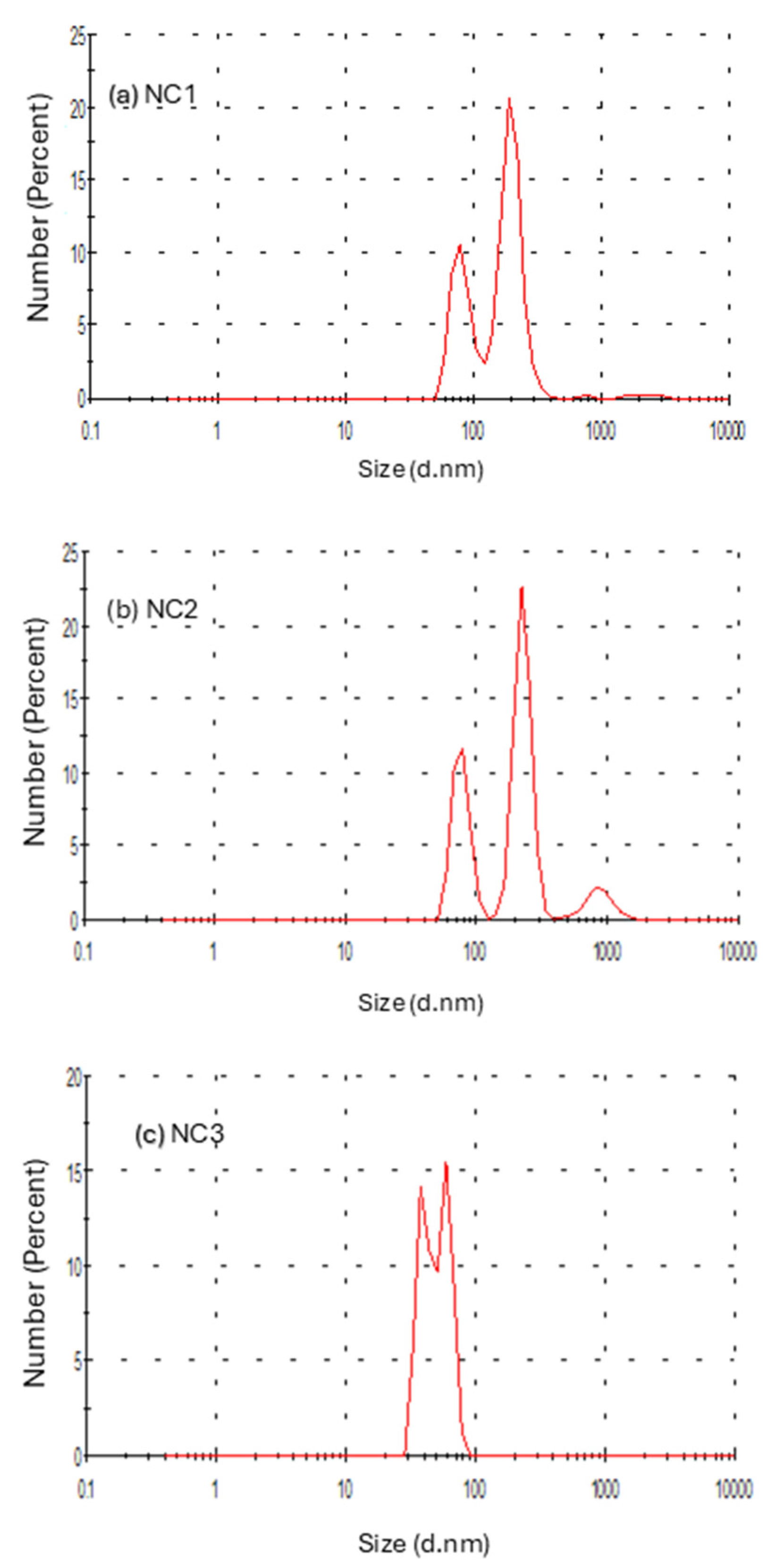
2.3.6. Dynamic Light Scattering (DLS)
3. Results
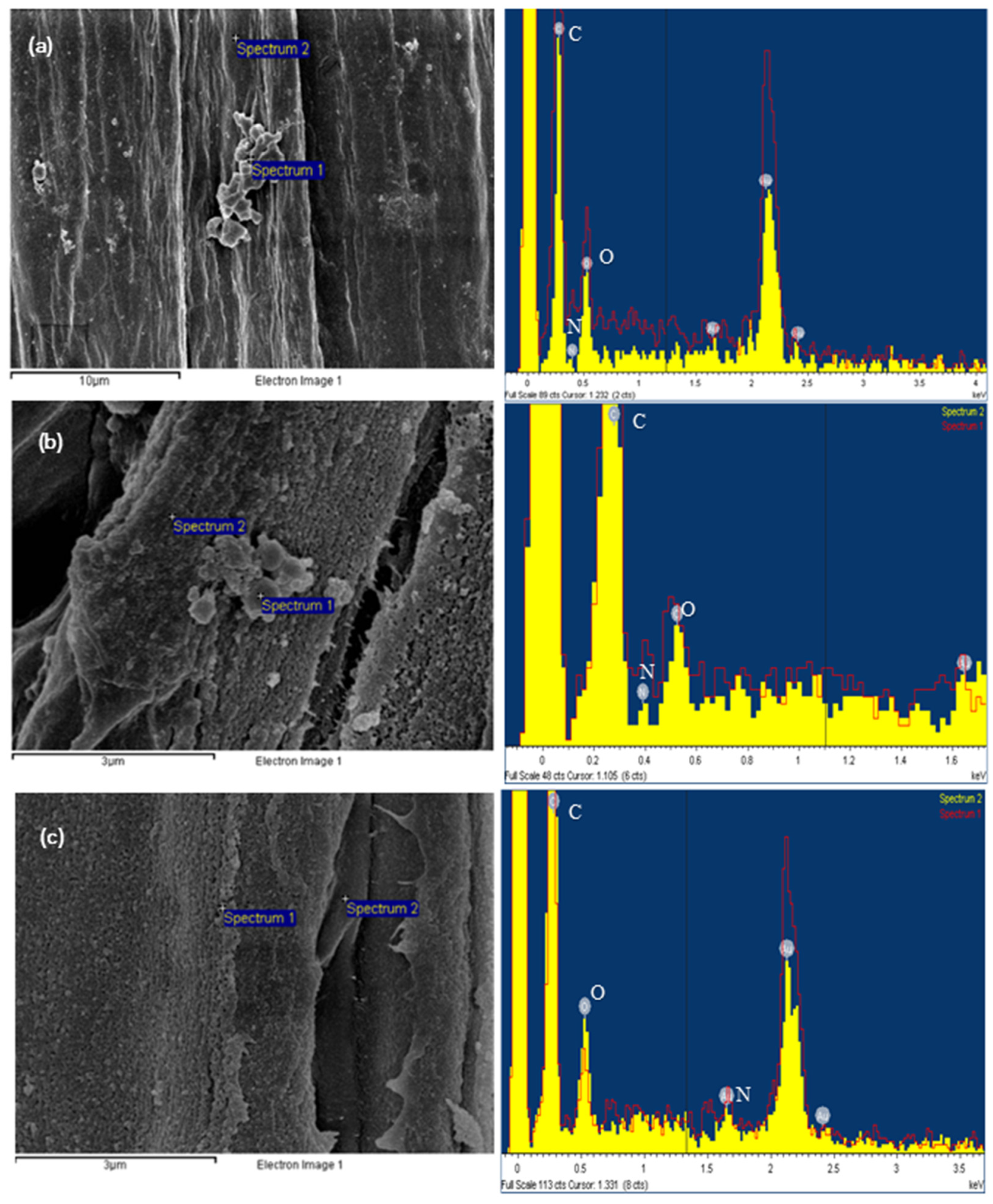
3.1. Microscopic Morphology Analysis by SEM
3.2. EDS Elemental Analysis
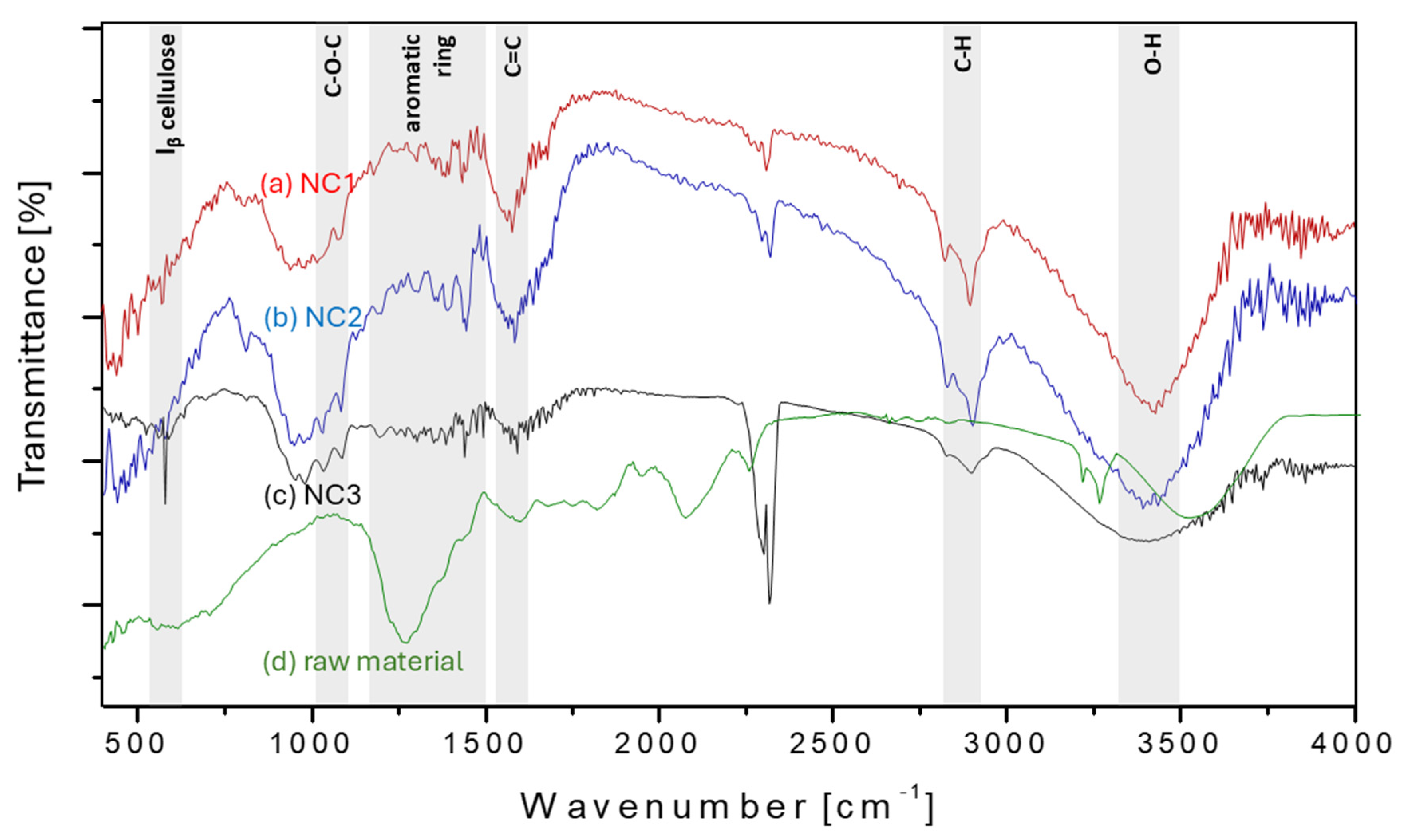
3.3. FT-IR Analysis
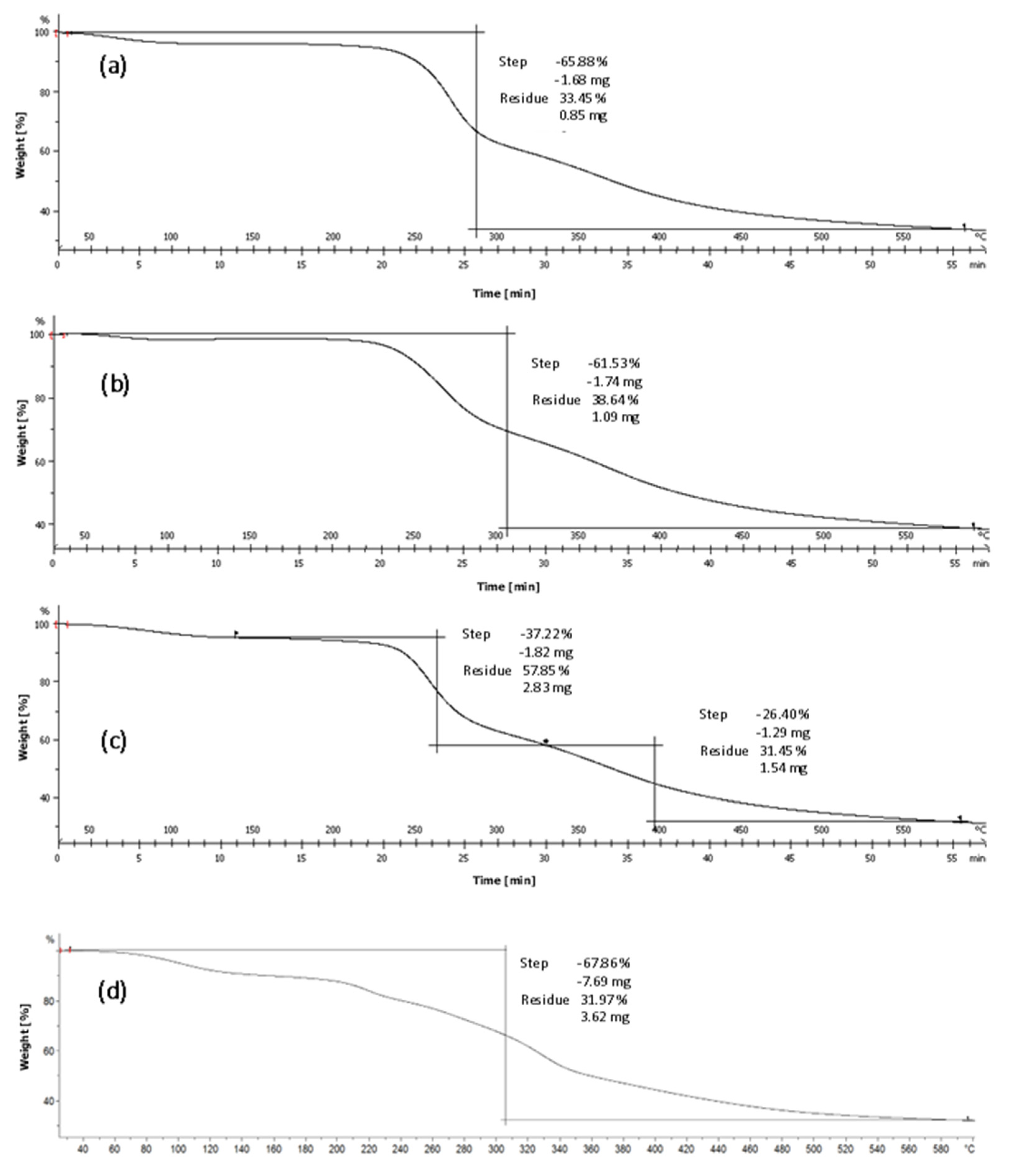
3.4. Thermogravimetric Analysis
3.5. Differential Scanning Calorimetry (DSC) Analysis
3.6. Particle Size Distribution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, J.; Wang, B.; Zeng, J.; Xu, J.; Zhu, S.; Duan, C.; Chen, K. Comparative Study on Properties of Nanocellulose Derived from Sustainable Biomass Resources. Cellulose 2022, 29, 7083–7098. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Shanks, R.A. 2—Chemistry and Structure of Cellulosic Fibres as Reinforcements in Natural Fibre Composites. In Natural Fibre Composites; Hodzic, A., Shanks, R., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 66–83. ISBN 978-0-85709-524-4. [Google Scholar]
- Susanto, A.; Fahma, F.; Jayanegara, A.; Djatna, T. Effect of Isolation Method on the Properties of Nanocellulose: A Meta-Analysis. Cellulose 2022, 29, 7211–7224. [Google Scholar] [CrossRef]
- Heise, K.; Delepierre, G.; King, A.W.T.; Kostiainen, M.A.; Zoppe, J.; Weder, C.; Kontturi, E. Chemical Modification of Reducing End-Groups in Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 66–87. [Google Scholar] [CrossRef]
- Park, N.-M.; Choi, S.; Oh, J.E.; Hwang, D.Y. Facile Extraction of Cellulose Nanocrystals. Carbohydr. Polym. 2019, 223, 115114. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of Nanocrystalline Cellulose from Lignocellulosic Biomass: Technology and Applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Jiang, Z.; Li, W.; Yu, Y. A Comparison Study on the Preparation of Nanocellulose Fibrils from Fibers and Parenchymal Cells in Bamboo (Phyllostachys pubescens). Ind. Crops Prod. 2015, 71, 80–88. [Google Scholar] [CrossRef]
- Lü, F.; Chai, L.; Shao, L.; He, P. Precise Pretreatment of Lignocellulose: Relating Substrate Modification with Subsequent Hydrolysis and Fermentation to Products and by-Products. Biotechnol. Biofuels 2017, 10, 88. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of Nanocellulose Isolated from Corncob Residue Using Sulfuric Acid, Formic Acid, Oxidative and Mechanical Methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Li, M.; Huang, Q.; Zhang, X.; Pan, W.; Yang, J.; Li, J. The Characteristic and Dispersion Stability of Nanocellulose Produced by Mixed Acid Hydrolysis and Ultrasonic Assistance. Carbohydr. Polym. 2017, 165, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Peretz, R.; Sterenzon, E.; Gerchman, Y.; Kumar Vadivel, V.; Luxbacher, T.; Mamane, H. Nanocellulose Production from Recycled Paper Mill Sludge Using Ozonation Pretreatment Followed by Recyclable Maleic Acid Hydrolysis. Carbohydr. Polym. 2019, 216, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Guan, G.; Ma, Y.; Hao, X.; Abudula, A. Effect of Ball Milling on the Production of Nanocellulose Using Mild Acid Hydrolysis Method. J. Taiwan. Inst. Chem. Eng. 2016, 60, 617–622. [Google Scholar] [CrossRef]
- Prado, K.S.; Gonzales, D.; Spinacé, M.A.S. Recycling of Viscose Yarn Waste through One-Step Extraction of Nanocellulose. Int. J. Biol. Macromol. 2019, 136, 729–737. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse Nanocelluloses Prepared from TEMPO-Oxidized Wood Cellulose Fibers: Nanonetworks, Nanofibers, and Nanocrystals. Curr. Opin. Solid. State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Indarti, E.; Rohaizu, R.; Wanrosli, W.D. Silylation of TEMPO Oxidized Nanocellulose from Oil Palm Empty Fruit Bunch by 3-Aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2019, 135, 106–112. [Google Scholar] [CrossRef]
- Assabjeu, A.C.; Noubissié, E.; Desobgo, S.C.Z.; Ali, A. Optimization of the Enzymatic Hydrolysis of Cellulose of Triplochiton Scleroxylon Sawdust in View of the Production of Bioethanol. Sci. Afr. 2020, 8, e00438. [Google Scholar] [CrossRef]
- He, S.; Shu, F.; Liu, X.; Yan, K.; Lei, S.; Liu, Y.; Zhou, M.; Yu, H.; Zhang, J.; Yang, F. Production of Lignin-Containing Nanocellulose by FeCl3-Catalyzed Ternary Deep Eutectic Solvent Pretreatment System: Structural Characteristics and Emulsification Capabilities. Ind. Crops Prod. 2023, 203, 117200. [Google Scholar] [CrossRef]
- Almeida, R.O.; Maloney, T.C.; Gamelas, J.A.F. Production of Functionalized Nanocelluloses from Different Sources Using Deep Eutectic Solvents and Their Applications. Ind. Crops Prod. 2023, 199, 116583. [Google Scholar] [CrossRef]
- Wang, S.; Zou, Q.; Zhang, L.; Zheng, W.; Huang, X.; Zhang, J. A New Nanocellulose Prepared from Waste Coconut Shell Fibers Based on a Novel Ultrasonic—Active Agent Combination Method: Preparation Principle and Performances in Cement Matrix. Ind. Crops Prod. 2023, 197, 116607. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, N.; Sun, Y.; Shao, J.; Liu, Q.; Zhuang, X.; Twebaze, C.B. Nanocellulose Aerogels from Banana Pseudo-Stem as a Wound Dressing. Ind. Crops Prod. 2023, 194, 116383. [Google Scholar] [CrossRef]
- Abu Bakar, N.F.; Abd Rahman, N.; Mahadi, M.B.; Mohd Zuki, S.A.; Mohd Amin, K.N.; Wahab, M.Z.; Wuled Lenggoro, I. Nanocellulose from Oil Palm Mesocarp Fiber Using Hydrothermal Treatment with Low Concentration of Oxalic Acid. Mater. Today Proc. 2022, 48, 1899–1904. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Chen, X.; Zhang, Y.; Yang, H.; Li, Q.; Jiang, J. Isolation and Characteristics of Nanocellulose from Hardwood Pulp via Phytic Acid Pretreatment. Ind. Crops Prod. 2022, 182, 114921. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S.; Gupta, S. Innovative Strategy for Rice Straw Valorization into Nanocellulose and Nanohemicellulose and Its Application. Ind. Crops Prod. 2022, 179, 114695. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and Characterization of Nanocellulose Crystals via Acid Hydrolysis from Agricultural Waste-Tea Stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A Review of Properties of Nanocellulose, Its Synthesis, and Potential in Biomedical Applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable Isolation of Nanocellulose from Cellulose and Lignocellulosic Feedstocks: Recent Progress and Perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef]
- Lekha, P.; Mtibe, A.; Motaung, T.E.; Andrew, J.E.; Sitholè, B.B.; Gibril, M. Effect of Mechanical Treatment on Properties of Cellulose Nanofibrils Produced from Bleached Hardwood and Softwood Pulps. Ciencia Y Tecnología 2016, 18, 457–466. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef] [PubMed]
- Sundue, M.; Kessler, M. New Species and New Records of the Fern Genus Terpsichore (Polypodiopsida: Polypodiaceae) from Bolivia. Org. Divers. Evol. 2008, 8, 163.e1–163.e10. [Google Scholar] [CrossRef][Green Version]
- Romagnoli, M.G.; Albornoz, P.L.; Arana, M.D. Comparative Morpho-Anatomy of the Sporophyte of the Most Austral American Species of Didymoglossum (Polypodiopsida: Hymenophyllaceae). Flora 2024, 310, 152440. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Parsian, H.; Majidinia, M.; Rahimi, M.; Mir, S.M.; Samadi Kafil, H.; Shafiei-Irannejad, V.; Kheyrollah, M.; Ostadi, H.; Yousefi, B. Nanocrystalline Cellulose: Preparation, Physicochemical Properties, and Applications in Drug Delivery Systems. Int. J. Biol. Macromol. 2019, 133, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding Nanocellulose Chirality and Structure–Properties Relationship at the Single Fibril Level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef]
- Feng, Z.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave Carbonized Cellulose for Trace Pharmaceutical Adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Karim, Z. Isolation and Characterization of Nanocrystalline Cellulose from Roselle-Derived Microcrystalline Cellulose. Int. J. Biol. Macromol. 2018, 114, 54–63. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of Nanocellulose Fibrils from Lignocellulosic Fibres: A Novel Approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and Characterisation of Cellulose Nanocrystals from Sago Seed Shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The Isolation of Cellulose Nanocrystals from Pistachio Shells via Acid Hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hasanulbasori, M.A.; Chiat, P.F.; Lee, H.V. Pyrus Pyrifolia Fruit Peel as Sustainable Source for Spherical and Porous Network Based Nanocellulose Synthesis via One-Pot Hydrolysis System. Int. J. Biol. Macromol. 2019, 123, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of Nanocellulose from Waste Sugarcane Bagasse (SCB) and Its Characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- de Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of Nanocellulose from Imperata Brasiliensis Grass Using Taguchi Method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef]
- Souza, A.G.; De Lima, G.F.; Rodrigues, R.C.L.B.; Cesarino, I.; Leão, A.L.; Rosa, D.S. A New Approach for Conversion of Eucalyptus Lignocellulosic Biomass into Cellulose Nanostructures: A Method That Can Be Applied in Industry. J. Nat. Fibers 2021, 18, 1501–1511. [Google Scholar] [CrossRef]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of High Crystallinity Cellulose Nanocrystals (CNCs) by Ionic Liquid Solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and Characterization of Cellulose Nanofibrils from Helicteres Isora Plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Chandra, J.; George, N.; Narayanankutty, S.K. Isolation and Characterization of Cellulose Nanofibrils from Arecanut Husk Fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Wang, L.-F.; Shankar, S.; Rhim, J.-W. Properties of Alginate-Based Films Reinforced with Cellulose Fibers and Cellulose Nanowhiskers Isolated from Mulberry Pulp. Food Hydrocoll. 2017, 63, 201–208. [Google Scholar] [CrossRef]
- Tang, S.; Liu, R.; Sun, F.F.; Dong, C.; Wang, R.; Gao, Z.; Zhang, Z.; Xiao, Z.; Li, C.; Li, H. Bioprocessing of Tea Oil Fruit Hull with Acetic Acid Organosolv Pretreatment in Combination with Alkaline H2O2. Biotechnol. Biofuels 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Boussaid, A.; Mansfield, S.D.; Gregg, D.J.; Saddler, J.N. Fast and Efficient Alkaline Peroxide Treatment to Enhance the Enzymatic Digestibility of Steam-Exploded Softwood Substrates. Biotechnol. Bioeng. 2002, 77, 678–684. [Google Scholar] [CrossRef]
- Liu, T.; Williams, D.L.; Pattathil, S.; Li, M.; Hahn, M.G.; Hodge, D.B. Coupling Alkaline Pre-Extraction with Alkaline-Oxidative Post-Treatment of Corn Stover to Enhance Enzymatic Hydrolysis and Fermentability. Biotechnol. Biofuels 2014, 7, 48. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.; Wan, C. Effects of Alkaline Hydrogen Peroxide Treatment on Cellulose Accessibility of Switchgrass Pretreated by Acidic Deep Eutectic Solvent. Cellulose 2019, 26, 9439–9446. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose Processing from Biomass and Its Derivatization into Carboxymethylcellulose: A Review. Sci. Afr. 2022, 15, e01078. [Google Scholar] [CrossRef]
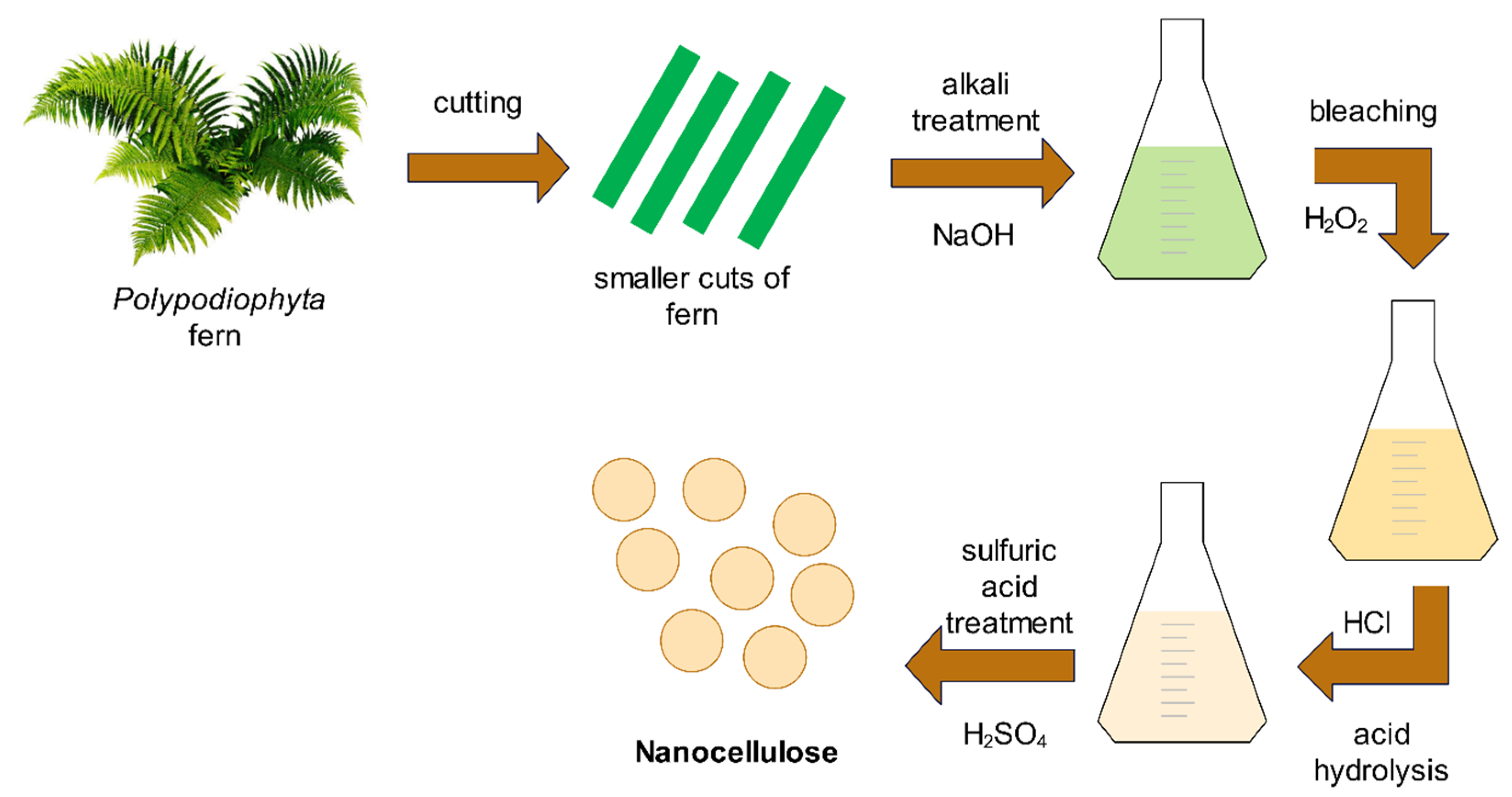

| Experiment | Alkali Treatment (NaOH) | Bleaching (H2O2) | Acid Hydrolysis (HCl) | Sulfuric Acid Treatment (H2SO4) |
|---|---|---|---|---|
| NC1 | 24 h | 4 h | 4 h | 1 h |
| NC2 | 4 h | 4 h | 24 h | 1 h |
| NC3 | 4 h | 24 h | 4 h | 1 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasić, K.; Dokl, M.; Knez, Ž.; Leitgeb, M. Isolation and Characterization of Nanocellulose from Polypodiophyta Fern Using Chemo-Mechanical Method. Biomimetics 2024, 9, 624. https://doi.org/10.3390/biomimetics9100624
Vasić K, Dokl M, Knez Ž, Leitgeb M. Isolation and Characterization of Nanocellulose from Polypodiophyta Fern Using Chemo-Mechanical Method. Biomimetics. 2024; 9(10):624. https://doi.org/10.3390/biomimetics9100624
Chicago/Turabian StyleVasić, Katja, Monika Dokl, Željko Knez, and Maja Leitgeb. 2024. "Isolation and Characterization of Nanocellulose from Polypodiophyta Fern Using Chemo-Mechanical Method" Biomimetics 9, no. 10: 624. https://doi.org/10.3390/biomimetics9100624
APA StyleVasić, K., Dokl, M., Knez, Ž., & Leitgeb, M. (2024). Isolation and Characterization of Nanocellulose from Polypodiophyta Fern Using Chemo-Mechanical Method. Biomimetics, 9(10), 624. https://doi.org/10.3390/biomimetics9100624








