Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases
Abstract
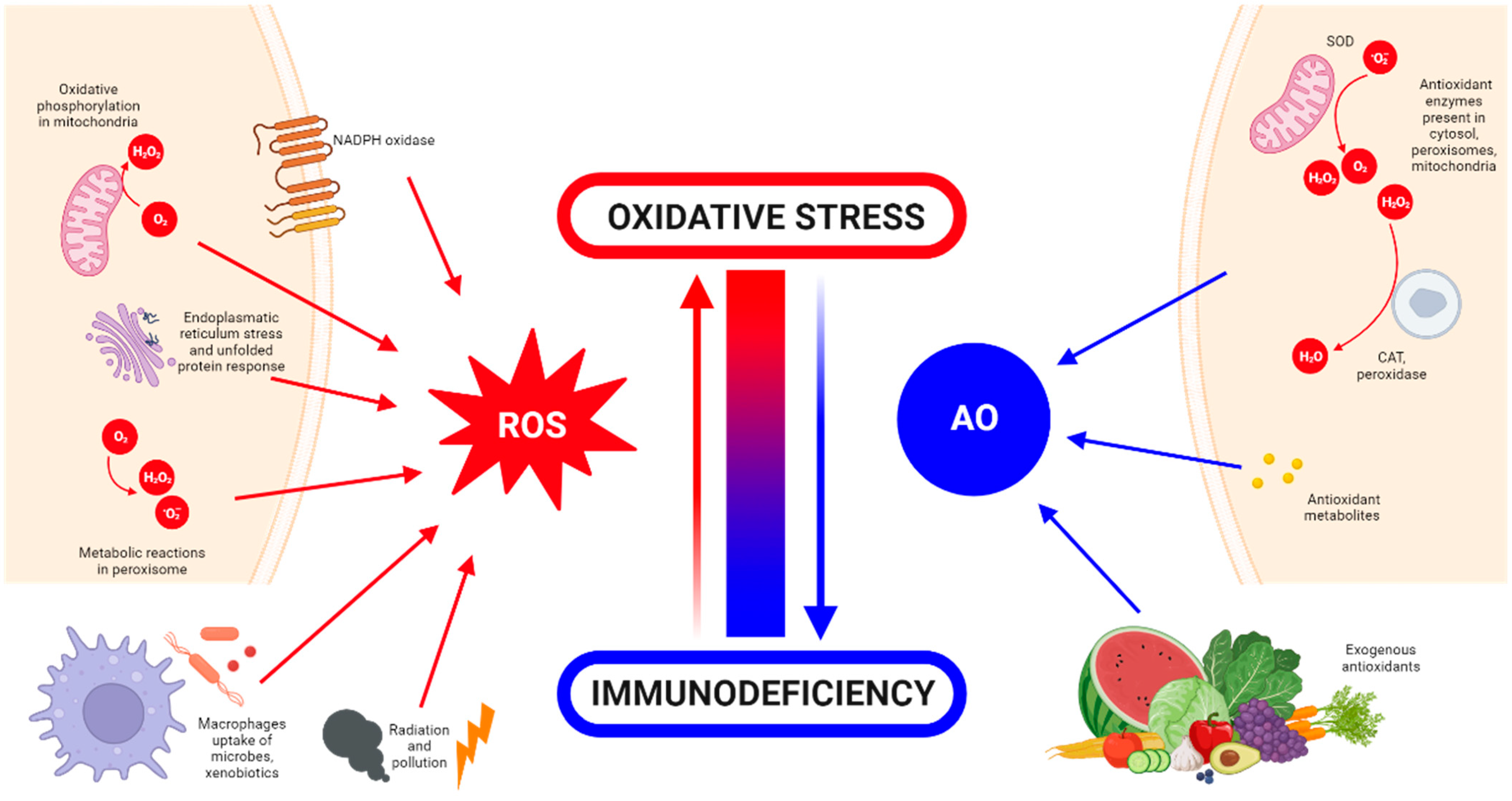
1. Introduction
What Is Oxidative Stress?
2. Detrimental Effects of ROS
3. Oxidative Stress and Tissue Engineering
4. Biomaterials Employed for Oxidative Stress Diseases
4.1. Wound Healing

| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Hydrogels | Chitosan, heparin and poly(γ-glutamic acid) | SOD | Diabetic rat model | Accelerating re-epithelialization and collagen deposition | [61] |
| Poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) | SOD | Diabetic rat model | Antioxidant activity and high wound closure rate | [62] | |
| GelMA with dopamine motifs | Cerium oxide NPs and AMP | Rats | (ROS) scavenging and antibacterial properties | [65] | |
| SBMA, CBMA and HEMA | Cerium oxide and microRNA-146 | Mice | Accelerating wound healing | [66] | |
| Chitosan-PEG | Silver NPs | Diabetic rabbits | Antioxidant and antibacterial activity | [67] | |
| Chitosan | Eugenol | - | Antioxidant activity | [71] | |
| Chitosan-g-polyaniline and benzaldehyde | PEG-co-poly(glycerol sebacate) | Mice | Good self-healing, electro-activity and free radical scavenging capacity | [72] | |
| Carboxybetaine dextran and sulphobetaine dextran | - | Mice | Self-healing, antioxidative and antifouling properties | [73] | |
| Alginate | Edudragit NPs and Edavarone | Mice | Wound healing promoting and efficient free radical scavenging | [74] | |
| Polyvinyl alcohol | Mupirocin and GM-CSF | Diabetic mice | Antibacterial activity and wound closure promoting | [75] | |
| Silk fibroin | Melanin and berberine | Diabetic rat | Re-epithelialization and wound repair promoting | [76] | |
| Inorganic NPs | Prussian Blue NPs | - | Mice | Antioxidant and collagen deposition | [64] |
| Liposomal particles | Lecithin nano-liposol | astaxanthin | NIH/3T3 cells | ROS scavenging and antioxidant capacity | [77] |
| Polymeric matrix | Cellulose | Nanochitosan dust | Human gingival cells | Antioxidant and antimicrobial activity | [70] |
| PLA | Asiatic acid | Diabetic mouse model | Accelerating re-epithelization, angiogenesis and ECM formation | [79] | |
| Poly(L-Lactic-co-caprolactone) (PLCL) | EGCG | Rat liver trauma model | Promoting wound healing and tissue organization | [81] |
4.2. Neurodegenerative Diseases
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Inorganic NPs | Cerium oxide (CeONPs) | - | P12 neuronal cells | Anti-amyloid aggregation, antioxidant activity | [93,94,95] |
| Ceria/Polyoxometalates | - | P12 neuronal cells | Inhibition of Aβ-induced microglial cell activation | [96] | |
| Iron oxide (IONPs) | - | Drosophila Alzheimer’s disease model | Anti-ROS activity | [97] | |
| Yttrium oxide | - | P12 neuronal cells | Reduction in oxidative stress and apoptosis | [98] | |
| Yttrium NPs and CeONPs | - | Wistar rats | Reduction in oxidative stress | [99] | |
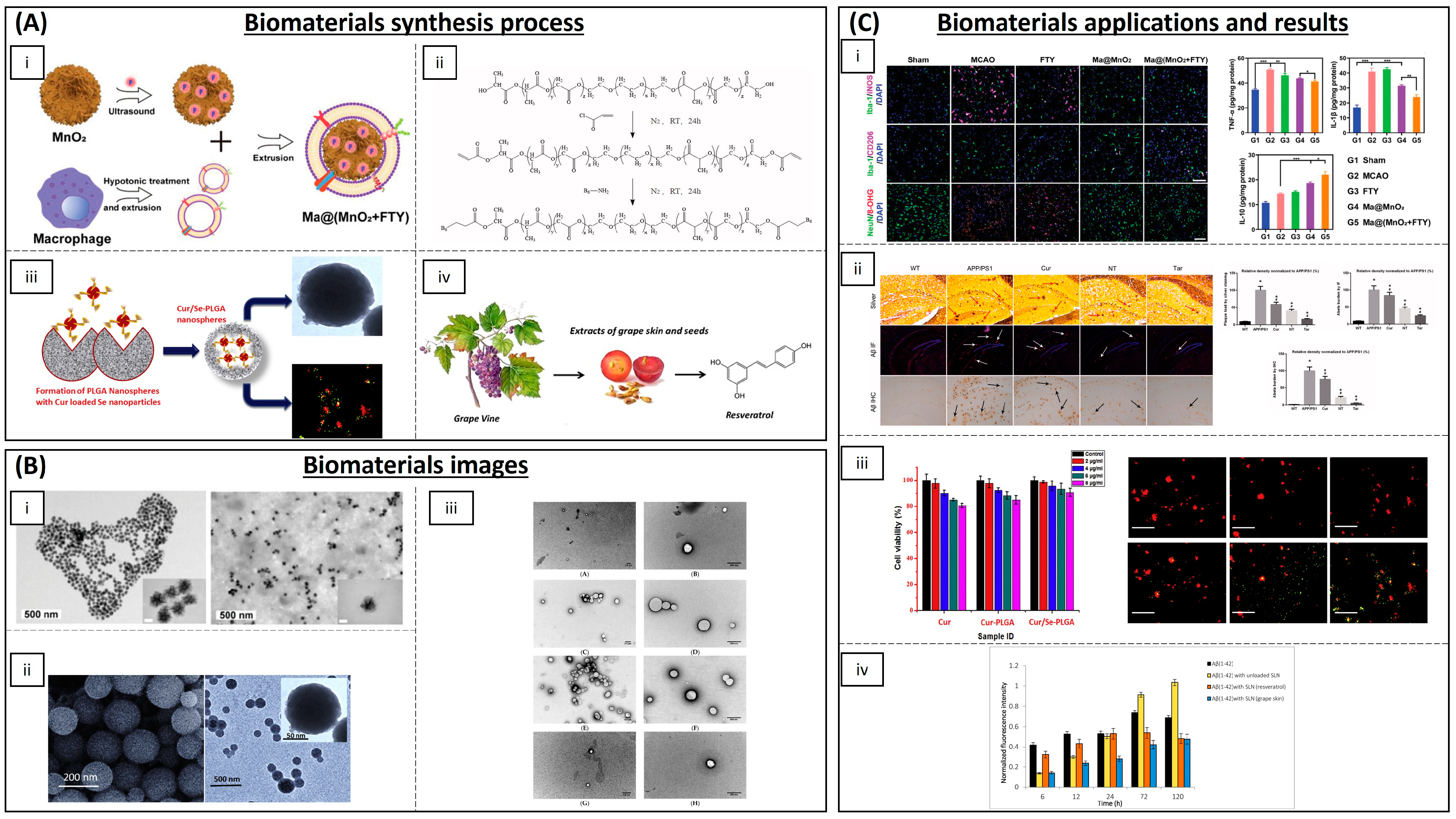
| MnO2 | Fingolimod | Mice | ROS and microglia pro-inflammatory state reduction | [100] | |
| Selenium NPs | Resveratrol | AD rat model | Anti-inflammatory activity | [116] | |
| Carbon materials | Partially reduced graphene oxide | - | Mouse-substantia-nigra-derived dopaminergic cell line | Prevention of dopaminergic neuron loss and α-syn depletion | [101] |
| PEG-HCCs | - | Brain endothelial cell line and primary cortical neuron cells | Protection against hydrogen peroxide | [102] | |
| Polymeric NPs | (PLGA-PEG) and B6 peptide | Curcumin | APP/PS1 Al transgenic mice | Improvement in spatial learning and memory | [104] |
| PLGA | Curcumin | Rats | Neuronal differentiation | [105,106,107,108,109] | |
| PEGylated PLGA NPs | Ascorbic acid and EGCG | Mice | Neuroinflammation and neuronal loss | [119] | |
| Solid lipid NPs | Glycerol behenate | Curcumin | AD mouse model | Cellular damage reduction in brain | [111] |
| Cetylpalmitate and OX26 mAb | Resveratrol | Human brain-like endothelial cells | Inhibition of protein aggregation | [117] | |
| Vitamin E and sefsol | Resveratrol | In vitro | Increasing the levels of GSH and SOD | [118] | |
| Unspecified | EGCG | Rat | Increasing bioavailability of EGCG | [120] |
4.3. Cardiovascular Diseases
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | Copolyoxalate | Vanillyl alcohol | I/R mouse model | Reduction in ROS | [130] |
| PEG and poly-(propylene sulphide) | Ginsenoside Rg3 | I/R rat model | Inhibition of oxidative stress, inflammation and fibrosis | [131] | |
| (PLL-PEG-PLL) | - | I/R rat model | Decreased oxidative stress and promoted myocardial function | [134] | |
| PLGA | Quercetin | H9C2 cells | Increased quercetin bioavailability | [135] | |
| PLGA | Resveratrol | H9C2 cells | ROS scavenging | [136] | |
| PLGA | Resveratrol | Rat | Preventing myocardial necrosis | [137] | |
| PLGA | Pioglitazone | Mouse and porcine model | Cardioprotection | [138] | |
| PLGA | Irbesartan | I/R mouse model | Anti-inflammatory activity and reduced infarct size | [139] | |
| PLGA | Mdivi-1 | I/R mouse model | Cardioprotection against I/R | [140] | |
| PLGA | CoQ-10 | Mice | Increased bioavailability | [142] | |
| PGMA | AID and cur/res | Rat | Decreased oxidative stress | [145] | |
| Solid lipid NPs | PEG-modified solid lipid NPs | Baicalin, schisandrin B | Rat | Reduction in the infarction size | [132,133] |
| Egg phosphatidylcholine, cholesterol, PEG2000-DSPE | CoQ-10 | I/R rabbit model | Limiting the fraction of damaged myocardium | [143] | |
| Inorganic NPs | Ceria NPs | - | Murine cardiac progenitor cells | Protecting cardiac progenitor cells | [147] |
| AuNP-MIBI | - | I/R rat model | Reduction in inflammation | [150] | |
| Inorganic fibres | Polyurethane | Methylprednisolone | Rat | Reconstruction of cardiac function | [152] |
| Hydrogels | Modified chitosan (CS-B-NO) | NO | I/R mouse model | Attenuation of cardiac damage | [154] |
| PMNT-PEG-PMNT | - | Mouse | ROS scavenging | [155] | |
| PVA/Dex | Astaxanthin | Rat | Reduction in oxidative stress | [156] | |
| Chitosan | α-tocopherol | Neonatal rat cardiomyocytes | Suppression of oxidative stress | [157] | |
| Chitosan chloride–glutathione | - | Neonatal rat cardiomyocytes | Scavenging superoxide anion and hydroxyl radical | [158] | |
| Chitosan–vitamin E | - | Neonatal rat cardiomyocytes | Reducing ROS | [159] | |
| Chitosan | Ferulic acid | Rabbit | Protection from oxidative stress | [160] | |
| CMC-BA | Curcumin, collagen III | Rat | Anti-inflammatory | [161] | |
| Alginate | Fullerenol nps | Brown adipose-derived stem cells | Scavenging the superoxide anion and hydroxyl radicals | [162] | |
| N-isopropyl acrylamide and methoxy-PEG methacrylate | - | Sheep | Increased contractile function and decreased ROS | [163] | |
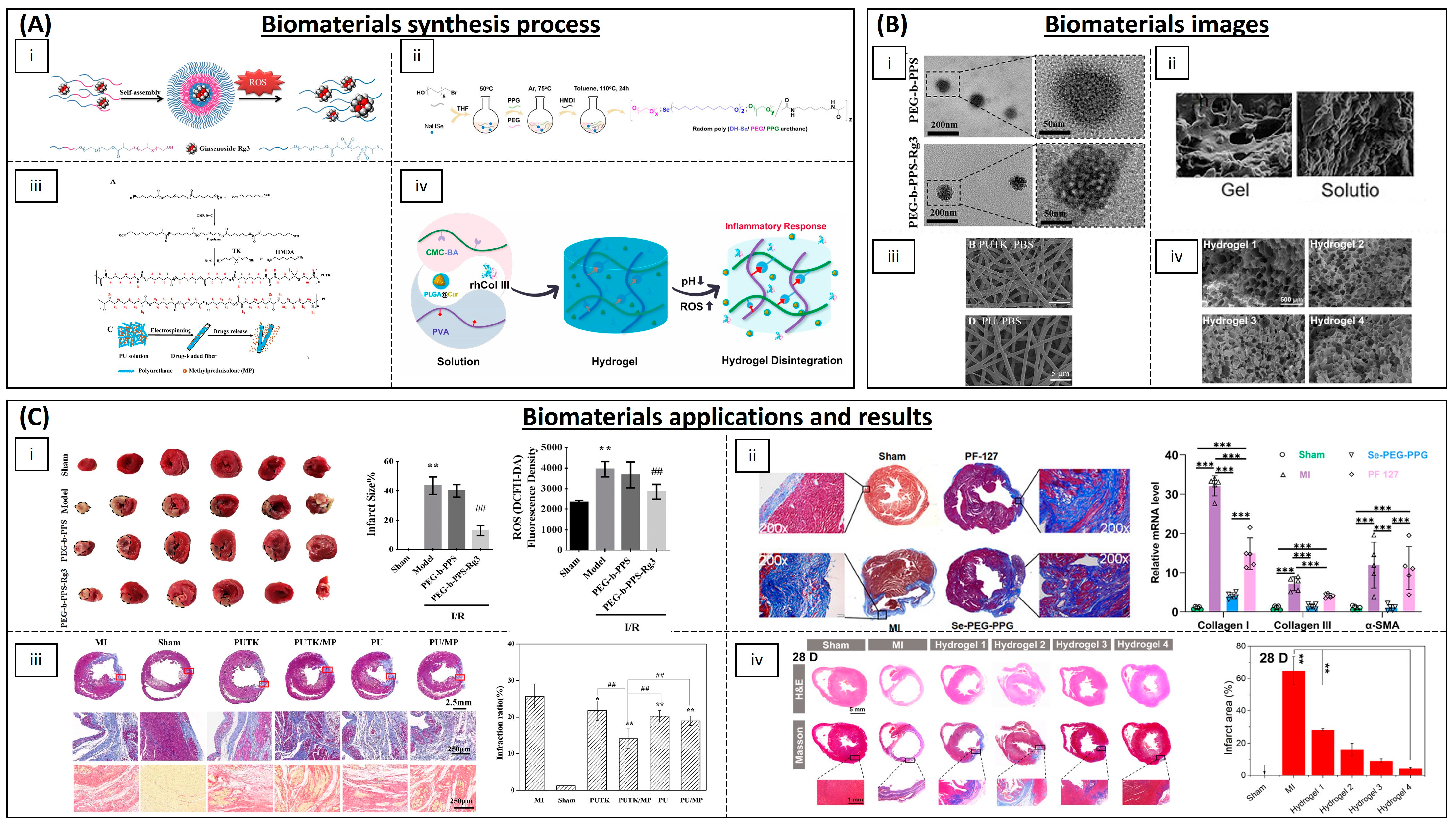
| Poly(DH-SE/PEG/PPG urethane | - | Mouse | Inhibition of inflammation and fibrosis | [164] | |
| GO-IG | MSCs | WJ-MSCs and rat cardiomyocytes | Decreasing the oxidative damage | [166] | |
| Hyaluronic acid and 2-hydroxy-β-cyclodextrin | Resveratrol and MSCs | Rat | Proangiogenic, anti-inflammatory and anti-apoptotic activity | [167] | |
| Polymeric scaffolds | Cellulose | Statin and heparin | - | Anti-thrombogenic and anti-inflammatory functions | [168] |
| PLA/PVA | TEMPOL, rapamycin | Porcine model | Favours endothelialization and mitigates local inflammation | [169] |
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | Chitosan | EGCG | THP-1 cells | Decreasing cholesterol content and chemoattractant protein expression in macrophages | [179] |
| PLGA | Curcumin–bioperine | THP-1 cells | Anti-inflammatory activity | [180] | |
| Poly lactide–glycolidechitin | Diosmin | Rat | Downregulation of inflammatory molecules levels | [182] | |
| Chitosan | Selenium | Mice | Alleviation of early atherosclerotic lesions | [183] | |
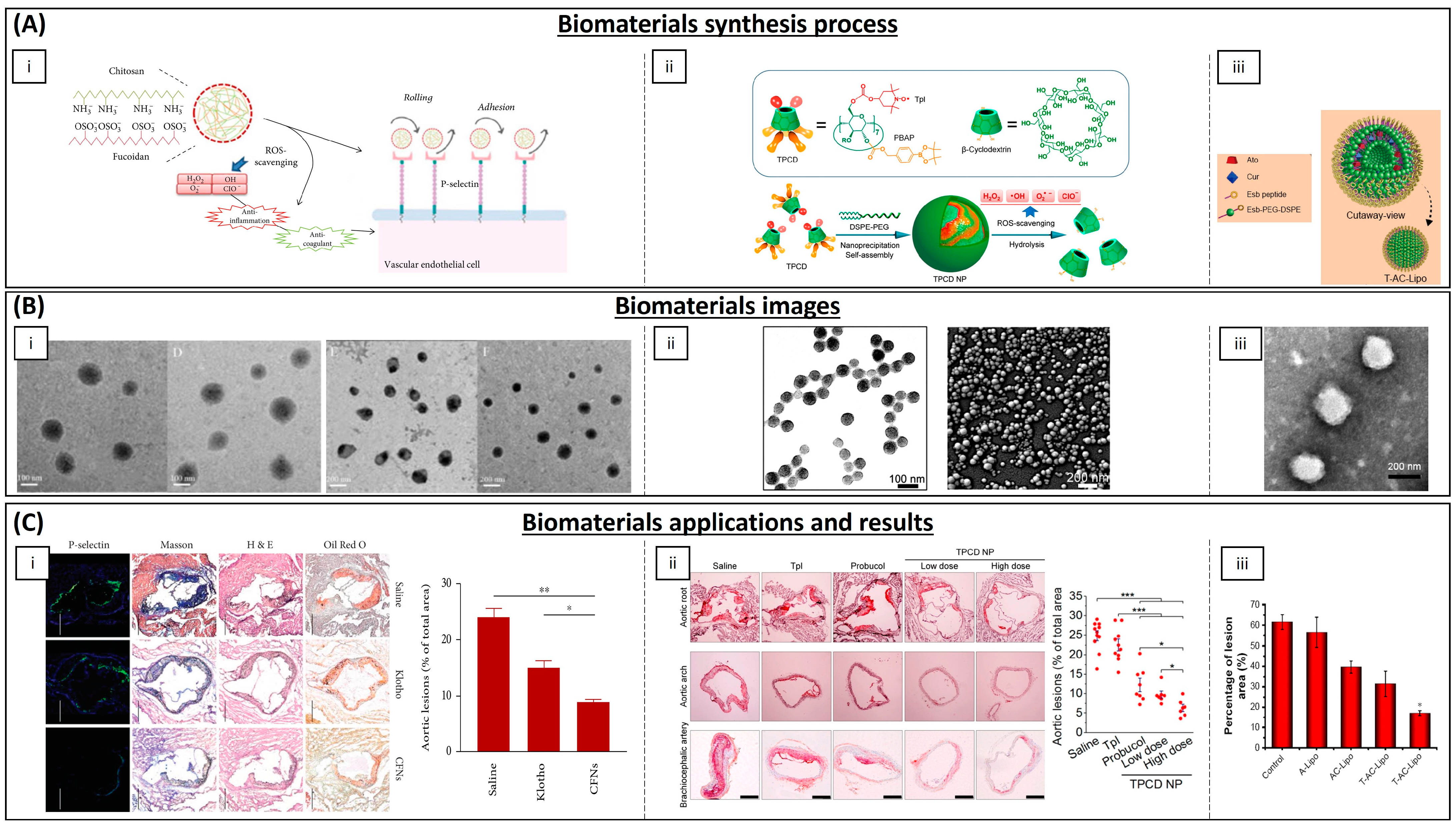
| Chitosan–fucoidan | - | Mice | Suppression of local oxidative stress and inflammation | [185] | |
| β-cyclodextrin | Tempol, phenylboronic acid pinacol ester | Mice | Antioxidant and anti-inflammatory properties | [186] | |
| Lipid NPs | Phosphatidylcholines | EGCG and α-tocopherol | Mice | Smaller lesion surface areas on aortic arches | [178] |
| Cholesterol, DPPC and Mal-PEG2000-DSPE | Atorvastatin calcium and curcumin | Mice | Reduction in plasma lipid levels | [181] | |
| Inorganic NPs | Iron oxide | Spinacia oleracea | Mice | Increased activity of SOD and catalase enzymes | [184] |
4.4. Bone Diseases
| Category | Material | Load | Model | Properties | Ref. |
|---|---|---|---|---|---|
| Hydrogel | Poloxamer 407 and selenium | Silibinin | Rat | Bone regeneration and mineralization | [201] |
| EGCG, 3-acrylamido phenylboronic acid and acrylamide | MSCs | Rabbit | Antioxidant and anti-inflammatory activity, and improved osteogenesis | [220] | |
| gelatine methacryloyl–dopamine | Melatonin | Rat | Promotion of osteogenesis and improved bone quality | [221] | |
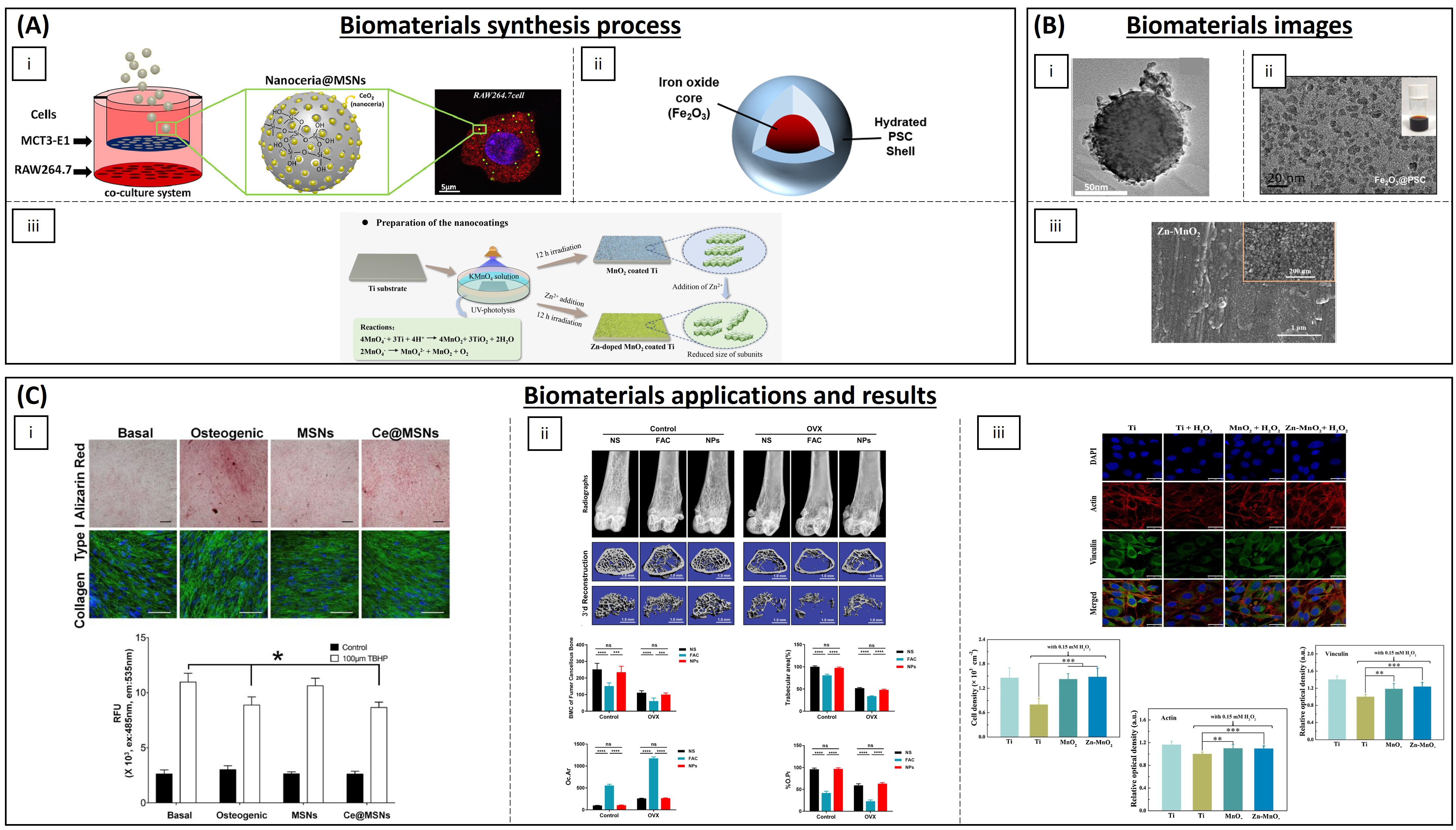
| Polymers | Silica NPs | Cerium oxide | RAW264.7 and MC3T3-E1 cells | Antioxidant capability and stimulated cell proliferation and osteogenic responses | [202] |
| Chitosan NPs | Shilajit water extract | Rat | Antioxidant and anti-inflammatory activity | [205] | |
| Fe2O3@PSC NPs | - | Mice | ROS scavenging, pro-osteogenic and inhibition of osteoclast differentiation | [206] | |
| Lycopene NPs | - | BMSCs | Pro-osteoblast differentiation | [211] | |
| PLGA NPs | Tocotrienol | Rat | Improvement in bone strength and mineralization | [207] | |
| polycaprolactone/gelatine NPs | Polaprezinc | Rat | Promotion of bone formation | [208] | |
| Titanium dioxide nanotubes | - | Rat calvarial osteoblasts | Improvement in osteoblast adhesion and osteogenic differentiation | [218] | |
| Inorganic NPs | Selenium | - | hESC-derived hMSCs | Increased antioxidant levels | [209] |
| Cerium oxide | - | MC3T3-E1 cells | Antioxidant activity | [203] | |
| Iron oxide | - | Mice | Antioxidant, osteogenic differentiation and inhibition of osteoclast differentiation | [212] | |
| Platinum | - | Mice | Decreased osteoclast activity levels and antioxidant capacity | [213] | |
| Manganese | β-tricalcium | Rat | Promotion of the differentiation of osteoblasts and accelerate bone regeneration | [214] | |
| Manganese oxide | Zn2+ | MC3T3-E1 cells | Catalase-like activity | [216] |
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Juan, C.; Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Jaimes, E.A. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Mattevi, A. Structure and Mechanisms of ROS Generation by NADPH Oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Pinchuk, I. Oxidative Stress, the Term and the Concept. Biochem. Biophys. Res. Commun. 2015, 461, 441–444. [Google Scholar] [CrossRef]
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A.; Chan, D.A.; Giaccia, A.J.; Richard, D.E. Hypoxia-Inducible Factor-1 Activation in Nonhypoxic Conditions: The Essential Role of Mitochondrial-Derived Reactive Oxygen Species. Mol. Biol. Cell 2010, 21, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-Dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef]
- Gao, Q. Oxidative Stress and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 179–198. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.C.; Sasai, M.; Lee, H.K.; Yordy, B.; Shadel, G.S.; Iwasaki, A. Absence of Autophagy Results in Reactive Oxygen Species-Dependent Amplification of RLR Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 2770–2775. [Google Scholar] [CrossRef]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR Signalling Augments Macrophage Bactericidal Activity through Mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef]
- Guo, C.; Dong, G.; Liang, X.; Dong, Z. NOX2-Dependent Regulation of Inflammation. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging In Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Allen, R.G. Oxidative Stress as a Causal Factor in Differentiation and Aging: A Unifying Hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Abuja, P.M.; Albertini, R. Methods for Monitoring Oxidative Stress, Lipid Peroxidation and Oxidation Resistance of Lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Env. Sci. Health C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Mori, M.P.; Souza-Pinto, N.C. de Formation and Repair of Oxidative Damage in the Mitochondrial DNA. Mitochondrion 2014, 17, 164–181. [Google Scholar] [CrossRef]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative Stress, DNA Methylation and Carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef]
- Ramana, K.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease. Oxid. Med. Cell. Longev. 2013, 2013, 583438. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular Dynamic Simulations of Oxidized Skin Lipid Bilayer and Permeability of Reactive Oxygen Species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in Cardiovascular Therapy: Panacea or False Hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Feric, N.T.; Pallotta, I.; Singh, R.; Bogdanowicz, D.R.; Gustilo, M.M.; Chaudhary, K.W.; Willette, R.N.; Chendrimada, T.P.; Xu, X.; Graziano, M.P.; et al. Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicol. Sci. 2019, 172, 89–97. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Mastikhina, O.; Moon, B.U.; Williams, K.; Hatkar, R.; Gustafson, D.; Mourad, O.; Sun, X.; Koo, M.; Lam, A.Y.L.; Sun, Y.; et al. Human Cardiac Fibrosis-on-a-Chip Model Recapitulates Disease Hallmarks and Can Serve as a Platform for Drug Testing. Biomaterials 2020, 233, 119741. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Desai, R.M.; Joly, P.; Li, J.; Bagrodia, R.K.; Lewin, S.A.; Joshi, N.S.; Mooney, D.J. Click-Crosslinked Injectable Gelatin Hydrogels. Adv. Healthc. Mater. 2016, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Ott, H.C. Organ Engineering Based on Decellularized Matrix Scaffolds. Trends Mol. Med. 2011, 17, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Belviso, I.; Romano, V.; Sacco, A.M.; Ricci, G.; Massai, D.; Cammarota, M.; Catizone, A.; Schiraldi, C.; Nurzynska, D.; Terzini, M.; et al. Decellularized Human Dermal Matrix as a Biological Scaffold for Cardiac Repair and Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Montero, P.; Flandes, M.; Musquiz, S.; Araluce, M.P.; Plano, D.; Sanmartín, C.; Gavira, J.J.; Mazo, M.M.; Prosper, F. Cells, Materials and Fabrication Processes for Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer-Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Montero-Calle, P.; Flandes-Iparraguirre, M.; Mountris, K.; de la Nava, A.S.; Laita, N.; Rosales, R.M.; Iglesias-García, O.; de-Juan-Pardo, E.M.; Atienza, F.; Fernández-Santos, M.E.; et al. Fabrication of Human Myocardium Using Multidimensional Modelling of Engineered Tissues. Biofabrication 2022, 14, 045017. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS:Gelatin Nanofibrous Scaffolds with Tunable Mechanical and Structural Properties for Engineering Cardiac Tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing Structural Integrity of Hydrogels by Using Highly Organised Melt Electrospun Fibre Constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Shakiba, N.; Zandstra, P.W. Engineering Cell Fitness: Lessons for Regenerative Medicine. Curr. Opin. Biotechnol. 2017, 47, 7–15. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Gamna, F.; Yamaguchi, S.; Cochis, A.; Ferraris, S.; Kumar, A.; Rimondini, L.; Spriano, S. Conferring Antioxidant Activity to an Antibacterial and Bioactive Titanium Surface through the Grafting of a Natural Extract. Nanomaterials 2023, 13, 479. [Google Scholar] [CrossRef]
- Bommakanti, V.; Banerjee, M.; Shah, D.; Manisha, K.; Sri, K.; Banerjee, S. An Overview of Synthesis, Characterization, Applications and Associated Adverse Effects of Bioactive Nanoparticles. Environ. Res. 2022, 214, 113919. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Xu, G.M.; Zhang, G.J.; Liu, J.R.; Wu, Y.M.; Gao, L.G.; Yang, Y.; Chang, Z.S.; Yao, C.W. Low-Temperature Plasma Promotes Fibroblast Proliferation in Wound Healing by ROS-Activated NF-ΚB Signaling Pathway. Curr. Med. Sci. 2018, 38, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Koo, M.A.; Hee Hong, S.; Hee Lee, M.; Kwon, B.J.; Mi Seon, G.; Sung Kim, M.; Kim, D.; Chang Nam, K.; Park, J.C. Effective Stacking and Transplantation of Stem Cell Sheets Using Exogenous ROS-Producing Film for Accelerated Wound Healing. Acta Biomater. 2019, 95, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Zhu, Y.; Hoshi, R.; Chen, S.; Yi, J.; Duan, C.; Galiano, R.D.; Zhang, H.F.; Ameer, G.A. Sustained Release of Stromal Cell Derived Factor-1 from an Antioxidant Thermoresponsive Hydrogel Enhances Dermal Wound Healing in Diabetes. J. Control. Release 2016, 238, 114–122. [Google Scholar] [CrossRef]
- Helary, C.; Abed, A.; Mosser, G.; Louedec, L.; Letourneur, D.; Coradin, T.; Giraud-Guille, M.M.; Meddahi-Pellé, A. Evaluation of Dense Collagen Matrices as Medicated Wound Dressing for the Treatment of Cutaneous Chronic Wounds. Biomater. Sci. 2015, 3, 373–382. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A Composite Hydrogel of Chitosan/Heparin/Poly (γ-Glutamic Acid) Loaded with Superoxide Dismutase for Wound Healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhuang, H.; Hao, Y.; Zhang, L.; Yang, Q.; Liu, Y.; Qi, C.; Wang, S. Poly(N-Isopropyl-Acrylamide)/Poly(γ-Glutamic Acid) Thermo-Sensitive Hydrogels Loaded with Superoxide Dismutase for Wound Dressing Application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, S.; Yin, J.J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Jeon, J.; Lee, M.S.; Yang, H.S.; Tae, G. Antioxidant and Anti-Inflammatory Activities of Prussian Blue Nanozyme Promotes Full-Thickness Skin Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Shi, Z.; Yue, K.; Huang, X.; Xu, Y.; Gao, C.; Yao, Z.; Zhang, Y.S.; Wang, J. Sprayable Hydrogel Dressing Accelerates Wound Healing with Combined Reactive Oxygen Species-Scavenging and Antibacterial Abilities. Acta Biomater. 2021, 124, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Hilton, S.A.; Osmond, M.J.; Zgheib, C.; Newsom, J.P.; Dewberry, L.; Singh, S.; Sakthivel, T.S.; Seal, S.; Liechty, K.W.; et al. Injectable, Self-Healable Zwitterionic Cryogels with Sustained MicroRNA-Cerium Oxide Nanoparticle Release Promote Accelerated Wound Healing. Acta Biomater. 2020, 101, 262–272. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and Antioxidant Nanochitosan Dots/Biocellulose Hydrogels for Wound Healing Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111925. [Google Scholar] [CrossRef]
- Jung, B.O.; Chung, S.J.; Lee, S.B. Preparation and Characterization of Eugenol-Grafted Chitosan Hydrogels and Their Antioxidant Activities. J. Appl. Polym. Sci. 2006, 99, 3500–3506. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, J.; Cao, L.; Jiao, Q.; Zhou, J.; Yang, L.; Zhang, H.; Wei, Y. Antifouling Antioxidant Zwitterionic Dextran Hydrogels as Wound Dressing Materials with Excellent Healing Activities. ACS Appl. Mater. Interfaces 2021, 13, 7060–7069. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, W.; Lei, Y.; Gaucher, C.; Pei, S.; Zhang, J.; Xia, X. Edaravone-Loaded Alginate-Based Nanocomposite Hydrogel Accelerated Chronic Wound Healing in Diabetic Mice. Mar. Drugs 2019, 17, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.; Alam, S.; Samanta, S.; Prakash, R.G.; Govindaraju, T. Antioxidant Silk Fibroin Composite Hydrogel for Rapid Healing of Diabetic Wound. Macromol. Biosci. 2022, 22, e2200097. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.M.; Choi, W. Il Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Sharma, A.; Zhang, T.; Wu, Y.; Ding, X. Pharmacological Review on Asiatic Acid and Its Derivatives: A Potential Compound. SLAS Technol. 2018, 23, 111–127. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Li, Y.; Wang, M.; Fan, T.; Liu, M.; Ke, Q.; Xu, H.; Yi, Z. An Aligned Porous Electrospun Fibrous Scaffold with Embedded Asiatic Acid for Accelerating Diabetic Wound Healing. J. Mater. Chem. B 2019, 7, 6125–6138. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green Tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Zhao, B.; Li, X.; Liang, W.; Lang, M.; Cheng, B.; Li, J. Antibacterial, Antioxidant and Anti-Inflammatory PLCL/Gelatin Nanofiber Membranes to Promote Wound Healing. Int. J. Biol. Macromol. 2022, 194, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.A. Economic Burden of Alzheimer Disease and Managed Care Considerations. Am. J. Manag. Care 2020, 26, S171–S183. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and Away from Alzheimer’s Disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s Disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M.Y. Initiation and Synergistic Fibrillization of Tau and Alpha-Synuctein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Mizuno, Y. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 406–411. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Aguilera, G.; Colín-González, A.L.; Rangel-López, E.; Chavarría, A.; Santamaría, A. Redox Signaling, Neuroinflammation, and Neurodegeneration. Antioxid. Redox Signal. 2018, 28, 1626–1651. [Google Scholar] [CrossRef]
- Bello-Medina, P.C.; González-Franco, D.A.; Vargas-Rodríguez, I.; Díaz-Cintra, S. Oxidative Stress, the Immune Response, Synaptic Plasticity, and Cognition in Transgenic Models of Alzheimer Disease. Neurologia 2022, 37, 682–690. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The Impact of Reactive Oxygen Species and Genetic Mitochondrial Mutations in Parkinson’s Disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium Oxide Nanoparticles in Neuroprotection and Considerations for Efficacy and Safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef] [PubMed]
- Zand, Z.; Khaki, P.A.; Salihi, A.; Sharifi, M.; Nanakali, N.M.Q.; Alasady, A.A.B.; Aziz, F.M.; Shahpasand, K.; Hasan, A.; Falahati, M. Cerium Oxide NPs Mitigate the Amyloid Formation of α-Synuclein and Associated Cytotoxicity. Int. J. Nanomed. 2019, 14, 6989–7000. [Google Scholar] [CrossRef] [PubMed]
- Siposova, K.; Huntosova, V.; Garcarova, I.; Shlapa, Y.; Timashkov, I.; Belous, A.; Musatov, A. Dual-Functional Antioxidant and Antiamyloid Cerium Oxide Nanoparticles Fabricated by Controlled Synthesis in Water-Alcohol Solutions. Biomedicines 2022, 10, 942. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Cappello, V.; Gemmi, M.; Athanassiou, A.; Mazzolai, B.; Mattoli, V. Effects of Cerium Oxide Nanoparticles on PC12 Neuronal-like Cells: Proliferation, Differentiation, and Dopamine Secretion. Pharm. Res. 2013, 30, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs Hybrid Nanoparticles as a Mimicking Metallopeptidase for Treatment of Neurotoxicity of Amyloid-β Peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, X.; Wang, L.; Yin, M.; Wang, L.; Chen, N.; Fan, C.; Song, H. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv. Mater. 2016, 28, 1387–1393. [Google Scholar] [CrossRef]
- Ghaznavi, H.; Najafi, R.; Mehrzadi, S.; Hosseini, A.; Tekyemaroof, N.; Shakeri-Zadeh, A.; Rezayat, M.; Sharifi, A.M. Neuro-Protective Effects of Cerium and Yttrium Oxide Nanoparticles on High Glucose-Induced Oxidative Stress and Apoptosis in Undifferentiated PC12 Cells. Neurol. Res. 2015, 37, 624–632. [Google Scholar] [CrossRef]
- Hosseini, A.; Mohammad Sharifi, A.; Abdollahi, M.; Najafi, R.; Baeeri, M.; Rayegan, S.; Cheshmehnour, J.; Hassani, S.; Bayrami, Z.; Safa, M. Cerium and Yttrium Oxide Nanoparticles Against Lead-Induced Oxidative Stress and Apoptosis in Rat Hippocampus. Biol. Trace Elem. Res. 2015, 164, 80–89. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, 2101526. [Google Scholar] [CrossRef]
- Rodriguez-Losada, N.; Wendelbob, R.; Ocaña, M.C.; Casares, A.D.; Guzman de Villoría, R.; Aguirre Gomez, J.A.; Arraez, M.A.; Gonzalez-Alegre, P.; Medina, M.A.; Arenas, E.; et al. Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci. 2020, 14, 570409. [Google Scholar] [CrossRef] [PubMed]
- Fabian, R.H.; Derry, P.J.; Rea, H.C.; Dalmeida, W.V.; Nilewski, L.G.; Sikkema, W.K.A.; Mandava, P.; Tsai, A.L.; Mendoza, K.; Berka, V.; et al. Efficacy of Novel Carbon Nanoparticle Antioxidant Therapy in a Severe Model of Reversible Middle Cerebral Artery Stroke in Acutely Hyperglycemic Rats. Front. Neurol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chena, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1044–1055. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A Novel Synthesis of Selenium Nanoparticles Encapsulated PLGA Nanospheres with Curcumin Molecules for the Inhibition of Amyloid β Aggregation in Alzheimer’s Disease. J. Photochem. Photobiol. B 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Barbara, R.; Belletti, D.; Pederzoli, F.; Masoni, M.; Keller, J.; Ballestrazzi, A.; Vandelli, M.A.; Tosi, G.; Grabrucker, A.M. Novel Curcumin Loaded Nanoparticles Engineered for Blood-Brain Barrier Crossing and Able to Disrupt Abeta Aggregates. Int. J. Pharm. 2017, 526, 413–424. [Google Scholar] [CrossRef]
- Iemolo, A.; Moein Moghimi, S.; Decuzzi, P.; Ameruoso, A.; Palomba, R.; Lisa Palange, A.; Cervadoro, A.; Lee, A.; Di Mascolo, D. Ameliorating Amyloid-β Fibrils Triggered Inflammation via Curcumin-Loaded Polymeric Nanoconstructs. Front. Immunol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Huang, N.; Lu, S.; Liu, X.G.; Zhu, J.; Wang, Y.J.; Liu, R.T. PLGA Nanoparticles Modified with a BBB-Penetrating Peptide Co-Delivering Aβ Generation Inhibitor and Curcumin Attenuate Memory Deficits and Neuropathology in Alzheimer’s Disease Mice. Oncotarget 2017, 8, 81001–81013. [Google Scholar] [CrossRef]
- Maiti, P.; Paladugu, L.; Dunbar, G.L. Solid Lipid Curcumin Particles Provide Greater Anti-Amyloid, Anti-Inflammatory and Neuroprotective Effects than Curcumin in the 5xFAD Mouse Model of Alzheimer’s Disease BMC Neuroscience. BMC Neurosci. 2018, 19, 7. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of Unloaded and Curcumin-Loaded Solid Lipid Nanoparticles on Tissue Transglutaminase Isoforms Expression Levels in an Experimental Model of Alzheimer’s Disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Esmaeili, A.; Zarrabi, A.; Zarepour, A. Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics 2022, 14, 2692. [Google Scholar] [CrossRef] [PubMed]
- Palmal, S.; Maity, A.R.; Singh, B.K.; Basu, S.; Jana, N.R.; Jana, N.R. Inhibition of Amyloid Fibril Growth and Dissolution of Amyloid Fibrils by Curcumin-Gold Nanoparticles. Chemistry 2014, 20, 6184–6191. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Redox Nanoparticles: Synthesis, Properties and Perspectives of Use for Treatment of Neurodegenerative Diseases. J. Nanobiotechnol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Abozaid, O.A.R.; Sallam, M.W.; El-Sonbaty, S.; Aziza, S.; Emad, B.; Ahmed, E.S.A. Resveratrol-Selenium Nanoparticles Alleviate Neuroinflammation and Neurotoxicity in a Rat Model of Alzheimer’s Disease by Regulating Sirt1/MiRNA-134/GSK3β Expression. Biol. Trace Elem. Res. 2022, 200, 5104–5114. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.N.; et al. Resveratrol and Grape Extract-Loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E Loaded Resveratrol Nanoemulsion for Brain Targeting for the Treatment of Parkinson’s Disease by Reducing Oxidative Stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kühne, B.A.; Barenys, M.; Sánchez-López, E.; Souto, E.B.; García, M.L.; et al. Epigallocatechin-3-Gallate PEGylated Poly(Lactic-Co-Glycolic) Acid Nanoparticles Mitigate Striatal Pathology and Motor Deficits in 3-Nitropropionic Acid Intoxicated Mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic Particles Improve the Bioavailability and Alpha-Secretase Inducing Ability of Epigallocatechin-3-Gallate (EGCG) for the Treatment of Alzheimer’s Disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef]
- Vishwas, S.; Kumar, R.; Khursheed, R.; Ramanunny, A.K.; Kumar, R.; Awasthi, A.; Corrie, L.; Porwal, O.; Arshad, M.F.; Alshammari, M.K.; et al. Expanding Arsenal against Neurodegenerative Diseases Using Quercetin Based Nanoformulations: Breakthroughs and Bottlenecks. Curr. Neuropharmacol. 2022, 20, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic Acid Inhibits Oxidative Stress and Cell Death Induced by Ab Oligomers: Improved Delivery by Solid Lipid Nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; Prasad, P.N.; Mahajan, S.D. Neuroprotective Effects of a Biodegradable Poly(Lactic-Co-Glycolic Acid)-Ginsenoside Rg3 Nanoformulation: A Potential Nanotherapy for Alzheimer’s Disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Soudi, S.A.; Nounou, M.I.; Sheweita, S.A.; Ghareeb, D.A.; Younis, L.K.; El-Khordagui, L.K. Protective Effect of Surface-Modified Berberine Nanoparticles against LPS-Induced Neurodegenerative Changes: A Preclinical Study. Drug Deliv. Transl. Res. 2019, 9, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Lanthier, P.; Miller, H.; Beyers, M.; Sodja, C.; Zurakowski, B.; Gangaraju, S.; Pandey, S.; Sandhu, J.K. Nanomicellar Formulation of Coenzyme Q10 (Ubisol-Q10) Effectively Blocks Ongoing Neurodegeneration in the Mouse 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Model: Potential Use as an Adjuvant Treatment in Parkinson’s Disease. Neurobiol. Aging 2014, 35, 2329–2346. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M.T.B.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid Med Cell Longev 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, 2181–2190. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative Stress and Inflammation in the Evolution of Heart Failure: From Pathophysiology to Therapeutic Strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Bae, S.; Park, M.; Kang, C.; Dilmen, S.; Kang, T.H.; Kang, D.G.; Ke, Q.; Lee, S.U.; Lee, D.; Kang, P.M. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003697. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Guo, R.; Li, S.; Ni, J.; Gao, S.; Gao, X.; Mao, J.; Zhu, Y.; Wu, P.; et al. Ginsenoside Rg3-Loaded, Reactive Oxygen Species-Responsive Polymeric Nanoparticles for Alleviating Myocardial Ischemia-Reperfusion Injury. J. Control. Release 2020, 317, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Pan, J. Drug Delivery Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats Baicalin-Loaded PEGylated Lipid Nanoparticles: Characterization, Pharmacokinetics, and Protective Effects on Acute Myocardial Ischemia in Rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Yang, W.; Han, G. Protective Effects on Myocardial Infarction Model: Delivery of Schisandrin B Using Matrix Metalloproteinase-Sensitive Peptide-Modified, Pegylated Lipid Nanoparticles. Int. J. Nanomed. 2017, 12, 7121. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, P.; Zhou, H.; Shen, R.; Hu, B.; Shen, Y.; Zhang, X.; Shen, X.; Xu, G.; Jin, L. Pharmacological Signatures of the Exenatide Nanoparticles Complex Against Myocardial Ischemia Reperfusion Injury. Kidney Blood Press. Res. 2018, 43, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Lozano, O.; Lázaro-Alfaro, A.; Silva-Platas, C.; Oropeza-Almazán, Y.; Torres-Quintanilla, A.; Bernal-Ramírez, J.; Alves-Figueiredo, H.; García-Rivas, G. Nanoencapsulated Quercetin Improves Cardioprotection during Hypoxia-Reoxygenation Injury through Preservation of Mitochondrial Function. Oxid. Med. Cell. Longev. 2019, 2019, 7683051. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, D.Z.; Zhang, C.X.; Cui, H.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Lu, Z.F.; Zhou, S.Y. Mitochondria-Targeted Antioxidant Delivery for Precise Treatment of Myocardial Ischemia–Reperfusion Injury through a Multistage Continuous Targeted Strategy. Nanomedicine 2019, 16, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, Y.; Mishra, A.; Sreeharsha, N.; Moktan, J.B.; Kumar, P.; Wang, L. Protective Role of Poly(Lactic-Co-Glycolic) Acid Nanoparticle Loaded with Resveratrol against Isoproterenol-Induced Myocardial Infarction. Biofactors 2020, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Tokutome, M.; Matoba, T.; Nakano, Y.; Okahara, A.; Fujiwara, M.; Koga, J.I.; Nakano, K.; Tsutsui, H.; Egashira, K. Peroxisome Proliferator-Activated Receptor-Gamma Targeting Nanomedicine Promotes Cardiac Healing after Acute Myocardial Infarction by Skewing Monocyte/Macrophage Polarization in Preclinical Animal Models. Cardiovasc. Res. 2019, 115, 419–431. [Google Scholar] [CrossRef]
- Nakano, Y.; Matoba, T.; Tokutome, M.; Funamoto, D.; Katsuki, S.; Ikeda, G.; Nagaoka, K.; Ishikita, A.; Nakano, K.; Koga, J.-I.; et al. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci. Rep. 2016, 6, 29601. [Google Scholar] [CrossRef]
- Ishikita, A.; Matoba, T.; Ikeda, G.; Koga, J.I.; Mao, Y.; Nakano, K.; Takeuchi, O.; Sadoshima, J.; Egashira, K. Nanoparticle-Mediated Delivery of Mitochondrial Division Inhibitor 1 to the Myocardium Protects the Heart From Ischemia-Reperfusion Injury Through Inhibition of Mitochondria Outer Membrane Permeabilization: A New Therapeutic Modality for Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003872. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Simoń-Yarza, T.; Tamayo, E.; Benavides, C.; Lana, H.; Formiga, F.R.; Grama, C.N.; Ortiz-de-Solorzano, C.; Ravi Kumar, M.N.V.; Prosper, F.; Blanco-Prieto, M.J. Functional Benefits of PLGA Particulates Carrying VEGF and CoQ10 in an Animal of Myocardial Ischemia. Int. J. Pharm. 2013, 454, 784–790. [Google Scholar] [CrossRef][Green Version]
- Verma, D.D.; Hartner, W.C.; Thakkar, V.; Levchenko, T.S.; Torchilin, V.P. Protective Effect of Coenzyme Q10-Loaded Liposomes on the Myocardium in Rabbits with an Acute Experimental Myocardial Infarction. Pharm. Res. 2007, 24, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Viola, H.M.; Arthur, P.G.; Hool, L.C. Transient Exposure to Hydrogen Peroxide Causes an Increase in Mitochondria-Derived Superoxide as a Result of Sustained Alteration in L-Type Ca2+ Channel Function in the Absence of Apoptosis in Ventricular Myocytes. Circ. Res. 2007, 100, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.; Viola, H.M.; Johnstone, V.P.A.; Clemons, T.D.; Cserne Szappanos, H.; Singh, R.; Smith, N.M.; Iyer, K.S.; Hool, L.C. Nanoparticle-Mediated Dual Delivery of an Antioxidant and a Peptide against the L-Type Ca2+ Channel Enables Simultaneous Reduction of Cardiac Ischemia-Reperfusion Injury. ACS Nano 2015, 9, 279–289. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria Nanoparticles That Can Protect against Ischemic Stroke. Angew. Chem. Int. Ed. Engl. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, F.; Mandoli, C.; Forte, G.; Magnani, E.; Pagliari, S.; Nardone, G.; Licoccia, S.; Minieri, M.; Di Nardo, P.; Traversa, E. Cerium Oxide Nanoparticles Protect Cardiac Progenitor Cells from Oxidative Stress. ACS Nano 2012, 6, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; DessÌ, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-Step Green Synthesis and Characterization of Gold-Conjugated Polyphenol Nanoparticles with Antioxidant and Biological Activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar] [CrossRef]
- Babu, B.; Palanisamy, S.; Vinosha, M.; Anjali, R.; Kumar, P.; Pandi, B.; Tabarsa, M.; You, S.G.; Prabhu, N.M. Bioengineered Gold Nanoparticles from Marine Seaweed Acanthophora Spicifera for Pharmaceutical Uses: Antioxidant, Antibacterial, and Anticancer Activities. Bioprocess Biosyst. Eng. 2020, 43, 2231–2242. [Google Scholar] [CrossRef]
- Tartuce, L.P.; Pacheco Brandt, F.; dos Santos Pedroso, G.; Rezende Farias, H.; Barros Fernandes, B.; da Costa Pereira, B.; Gonçalves Machado, A.; Feuser, P.E.; Lock Silveira, P.C.; Tiscoski Nesi, R.; et al. 2-Methoxy-Isobutyl-Isonitrile-Conjugated Gold Nanoparticles Improves Redox and Inflammatory Profile in Infarcted Rats. Colloids Surf. B Biointerfaces 2020, 192, 111012. [Google Scholar] [CrossRef]
- Jarrar, Q.; Al-Doaiss, A.; Jarrar, B.M.; Alshehri, M. On the Toxicity of Gold Nanoparticles: Histological, Histochemical and Ultrastructural Alterations. Toxicol. Ind. Health 2022, 38, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ding, J.; Wang, Z.; Zhang, H.; Xie, J.; Wang, Y.; Hong, L.; Mao, Z.; Gao, J.; Gao, C. ROS-Responsive Polyurethane Fibrous Patches Loaded with Methylprednisolone (MP) for Restoring Structures and Functions of Infarcted Myocardium in Vivo. Biomaterials 2020, 232, 119726. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, e2105408. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel Angiogenesis Therapeutics by Redox Injectable Hydrogel-Regulation of Local Nitric Oxide Generation for Effective Cardiovascular Therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Gregnanin, G.; Cencetti, C.; Di Meo, C.; Gueguen, V.; Letourneur, D.; Meddahi-Pellé, A.; Pavon-Djavid, G.; Matricardi, P. PVA/Dextran Hydrogel Patches as Delivery System of Antioxidant Astaxanthin: A Cardiovascular Approach. Biomed. Mater. 2017, 13, 015020. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.L.; Chen, S.; Gao, Y. α-Tocopherol Liposome Loaded Chitosan Hydrogel to Suppress Oxidative Stress Injury in Cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A Chitosan-Glutathione Based Injectable Hydrogel for Suppression of Oxidative Stress Damage in Cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Yu, J.; Song, L.; Chen, S.; Qin, Z.; Gong, J.; Zhan, H.; Gao, Y.; Zhang, J. A Chitosan-Vitamin C Based Injectable Hydrogel Improves Cell Survival under Oxidative Stress. Int. J. Biol. Macromol. 2022, 202, 102–111. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lin, F.H.; Wang, C.Y.; Hsiao, C.Y.; Chen, H.C.; Kuo, H.Y.; Tsai, T.F.; Chiou, S.H. Recovery of Oxidative Stress-Induced Damage in Cisd2-Deficient Cardiomyocytes by Sustained Release of Ferulic Acid from Injectable Hydrogel. Biomaterials 2016, 103, 207–218. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Zhang, W.; He, S.; Lu, L.; Fan, H.; Yang, L.; Wang, Y. Regeneration of Infarcted Hearts by Myocardial Infarction-Responsive Injectable Hydrogels with Combined Anti-Apoptosis, Anti-Inflammatory and pro-Angiogenesis Properties. Biomaterials 2022, 290, 121849. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, K.A.; Zhu, Y.; Takaba, K.; Ramasubramanian, A.; Badathala, A.; Haraldsson, H.; Collins, A.; Aguayo, E.; Shah, C.; Wallace, A.W.; et al. Myocardial Injection of a Thermoresponsive Hydrogel with Reactive Oxygen Species Scavenger Properties Improves Border Zone Contractility. J. Biomed. Mater. Res. A 2020, 108, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhu, C.; Li, Y.; Li, Z.; Zhang, Z.; Xu, J.; Chen, M.; Li, R.; Liu, S.; Wu, Y.; et al. Injectable Selenium-Containing Polymeric Hydrogel Formulation for Effective Treatment of Myocardial Infarction. Front. Bioeng. Biotechnol. 2022, 10, 912562. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Cheng, S.J.; Chen, H.H.; Hsu, W.C. Development of Injectable Graphene Oxide/Laponite/Gelatin Hydrogel Containing Wharton’s Jelly Mesenchymal Stem Cells for Treatment of Oxidative Stress-Damaged Cardiomyocytes. Colloids Surf. B Biointerfaces 2022, 209, 112150. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, X.; Liu, Y.; Cui, C.; Sun, Y.; Liu, W. Wet Adhesive Hydrogel Cardiac Patch Loaded with Anti-Oxidative, Autophagy-Regulating Molecule Capsules and MSCs for Restoring Infarcted Myocardium. Bioact. Mater. 2022, 21, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.; Pappa, F.; Karagkiozaki, V.; Gitas, C.; Ktenidis, K.; Logothetidis, S. Surface Modification of Endovascular Stents with Rosuvastatin and Heparin-Loaded Biodegradable Nanofibers by Electrospinning. Int. J. Nanomed. 2017, 12, 6343–6355. [Google Scholar] [CrossRef]
- Wang, R.; Lu, J.; Yin, J.; Chen, H.; Liu, H.; Xu, F.; Zang, T.; Xu, R.; Li, C.; Wu, Y.; et al. A TEMPOL and Rapamycin Loaded Nanofiber-Covered Stent Favors Endothelialization and Mitigates Neointimal Hyperplasia and Local Inflammation. Bioact. Mater. 2023, 19, 666–677. [Google Scholar] [CrossRef]
- Ghafoor, B.; Ali, N.; Riaz, Z. Synthesis and Appraisal of Natural Drug-Polymer-Based Matrices Relevant to the Application of Drug-Eluting Coronary Stent Coatings. Cardiol. Res. Pr. 2020, 2020, 4073091. [Google Scholar] [CrossRef]
- Wang, K.; Shang, T.; Zhang, L.; Zhou, L.; Liu, C.; Fu, Y.; Zhao, Y.; Li, X.; Wang, J. Application of a Reactive Oxygen Species-Responsive Drug-Eluting Coating for Surface Modification of Vascular Stents. ACS Appl. Mater. Interfaces 2021, 13, 35443. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Yang, H.; Wang, B.; Dai, Y.; Li, X.; Yan, K.; You, R.; Ma, L. Silk Fibroin/Chitosan Coating with Tunable Catalytic Nitric Oxide Generation for Surface Functionalization of Cardiovascular Stents. Int. J. Biol. Macromol. 2023, 228, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Venkata, N.; Pothineni, K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Genet. Genom. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S. Targeting Oxidative Stress for the Treatment of Ischemic Stroke: Upstream and Downstream Therapeutic Strategies. Brain Circ. 2016, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Zu, Y.; Abbasi, M.; Cao, J.; Li, C.; Wu, D.; Labib, S.; Brackee, G.; Shen, C.L.; et al. Anti-Atherogenic Effects of CD36-Targeted Epigallocatechin Gallate-Loaded Nanoparticles. J. Control. Release 2019, 303, 263–273. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation Enhances Epigallocatechin-3-Gallate Stability and Its Antiatherogenic Bioactivities in Macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef]
- Pillai, S.C.; Borah, A.; Le, M.N.T.; Kawano, H.; Hasegawa, K.; Sakthi Kumar, D. Co-Delivery of Curcumin and Bioperine via Plga Nanoparticles to Prevent Atherosclerotic Foam Cell Formation. Pharmaceutics 2021, 13, 1420. [Google Scholar] [CrossRef]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic Effects of Liposomes Encapsulating Atorvastatin Calcium and Curcumin and Targeting Dysfunctional Endothelial Cells in Reducing Atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Om, H.; El-Naggar, M.E.; El-Banna, M.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Combating Atherosclerosis with Targeted Diosmin Nanoparticles-Treated Experimental Diabetes. Investig. New Drugs 2020, 38, 1303–1315. [Google Scholar] [CrossRef]
- Xiao, J.; Li, N.; Xiao, S.; Wu, Y.; Liu, H. Comparison of Selenium Nanoparticles and Sodium Selenite on the Alleviation of Early Atherosclerosis by Inhibiting Endothelial Dysfunction and Inflammation in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11612. [Google Scholar] [CrossRef]
- Obidah Abert, H.; Umaru Aduwamai, H.; Shehu Adamu, S. Effect of Green Synthesized Iron Oxide Nanoparticles Using Spinach Extract on Triton X-100-Induced Atherosclerosis in Rats. Biochem. Res. Int. 2022, 2022, 9311227. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Ma, X.; Zhang, B.; Huang, Y.; Zhao, J.; Wang, S.; Li, Y.; Zhu, Y.; Xiong, J.; et al. Synthesis and Characterization of Fucoidan-Chitosan Nanoparticles Targeting P-Selectin for Effective Atherosclerosis Therapy. Oxid. Med. Cell. Longev. 2022, 2022, 8006642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhao, W.; Dou, Y.; An, H.; Tao, H.; Xu, X.; Jia, Y.; Lu, S.; Zhang, J.; et al. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-Inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef] [PubMed]
- Cymet, T.C.; Wood, B.; Orbach, N. Osteoporosis. J. Am. Osteopath Assoc. 2000, 100 Pt 1, S9–S15. [Google Scholar] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular Mechanisms of Bone Remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Prasad, G.; Dhillon, M.S.; Khullar, M.; Nagi, O.N. Evaluation of Oxidative Stress after Fractures. A Preliminary Study. Acta Orthop. Belg. 2003, 69, 546–551. [Google Scholar] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Yang, H.C. Effects of Hydrogen Peroxide (H2O2) on Alkaline Phosphatase Activity and Matrix Mineralization of Odontoblast and Osteoblast Cell Lines. Cell Biol. Toxicol. 2006, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal Involution by Age-Associated Oxidative Stress and Its Acceleration by Loss of Sex Steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative Stress Inhibits Osteoblastic Differentiation of Bone Cells by ERK and NF-ΚB. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Noble, B.; Weinstein, R.S. Osteocyte Apoptosis. Bone 2013, 54, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of Osteoclast Differentiation by the Redox-Dependent Modulation of Nuclear Import of Transcription Factors. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative Stress, Free Radicals and Bone Remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef]
- Sumi, D.; Hayashi, T.; Matsui-Hirai, H.; Jacobs, A.T.; Ignarro, L.J.; Iguchi, A. 17β-Estradiol Inhibits NADPH Oxidase Activity through the Regulation of P47phox MRNA and Protein Expression in THP-1 Cells. Biochim. Biophys. Acta Mol. Cell. Res. 2003, 1640, 113–118. [Google Scholar] [CrossRef]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant Status in Patients with Osteoporosis: A Controlled Study. Joint Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef]
- Tao, Z.; Li, T.L.; Yang, M.; Xu, H.G. Silibinin Can Promote Bone Regeneration of Selenium Hydrogel by Reducing the Oxidative Stress Pathway in Ovariectomized Rats. Calcif. Tissue Int. 2022, 110, 723–735. [Google Scholar] [CrossRef]
- Pinna, A.; Torki Baghbaderani, M.; Vigil Hernández, V.; Naruphontjirakul, P.; Li, S.; McFarlane, T.; Hachim, D.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Nanoceria Provides Antioxidant and Osteogenic Properties to Mesoporous Silica Nanoparticles for Osteoporosis Treatment. Acta Biomater. 2021, 122, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma Sprayed Cerium Oxide Coating Inhibits H2O2-Induced Oxidative Stress and Supports Cell Viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Sen, A.P.; Ghosal, S. Effects of Shilajit on Biogenic Free Radicals. Phytother. Res. 1995, 9, 56–59. [Google Scholar] [CrossRef]
- Alshubaily, F.A.; Jambi, E.J. Correlation between Antioxidant and Anti-Osteoporotic Activities of Shilajit Loaded into Chitosan Nanoparticles and Their Effects on Osteoporosis in Rats. Polymers 2022, 14, 3972. [Google Scholar] [CrossRef]
- Yu, P.; Zheng, L.; Wang, P.; Chai, S.; Zhang, Y.; Shi, T.; Zhang, L.; Peng, R.; Huang, C.; Guo, B.; et al. Development of a Novel Polysaccharide-Based Iron Oxide Nanoparticle to Prevent Iron Accumulation-Related Osteoporosis by Scavenging Reactive Oxygen Species. Int. J. Biol. Macromol. 2020, 165, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Mohd Noor, H.I.; Shuid, A.N.; Mohamad, S.; Abdul Malik, M.M.; Jayusman, P.A.; Shuid, A.N.; Naina Mohamed, I. Osteoprotective Effects in Postmenopausal Osteoporosis Rat Model: Oral Tocotrienol vs. Intraosseous Injection of Tocotrienol-Poly Lactic-Co-Glycolic Acid Combination. Front. Pharmacol. 2021, 12, 706747. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Al-Baadani, M.A.; Wu, M.; Tong, N.; Shen, X.; Ding, X.; Liu, J. Study on the Local Anti-Osteoporosis Effect of Polaprezinc-Loaded Antioxidant Electrospun Membrane. Int. J. Nanomed. 2022, 17, 17–29. [Google Scholar] [CrossRef]
- Fatima, S.; Alfrayh, R.; Alrashed, M.; Alsobaie, S.; Ahmad, R.; Mahmood, A. Selenium Nanoparticles by Moderating Oxidative Stress Promote Differentiation of Mesenchymal Stem Cells to Osteoblasts. Int. J. Nanomed. 2021, 16, 331–343. [Google Scholar] [CrossRef]
- Poleboina, S.; Sheth, V.G.; Sharma, N.; Sihota, P.; Kumar, N.; Tikoo, K. Selenium Nanoparticles Stimulate Osteoblast Differentiation via BMP-2/MAPKs/β-Catenin Pathway in Diabetic Osteoporosis. Nanomedicine 2022, 17, 607–625. [Google Scholar] [CrossRef]
- Ardawi, M.S.M.; Badawoud, M.H.; Hassan, S.M.; Ardawi, A.M.S.; Rouzi, A.A.; Qari, M.H.; Mousa, S.A. Lycopene Nanoparticles Promotes Osteoblastogenesis and Inhibits Adipogenesis of Rat Bone Marrow Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6894–6907. [Google Scholar] [CrossRef]
- Zheng, L.; Zhuang, Z.; Li, Y.; Shi, T.; Fu, K.; Yan, W.; Zhang, L.; Wang, P.; Li, L.; Jiang, Q. Bone Targeting Antioxidative Nano-Iron Oxide for Treating Postmenopausal Osteoporosis. Bioact. Mater. 2021, 14, 250–261. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, J.C.; Park, H.J.; Sul, O.J.; Lee, M.H.; Kim, J.S.; Choi, H.S. Platinum Nanoparticles Reduce Ovariectomy-Induced Bone Loss by Decreasing Osteoclastogenesis. Exp. Mol. Med. 2012, 44, 432–439. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-Containing Bioceramics Inhibit Osteoclastogenesis and Promote Osteoporotic Bone Regeneration via Scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef]
- Brandi, M.L. Healing of the Bone with Anti-Fracture Drugs. Expert Opin. Pharmacother 2013, 14, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Hu, T.; Shao, D.; Huang, S.; Xie, Y.; Zheng, X. Zn-Doped MnO2 Nanocoating with Enhanced Catalase-Mimetic Activity and Cytocompatibility Protects Pre-Osteoblasts against H2O2-Induced Oxidative Stress. Colloids Surf. B Biointerfaces 2021, 202, 111666. [Google Scholar] [CrossRef] [PubMed]
- Riccucci, G.; Ferraris, S.; Reggio, C.; Bosso, A.; Rlygsson, G.O.; Ng, C.H.; Spriano, S. Polyphenols from Grape Pomace: Functionalization of Chitosan-Coated Hydroxyapatite for Modulated Swelling and Release of Polyphenols. Langmuir 2021, 37, 14793–14804. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, X.; Luo, Z.; Hu, Y.; Li, M.; Ma, P.; Ran, Q.; Dai, L.; He, Y.; Cai, K. Osteogenesis Potential of Different Titania Nanotubes in Oxidative Stress Microenvironment. Biomaterials 2018, 167, 44–57. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, Q.; Lu, Y.; Wei, Q.; Tang, H.; Zhang, D.; Liu, Z.; Wang, G.; Wu, D. ROS-Scavenging Hydrogel as Protective Carrier to Regulate Stem Cells Activity and Promote Osteointegration of 3D Printed Porous Titanium Prosthesis in Osteoporosis. Front. Bioeng. Biotechnol. 2023, 11, 11103611. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; He, C. Melatonin Having Therapeutic Bone Regenerating Capacity in Biomaterials. Curr. Pharm. Biotechnol. 2022, 23, 707–718. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, J.; Chen, R.; Huang, Y.; Liu, Y.; Bai, J.; Ge, G.; Shi, X.; Chen, Y.; Shi, J.; et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, 6797154. [Google Scholar] [CrossRef] [PubMed]
- Ewart, L.; Apostolou, A.; Briggs, S.A.; Carman, C.V.; Chaff, J.T.; Heng, A.R.; Jadalannagari, S.; Janardhanan, J.; Jang, K.-J.; Joshipura, S.R.; et al. Performance Assessment and Economic Analysis of a Human Liver-Chip for Predictive Toxicology. Commun. Med. 2022, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Nicholson, M.W.; Wang, J.Y.; Ting, C.Y.; Tsai, M.H.; Cheng, Y.C.; Liu, C.L.; Chan, D.Z.H.; Lee, Y.C.; Hsu, C.C.; et al. Population-Based High-Throughput Toxicity Screen of Human IPSC-Derived Cardiomyocytes and Neurons. Cell Rep. 2022, 39, 110643. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Ahmed, S.M.; Shivnaraine, R.V.; Wu, J.C. FDA Modernization Act 2.0 Paves the Way to Computational Biology and Clinical Trials in a Dish. Circulation 2023, 148, 309–311. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. https://doi.org/10.3390/biomimetics9010023
Perez-Araluce M, Jüngst T, Sanmartin C, Prosper F, Plano D, Mazo MM. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics. 2024; 9(1):23. https://doi.org/10.3390/biomimetics9010023
Chicago/Turabian StylePerez-Araluce, Maria, Tomasz Jüngst, Carmen Sanmartin, Felipe Prosper, Daniel Plano, and Manuel M. Mazo. 2024. "Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases" Biomimetics 9, no. 1: 23. https://doi.org/10.3390/biomimetics9010023
APA StylePerez-Araluce, M., Jüngst, T., Sanmartin, C., Prosper, F., Plano, D., & Mazo, M. M. (2024). Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics, 9(1), 23. https://doi.org/10.3390/biomimetics9010023








