Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Route
2.2. Characterization
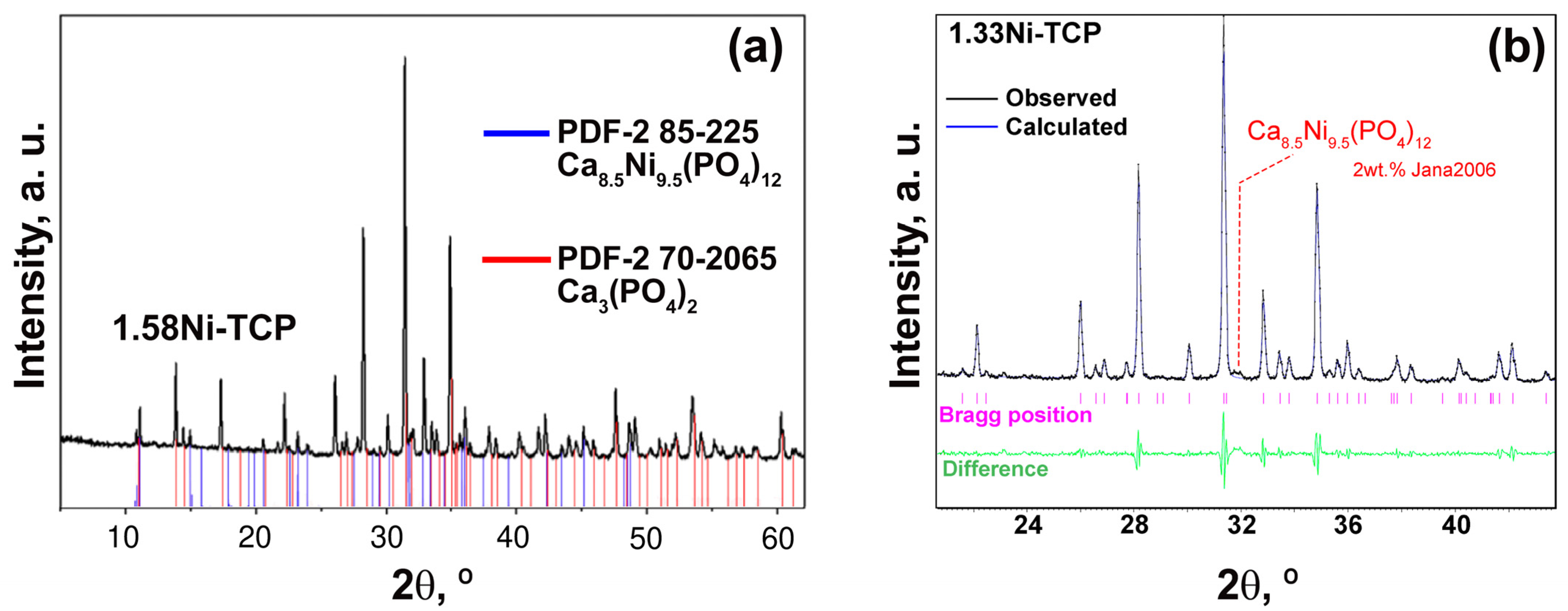
2.2.1. Powder X-ray Diffraction Study
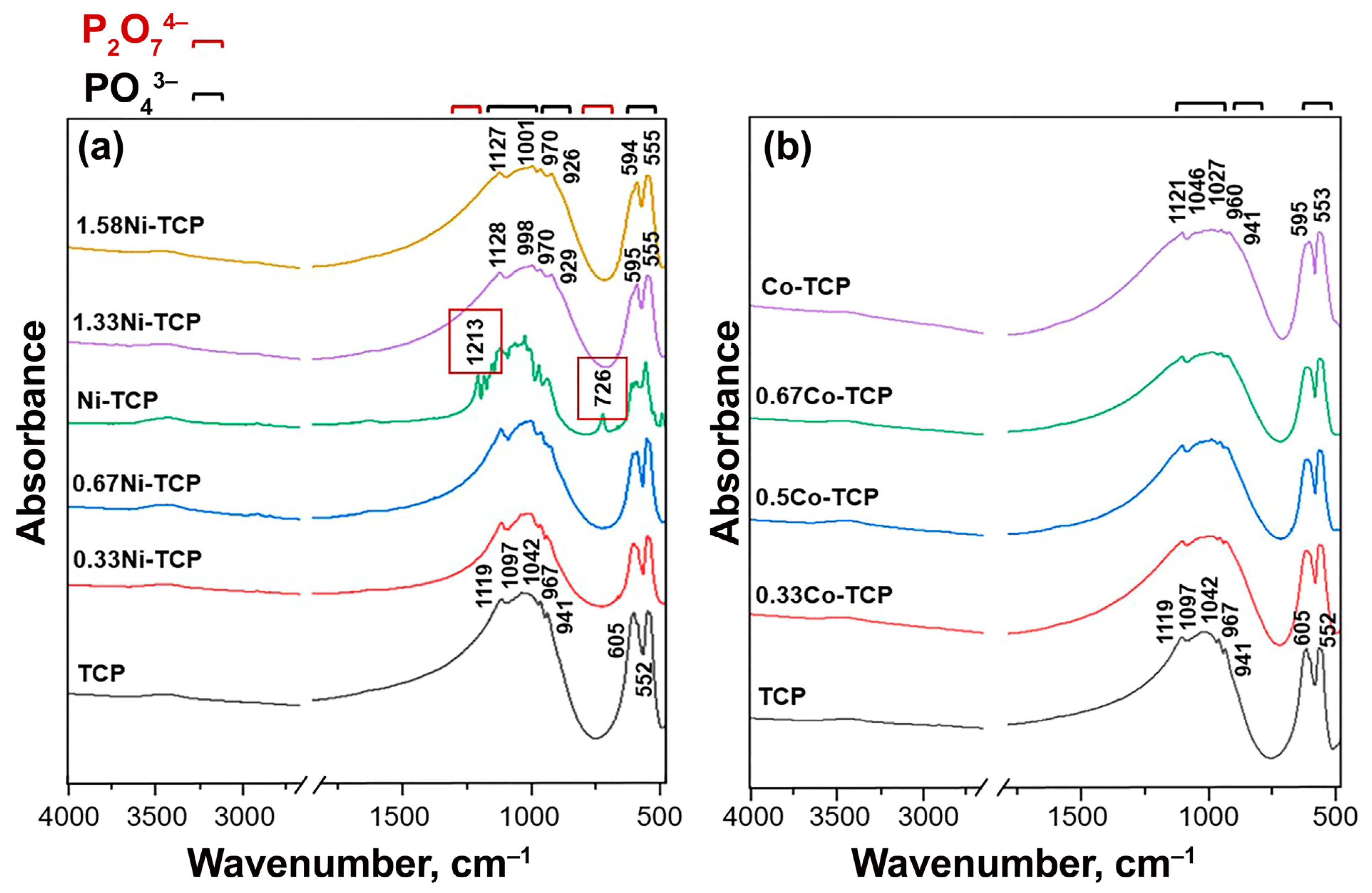
2.2.2. Fourier-Transform Infrared (FT-IR) Study
2.2.3. Second Harmonic Generation Study
2.2.4. Differential Scanning Calorimetry Measurements
2.2.5. The Release Behavior of Ions from β-TCP
2.2.6. Isolation and Growth of aMSCs
2.2.7. MTT Assay
2.2.8. Differentiation in the Osteogenic Lineage
2.2.9. Antimicrobial Activity
2.2.10. Statistical Analysis
3. Results
3.1. PXRD Study
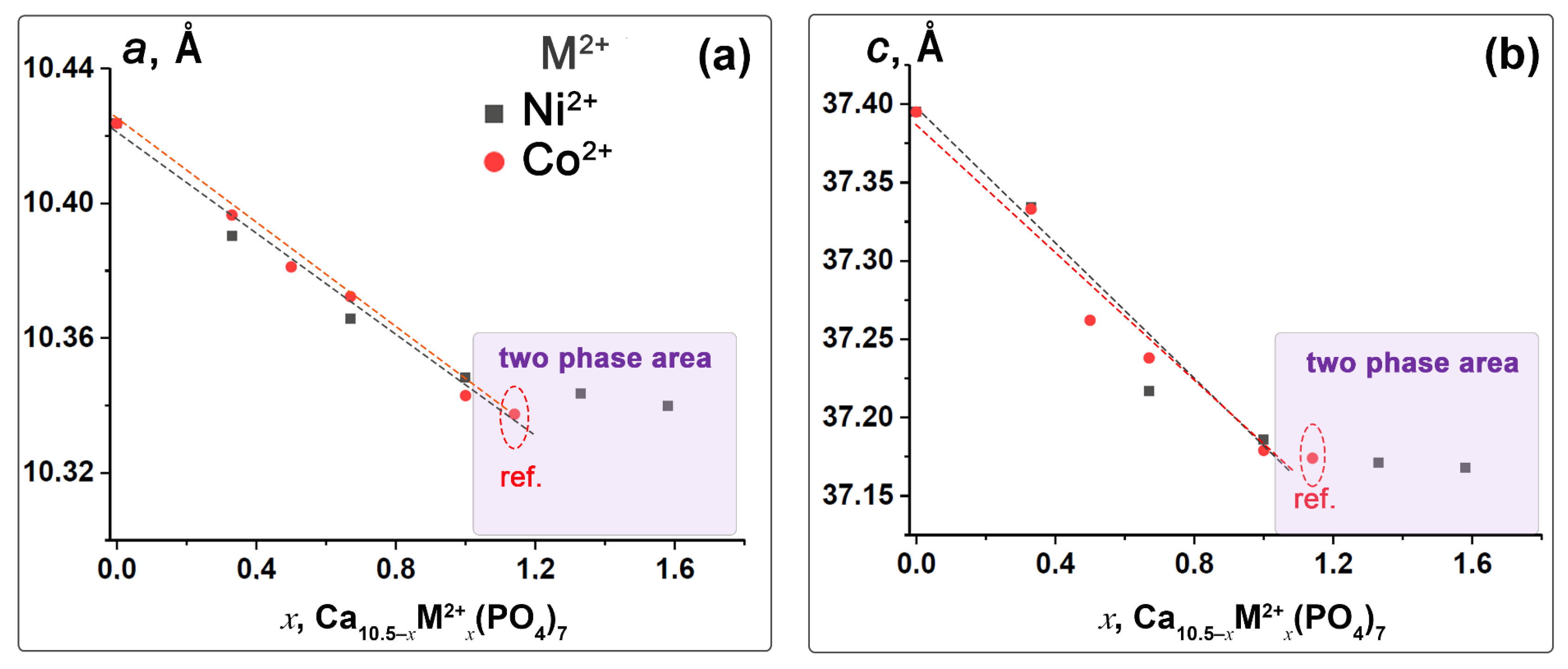
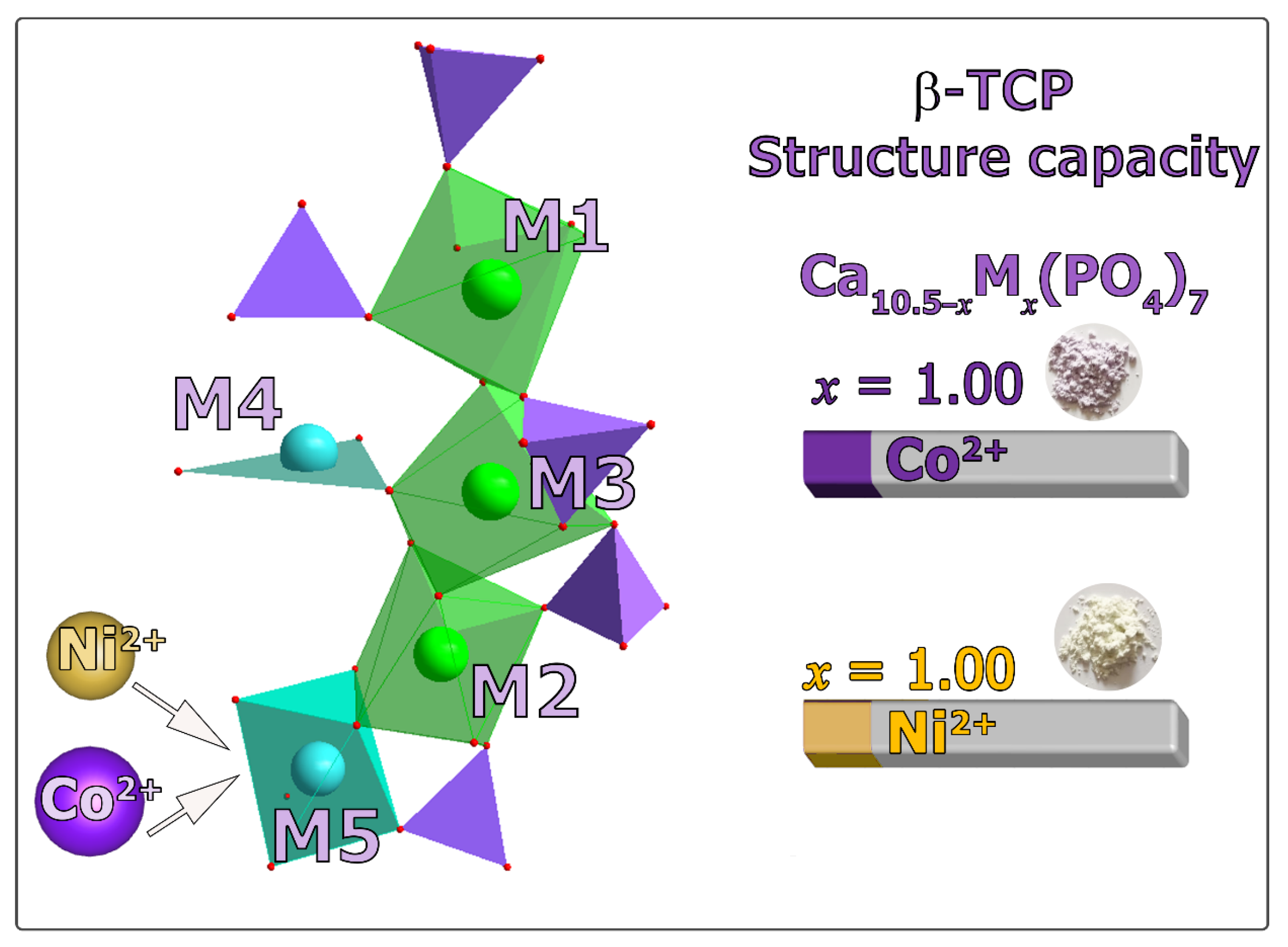
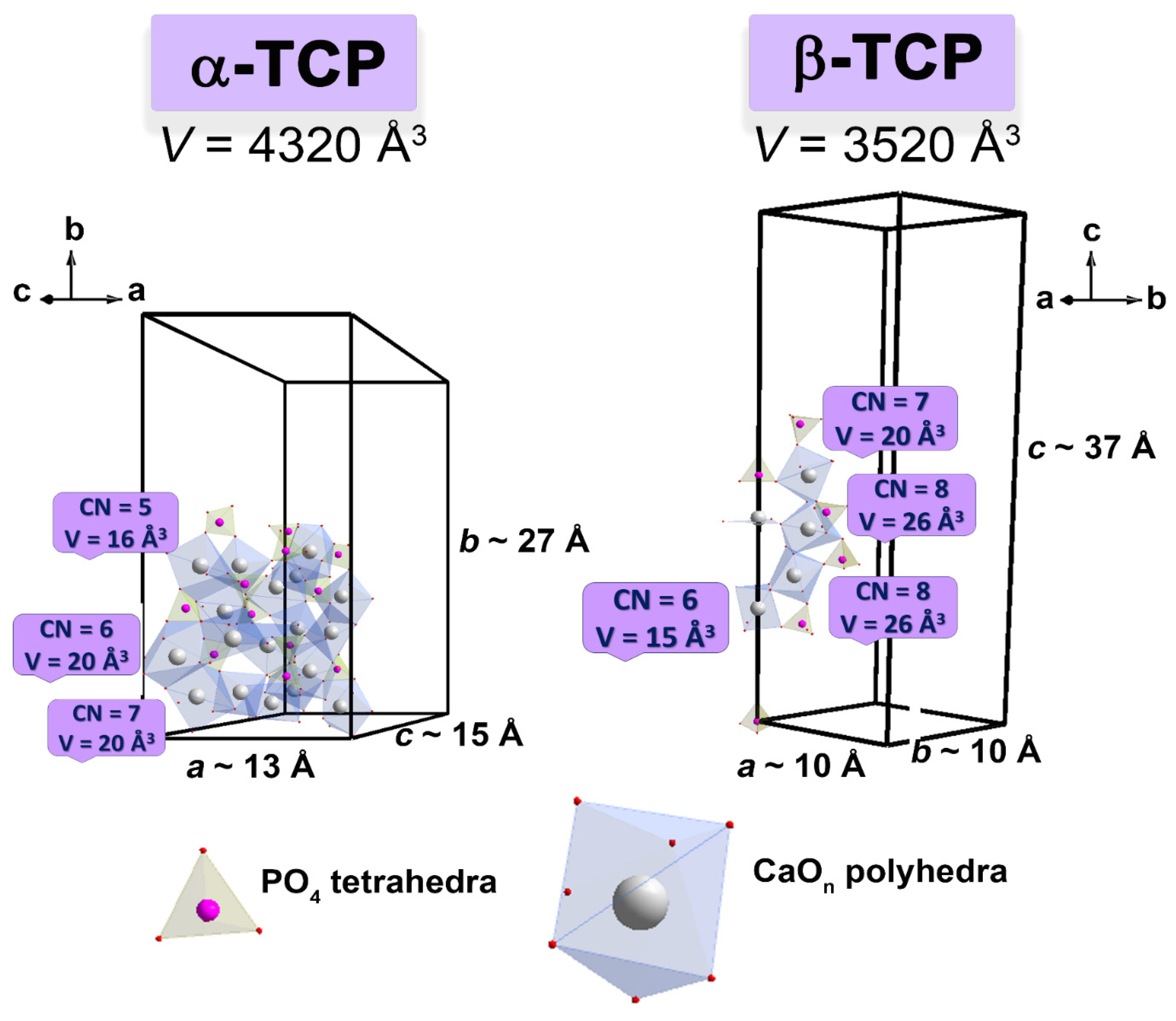
3.2. Crystal Structure Refinement
- for Ca3(PO4)2:M2+M3+
- for Sr3(PO4)2:M2+M3+
- C2/m Sr9□R3+(PO4)7 (R3+ = Sc, Cr, Fe, Ga)
- C2/c Sr9□In(PO4)7,
3.3. FT-IR Study
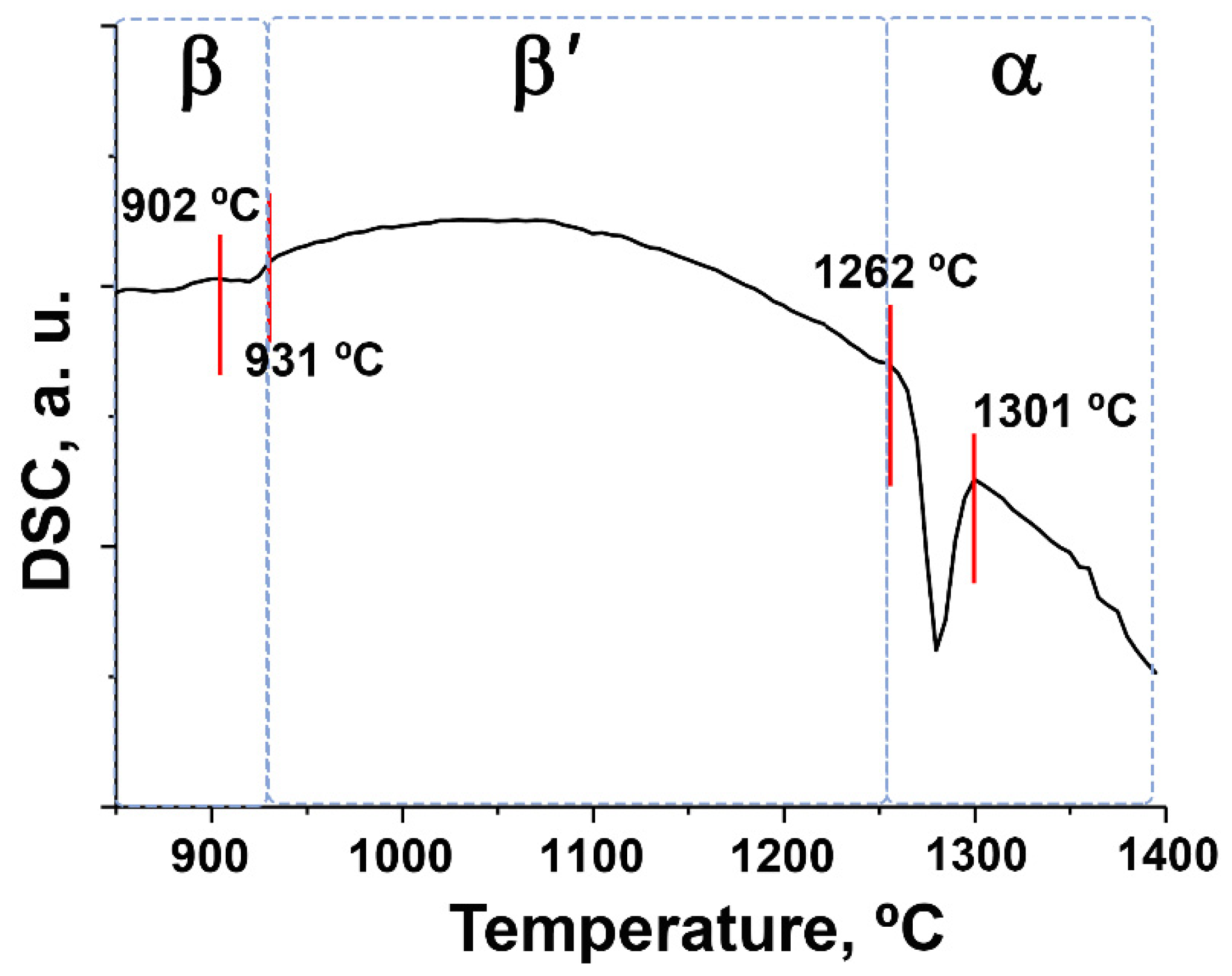
3.4. Phase Transitions
3.5. Ion Release Behavior and Soaking of the Ceramics
3.6. Bioactive Properties
3.6.1. Cell Viability Determination by MTT Assay
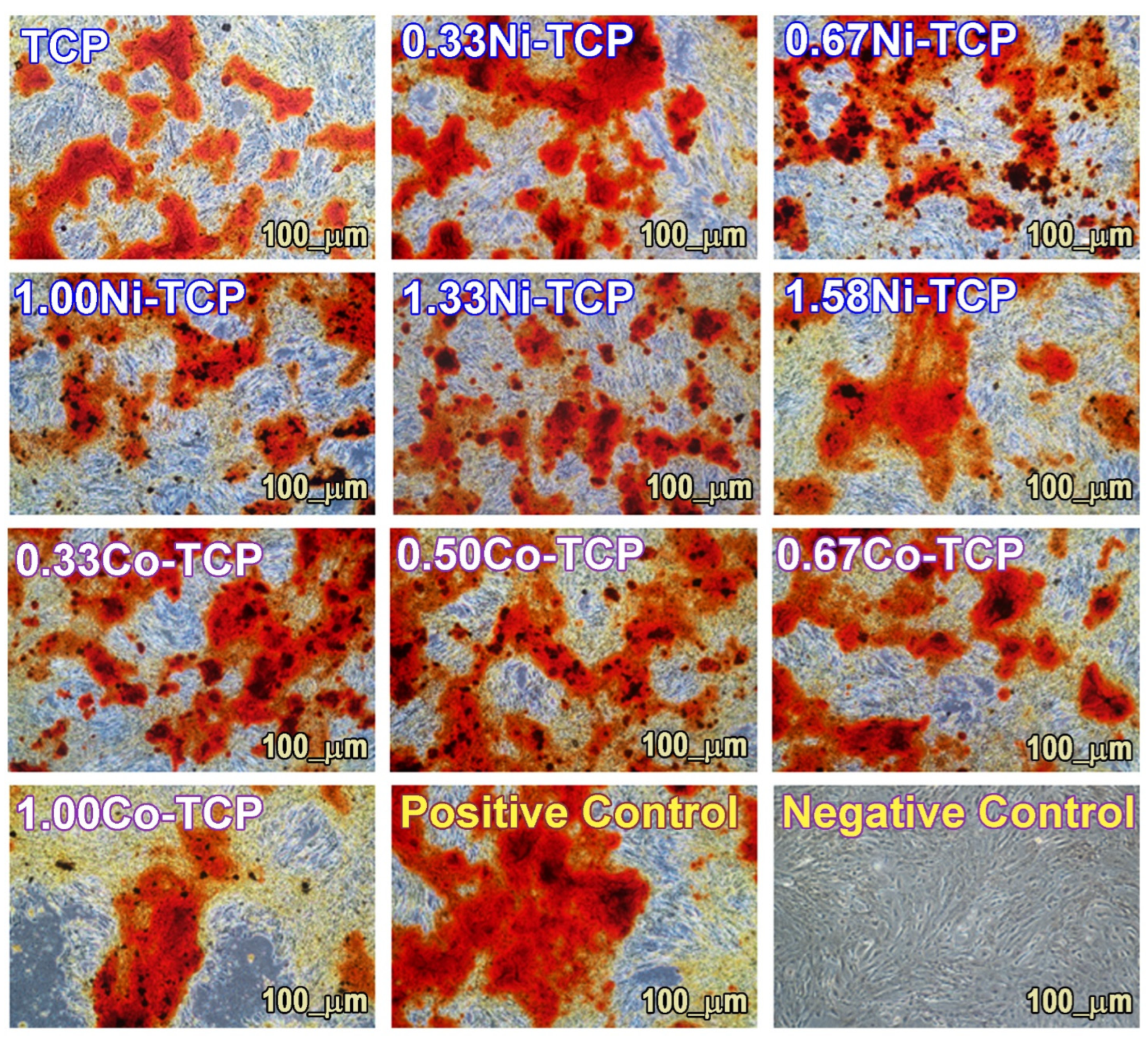
3.6.2. Differentiation in the Osteogenic Lineage
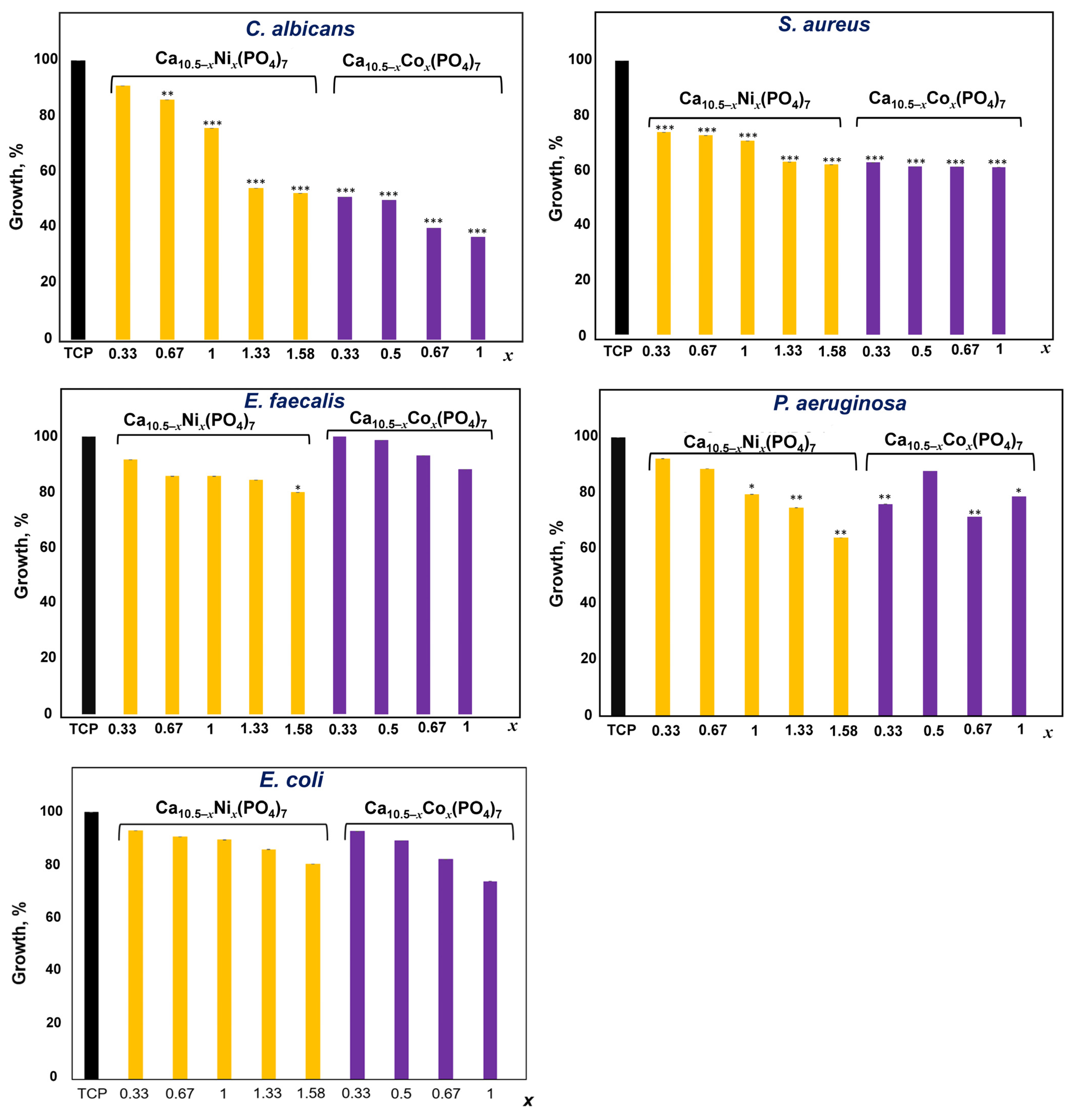
3.7. Inhibition of the Growth of Microorganisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Walsh, W.R.; Vizesi, F.; Michael, D.; Auld, J.; Langdown, A.; Oliver, R.; Yu, Y.; Irie, H.; Bruce, W. β-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials 2008, 29, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed]
- Saikia, K.; Bhattacharya, T.; Bhuyan, S.; Talukdar, D.; Saikia, S.; Jitesh, P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J. Orthop. 2008, 42, 169. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L.V.H. Autogenous Iliac Crest Bone Graft: Complications and Functional Assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Seidenstuecker, M.; Ruehe, J.; Suedkamp, N.P.; Serr, A.; Wittmer, A.; Bohner, M.; Bernstein, A.; Mayr, H.O. Composite material consisting of microporous β-TCP ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017, 51, 433–446. [Google Scholar] [CrossRef]
- Jiang, N.; Dusane, D.H.; Brooks, J.R.; Delury, C.P.; Aiken, S.S.; Laycock, P.A.; Stoodley, P. Antibiotic loaded β-tricalcium phosphate/calcium sulfate for antimicrobial potency, prevention and killing efficacy of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Sci. Rep. 2021, 11, 1446. [Google Scholar] [CrossRef]
- Sasaki, K.; Ninomiya, Y.; Takechi, M.; Tsuru, K.; Ishikawa, K.; Shigeishi, H.; Ohta, K.; Aikawa, T. Physical Properties and Antimicrobial Release Ability of Gentamicin-Loaded Apatite Cement/α-TCP Composites: An In Vitro Study. Materials 2023, 16, 995. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Urruth, G.; Balvay, S.; Gaillard, C.; Gremillard, L.; Leriche, A. Mg2+, Sr2+, Ag+, and Cu2+ co-doped β-tricalcium phosphate: Improved thermal stability and mechanical and biological properties. J. Am. Ceram. Soc. 2023, 106, 4061–4075. [Google Scholar] [CrossRef]
- Nikiforov, I.V.; Iliina, E.V.; Lazoryak, B.I.; Aksenov, S.M.; Slukin, P.V.; Deyneko, D.V. Photoluminescence, structural and antibacterial properties of co-doped β-Ca3(PO4)2-type phosphates Ca8CuRE(PO4)7 (RE = Eu–Er). J. Rare Earths 2023, 26, 125763. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Zheng, Y.; Barbaro, K.; Lebedev, V.N.; Aksenov, S.M.; Borovikova, E.Y.; Gafurov, M.R.; Fadeeva, I.V.; Lazoryak, B.I.; Di Giacomo, G.; et al. Dependence of antimicrobial properties on site-selective arrangement and concentration of bioactive Cu2+ ions in tricalcium phosphate. Ceram. Int. 2023, 49, 21308–21323. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, X.; YU, Y.; Yang, X.; Cai, Q. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2023, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Pang, N.; Xing, H. Preparation and antibacterial evaluation of a beta-tricalcium phosphate/collagen nanofiber biomimetic composite scaffold. Mater. Chem. Phys. 2021, 273, 125059. [Google Scholar] [CrossRef]
- Siek, D.; Ślósarczyk, A.; Przekora, A.; Belcarz, A.; Zima, A.; Ginalska, G.; Czechowska, J. Evaluation of antibacterial activity and cytocompatibility of α-TCP based bone cements with silver-doped hydroxyapatite and CaCO3. Ceram. Int. 2017, 43, 13997–14007. [Google Scholar] [CrossRef]
- Qin, L.; Yi, J.; Xuefei, L.; Li, L.; Kenan, X.; Lu, X. The preparation of a difunctional porous β-tricalcium phosphate scaffold with excellent compressive strength and antibacterial properties. RSC Adv. 2020, 10, 28397–28407. [Google Scholar] [CrossRef] [PubMed]
- Kamphof, R.; Lima, R.N.O.; Schoones, J.W.; Arts, J.J.; Nelissen, R.G.H.H.; Cama, G.; Pijls, B.G.C.W. Antimicrobial activity of ion-substituted calcium phosphates: A systematic review. Heliyon 2023, 9, e16568. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-J.; Ningsih, H.S.; Shih, S.-J. Preparation, characterization and investigation of antibacterial silver-zinc co-doped β-tricalcium phosphate by spray pyrolysis. Ceram. Int. 2020, 46, 16708–16715. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Ye, J.; He, F. Effects of zinc/gallium dual doping on the physicochemical properties and cell response of 3D printed β-tricalcium phosphate ceramic scaffolds. Ceram. Int. 2022, 48, 28557–28564. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater. Sci. Eng. C 2019, 99, 1199–1212. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Vieira, S.I.; Cerqueira, A.R.; Pina, S.; Cruz Silva, O.A.B.; Abrantes, J.C.C.; Ferreira, J.M.F. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef]
- Yoo, K.-H.; Kim, H.; Sun, W.G.; Kim, Y.I.; Yoon, S.Y. Fe-doped tricalcium phosphates: Crystal structure and degradation behavior. Mater. Res. Express 2020, 7, 125403. [Google Scholar] [CrossRef]
- Griesiute, D.; Sinusaite, L.; Kizalaite, A.; Antuzevics, A.; Mazeika, K.; Baltrunas, D.; Goto, T.; Sekino, T.; Kareiva, A.; Zarkov, A. The influence of Fe3+ doping on thermally induced crystallization and phase evolution of amorphous calcium phosphate. CrystEngComm 2021, 23, 4627–4637. [Google Scholar] [CrossRef]
- Zarkov, A.; Griesiute, D.; Dubnika, A.; Zakutna, D.; Tyrpekl, V. Low-Temperature Synthesis and Characterization of Iron Whitlockite (Ca18Fe2(HPO4)2(PO4)12). Proceedings. 2023, 92, 25. [Google Scholar] [CrossRef]
- Sikder, P.; Coomar, P.P.; Mewborn, J.M.; Bhaduri, S.B. Antibacterial calcium phosphate composite cements reinforced with silver-doped magnesium phosphate (newberyite) micro-platelets. J. Mech. Behav. Biomed. Mater. 2020, 110, 103934. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 2000, 50, 178–183. [Google Scholar] [CrossRef]
- Paterlini, V.; El Khouri, A.; Bettinelli, M.; Trucchi, D.M.; Capitelli, F. Spectroscopic and Structural Properties of β-Tricalcium Phosphates Ca9RE(PO4)7 (RE = Nd, Gd, Dy). Crystals 2021, 11, 1269. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Bose, S. Effects of Iron on Physical and Mechanical Properties, and Osteoblast Cell Interaction in β-Tricalcium Phosphate. Ann. Biomed. Eng. 2017, 45, 819–828. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Deyneko, D.V.; Barbaro, K.; Davydova, G.A.; Sadovnikova, M.A.; Murzakhanov, F.F.; Fomin, A.S.; Yankova, V.G.; Antoniac, I.V.; Barinov, S.M.; et al. Influence of Synthesis Conditions on Gadolinium-Substituted Tricalcium Phosphate Ceramics and Its Physicochemical, Biological, and Antibacterial Properties. Nanomaterials 2022, 12, 852. [Google Scholar] [CrossRef]
- Albayrak, O. Structural and mechanical characterization of boron doped biphasic calcium phosphate produced by wet chemical method and subsequent thermal treatment. Mater. Charact. 2016, 113, 82–89. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Filippov, Y.Y.; Fomin, A.S.; Shaposhnikov, M.E.; Davydova, G.A.; Antonova, O.S.; Selezneva, I.I.; Mikheev, A.Y.; Akhmetov, L.I.; Barinov, S.M.; et al. Synthesis of micro- and nanosized bioresorbing silicon-substituted tricalcium phosphates for bone tissue engineering and their biological safety using mesenchymal stem cells. Nanomechanics Sci. Technol. Int. J. 2015, 6, 305–317. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolanthai, E.; Nivethaa, E.A.K.; Pandian, M.S.; Ramasamy, P.; Catalani, L.H.; Kalkura, S.N. Enhanced in vitro inhibition of MCF-7 and magnetic properties of cobalt incorporated calcium phosphate (HAp and β-TCP) nanoparticles. Ceram. Int. 2023, 49, 855–861. [Google Scholar] [CrossRef]
- Liu, L.; Huang, R.; Zhang, L. Cobalt Element Doping for Biomedical Use: A Review. Mater. Sci. Forum 2020, 993, 811–819. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Gnanamozhi, P.; Renganathan, V.; Chen, S.-M.; Pandiyan, V.; Antony Arockiaraj, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alanzi, K.F. Influence of Nickel concentration on the photocatalytic dye degradation (methylene blue and reactive red 120) and antibacterial activity of ZnO nanoparticles. Ceram. Int. 2020, 46, 18322–18330. [Google Scholar] [CrossRef]
- Prashanth, G.K.; Prashanth, P.A.; Singh, P.; Nagabhushana, B.M.; Shivakumara, C.; Krishnaiah, G.M.; Nagendra, H.G.; Sathyananda, H.M.; Chaturvedi, V. Effect of doping (with cobalt or nickel) and UV exposure on the antibacterial, anticancer, and ROS generation activities of zinc oxide nanoparticles. J. Asian Ceram. Soc. 2020, 8, 1175–1187. [Google Scholar] [CrossRef]
- Singh, R.K.; Srivastava, M.; Prasad, N.K.; Kannan, S. Structural analysis and hyperthermia effect of Fe3+/Ni2+ co-substitutions in β-Ca3(PO4)2. J. Alloy. Compd. 2017, 725, 393–402. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Liu, C.; Wang, Z.; Ling, Z.; Lin, B.; Tan, B.; Zhou, L.; Chen, Y.; Liu, D.; et al. Cobalt-doped bioceramic scaffolds fabricated by 3D printing show enhanced osteogenic and angiogenic properties for bone repair. Biomed. Eng. Online 2021, 20, 1–24. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Li, H.; Yuen, J.; Chang, J.; Xiao, Y. Preparation, characterization and in vitro angiogenic capacity of cobalt substituted β-tricalcium phosphate ceramics. J. Mater. Chem. 2012, 22, 21686. [Google Scholar] [CrossRef]
- Tahmasebi Birgani, Z.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory effect of cobalt ions incorporated into calcium phosphate coatings on neovascularization in an in vivo intramuscular model in goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef]
- Grasselli, F.; Basini, G.; Bussolati, S.; Bianco, F. Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod. Fertil. Dev. 2005, 17, 715. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Nasiri-Tabrizi, B.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Alias, Y. Characterization of nickel-doped biphasic calcium phosphate/graphene nanoplatelet composites for biomedical application. Mater. Sci. Eng. C 2015, 49, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Kurinjinathan, P.; Thanigai Arul, K.; Ramana Ramya, J.; Manikandan, E.; Hegazy, H.H.; Umar, A.; Algarni, H.; Ahmad, N. Effect of Nickel Doping on the Properties of Hydroxyapatite Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Guerra-López, J.; González, R.; Gómez, A.; Pomés, R.; Punte, G.; Della Védova, C.O. Effects of nickel on calcium phosphate formation. J. Solid State Chem. 2000, 151, 163–169. [Google Scholar] [CrossRef]
- Belik, A.; Morozov, V.; Khasanov, S.; Lazoryak, B. Crystal structures of new double calcium and cobalt phosphates. Mater. Res. Bull. 1998, 33, 987–995. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Deng, Y. Materials Science & Engineering C Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 770–782. [Google Scholar] [CrossRef]
- Ates, T.; Dorozhkin, S.V.; Kaygili, O.; Kom, M.; Ercan, I.; Bulut, N.; Firdolas, F.; Keser, S.; Gursoy, N.C.; Ozercan, I.H.; et al. The effects of Mn and/or Ni dopants on the in vitro/in vivo performance, structural and magnetic properties of β-tricalcium phosphate bioceramics. Ceram. Int. 2019, 45, 22752–22758. [Google Scholar] [CrossRef]
- Altomare, A.; Rizzi, R.; Rossi, M.; El Khouri, A.; Elaatmani, M.; Paterlini, V.; Della Ventura, G.; Capitelli, F. New Ca2.90(Me2+)0.10(PO4)2 β-tricalcium Phosphates with Me2+ = Mn, Ni, Cu: Synthesis, Crystal-Chemistry, and Luminescence Properties. Crystals 2019, 9, 288. [Google Scholar] [CrossRef]
- Schamel, M.; Bernhardt, A.; Quade, M.; Würkner, C.; Gbureck, U.; Moseke, C.; Gelinsky, M.; Lode, A. Cu2+, Co2+ and Cr3+ doping of a calcium phosphate cement influences materials properties and response of human mesenchymal stromal cells. Mater. Sci. Eng. C 2017, 73, 99–110. [Google Scholar] [CrossRef]
- TenHuisen, K.S.; Brown, P.W. Phase evolution during the formation of α-tricalcium phosphate. J. Am. Ceram. Soc. 1999, 82, 2813–2818. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L.; Petrícek, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Fur Krist. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Legrouri, A.; Romdhane, S.S.; Lenzi, J.; Lenzi, M.; Bonel, G. Influence of preparation method on catalytic properties of mixed calcium-cobalt orthophosphates. J. Mater. Sci. 1996, 31, 2469–2473. [Google Scholar] [CrossRef]
- Mosafer, H.S.R.; Paszkowicz, W.; Minikayev, R.; Martin, C.; Kozłowski, M.; Chukova, O.; Zhydachevskyy, Y.; Nedilko, S. Crystal Structure, Thermal Expansion and Luminescence of Ca10.5−xNix(VO4)7. Crystals 2023, 13, 853. [Google Scholar] [CrossRef]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfllung der Atome. Z. Fur Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Goto, T.; Katsui, H. Chemical Vapor Deposition of Ca–P–O Film Coating. In Interface Oral Health Science 2014; Springer: Tokyo, Japan, 2015; pp. 103–115. [Google Scholar]
- Deyneko, D.V.; Aksenov, S.M.; Nikiforov, I.; Stefanovich, S.Y.; Lazoryak, B.I. Symmetry Inhomogeneity of Ca9–xZnxEu(PO4)7 Phosphor Determined by Second-Harmonic Generation and Dielectric and Photoluminescence Spectroscopy. Cryst. Growth Des. 2020, 20, 6461–6468. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Stachowicz, M.; Galuskina, I.O.; Woźniak, K.; Vapnik, Y.; Murashko, M.N.; Zieliński, G. Deynekoite, Ca9Fe 3+(PO4)7—A new mineral of the merrillite group from phosphide-bearing contact facies of paralava, Hatrurim Complex, Daba-Siwaqa, Jordan. Mineral. Mag. 2023, 1, 12. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Junaid Bushiri, M.; Antony, C.J.; Aatiq, A. Raman and FTIR studies of the structural aspects of Nasicon-type crystals; AFeTi(PO4)3 [A=Ca, Cd]. J. Phys. Chem. Solids 2008, 69, 1985–1989. [Google Scholar] [CrossRef]
- Antony, C.J.; Aatiq, A.; Panicker, C.Y.; Bushiri, M.J.; Varghese, H.T.; Manojkumar, T.K. FT-IR and FT-Raman study of Nasicon type phosphates, ASnFe(PO4)3 [A=Na2, Ca, Cd]. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 415–419. [Google Scholar] [CrossRef]
- Bogdanoviciene, I.; Beganskiene, A.; Tõnsuaadu, K.; Glaser, J.; Meyer, H.-J.; Kareiva, A. Calcium hydroxyapatite, Ca10(PO4)6(OH)2 ceramics prepared by aqueous sol–gel processing. Mater. Res. Bull. 2006, 41, 1754–1762. [Google Scholar] [CrossRef]
- Szyszka, K.; Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Ledwa, K.A.; Piecuch, A.; Giersig, M.; Drulis-Kawa, Z.; Wiglusz, R.J. Structural modification of nanohydroxyapatite Ca10(PO4)6(OH)2 related to Eu3+ and Sr2+ ions doping and its spectroscopic and antimicrobial properties. J. Inorg. Biochem. 2019, 203, 110884. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-S.; Youn, H.-J.; Sun Hong, K.; Chang, B.-S.; Lee, C.-K.; Chung, S.-S. An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 2002, 23, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, M.; Sglavo, V.M. Effect of Mg2+ doping on beta–alpha phase transition in tricalcium phosphate (TCP) bioceramics. Acta Biomater. 2016, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sinusaite, L.; Kareiva, A.; Zarkov, A. Thermally Induced Crystallization and Phase Evolution of Amorphous Calcium Phosphate Substituted with Divalent Cations Having Different Sizes. Cryst. Growth Des. 2021, 21, 1242–1248. [Google Scholar] [CrossRef]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.K.; Hwang, N.S.; et al. In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- De Oliveira Lomelino, R.; Castro-Silva, I.I.; Linhares, A.B.R.; Alves, G.G.; de Albuquerque Santos, S.R.; Gameiro, V.S.; Rossi, A.M.; Granjeiro, J.M. The association of human primary bone cells with biphasic calcium phosphate (β-TCP/HA 70:30) granules increases bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 781–788. [Google Scholar] [CrossRef]



| Formula |
| Physical Properties | Bioactive Properties | Optimum Concentration of Dopant | Ref. |
|---|---|---|---|---|---|
|
|
| N/a | N/a | [44] |
| (Co0.0xCa1−0.0x)3(PO4)2 |
|
|
| 2 and 5 mol.% | [38] |
| (Co0.0xCa1−0.0x)3(PO4)2 |
|
|
| 2 and 5 mol.% | [45] |
| Ca10Li(PO4)7 |
|
|
| 0.25 mol.% | [37] |
| CPs:Co2+ |
| N/a |
| N/a | [39] |
| β-Ca3(PO4)2:Ni |
|
|
| N/a | [46] |
| HAP:Ni2+ |
|
| 6 wt.% Ni2+ showed cytotoxicity for hFOB cells | 6 wt.% Ni2+ with 1.5 wt.% GNPs | [41] |
| Ca2.9Ni0.1(PO4)2 |
| Ni2+ occupies the M5 site | N/a | N/a | [47] |
| β-Ca3(PO4)2:Ni |
| Ni2+ occupies the M5 and M4 sites. |
| Ni/Fe co-doping | [36] |
| Chemical Formula | x, Ni2+ or Co2+ | M2+, mol.% | Sample Code | a, Å | c, Å | V, Å3 |
|---|---|---|---|---|---|---|
| Ca10.5(PO4)7 | 0 | TCP | 10.4237(3) | 37.395(2) | 4063.1(7) | |
| Ca10.17Ni0.33(PO4)7 | 0.33 | 3.1 | 0.33Ni-TCP | 10.3903(5) | 37.334(1) | 4030.5(4) |
| Ca9.83Ni0.67(PO4)7 | 0.67 | 6.4 | 0.67Ni-TCP | 10.3557(5) | 37.217(8) | 3991.2(3) |
| Ca9.5Ni(PO4)7 | 1.0 | 9.5 | 1.00Ni-TCP | 10.3483(4) | 37.186(9) | 3982.1(8) |
| Ca9.17Ni1.33(PO4)7 | 1.33 | 12.7 | 1.33Ni-TCP | 10.3135(6) | 37.171(3) | 3953.8(8) |
| Ca8.92Ni1.58(PO4)7 | 1.58 | 15 | 1.58Ni-TCP | 10.3109(5) | 37.168(8) | 3951.5(1) |
| Ca10.17Co0.33(PO4)7 | 0.33 | 3.1 | 0.33Co-TCP | 10.3965(6) | 37.333(1) | 4035.2(9) |
| Ca10.0Co0.5(PO4)7 | 0.5 | 4.8 | 0.5Co-TCP | 10.3811(9) | 37.262(6) | 4015.7(1) |
| Ca9.83Co0.67(PO4)7 | 0.67 | 6.4 | 0.67Co-TCP | 10.3723(3) | 37.238(8) | 4006.2(6) |
| Ca9.5Co(PO4)7 | 1.0 | 9.5 | 1.00Co-TCP | 10.3429(2) | 37.179(5) | 3997.3(4) |
| Sample | OD Media | Growth, % | SD |
|---|---|---|---|
| Cell control | 0.151 | 100 | 0.021 |
| TCP | 0.151 | 100.24 | 0.008 |
| 0.33Ni-TCP | 0.157 | 103.95 | 0.008 |
| 0.67Ni-TCP | 0.154 | 101.90 | 0.009 |
| 1.00Ni-TCP | 0.152 | 100.38 | 0.003 |
| 1.33Ni-TCP | 0.151 | 100.11 | 0.005 |
| 1.58Ni-TCP | 0.146 | 96.53 | 0.006 |
| 0.33Co-TCP | 0.149 | 98.68 | 0.001 |
| 0.50Co-TCP | 0.146 | 96.47 | 0.004 |
| 0.67Co-TCP | 0.135 | 89.38 | 0.013 |
| 1.00Co-TCP | 0.135 | 89.43 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deyneko, D.V.; Lebedev, V.N.; Barbaro, K.; Titkov, V.V.; Lazoryak, B.I.; Fadeeva, I.V.; Gosteva, A.N.; Udyanskaya, I.L.; Aksenov, S.M.; Rau, J.V. Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics. Biomimetics 2024, 9, 14. https://doi.org/10.3390/biomimetics9010014
Deyneko DV, Lebedev VN, Barbaro K, Titkov VV, Lazoryak BI, Fadeeva IV, Gosteva AN, Udyanskaya IL, Aksenov SM, Rau JV. Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics. Biomimetics. 2024; 9(1):14. https://doi.org/10.3390/biomimetics9010014
Chicago/Turabian StyleDeyneko, Dina V., Vladimir N. Lebedev, Katia Barbaro, Vladimir V. Titkov, Bogdan I. Lazoryak, Inna V. Fadeeva, Alevtina N. Gosteva, Irina L. Udyanskaya, Sergey M. Aksenov, and Julietta V. Rau. 2024. "Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics" Biomimetics 9, no. 1: 14. https://doi.org/10.3390/biomimetics9010014
APA StyleDeyneko, D. V., Lebedev, V. N., Barbaro, K., Titkov, V. V., Lazoryak, B. I., Fadeeva, I. V., Gosteva, A. N., Udyanskaya, I. L., Aksenov, S. M., & Rau, J. V. (2024). Antimicrobial and Cell-Friendly Properties of Cobalt and Nickel-Doped Tricalcium Phosphate Ceramics. Biomimetics, 9(1), 14. https://doi.org/10.3390/biomimetics9010014










