Preparation of Natural Plant Polyphenol Catechin Film for Structural Coloration of Silk Fabrics
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Section
2.2.1. Preparation of S-PCC-n
2.2.2. Preparation of S-PCC-mPVP-n
2.3. Characterization
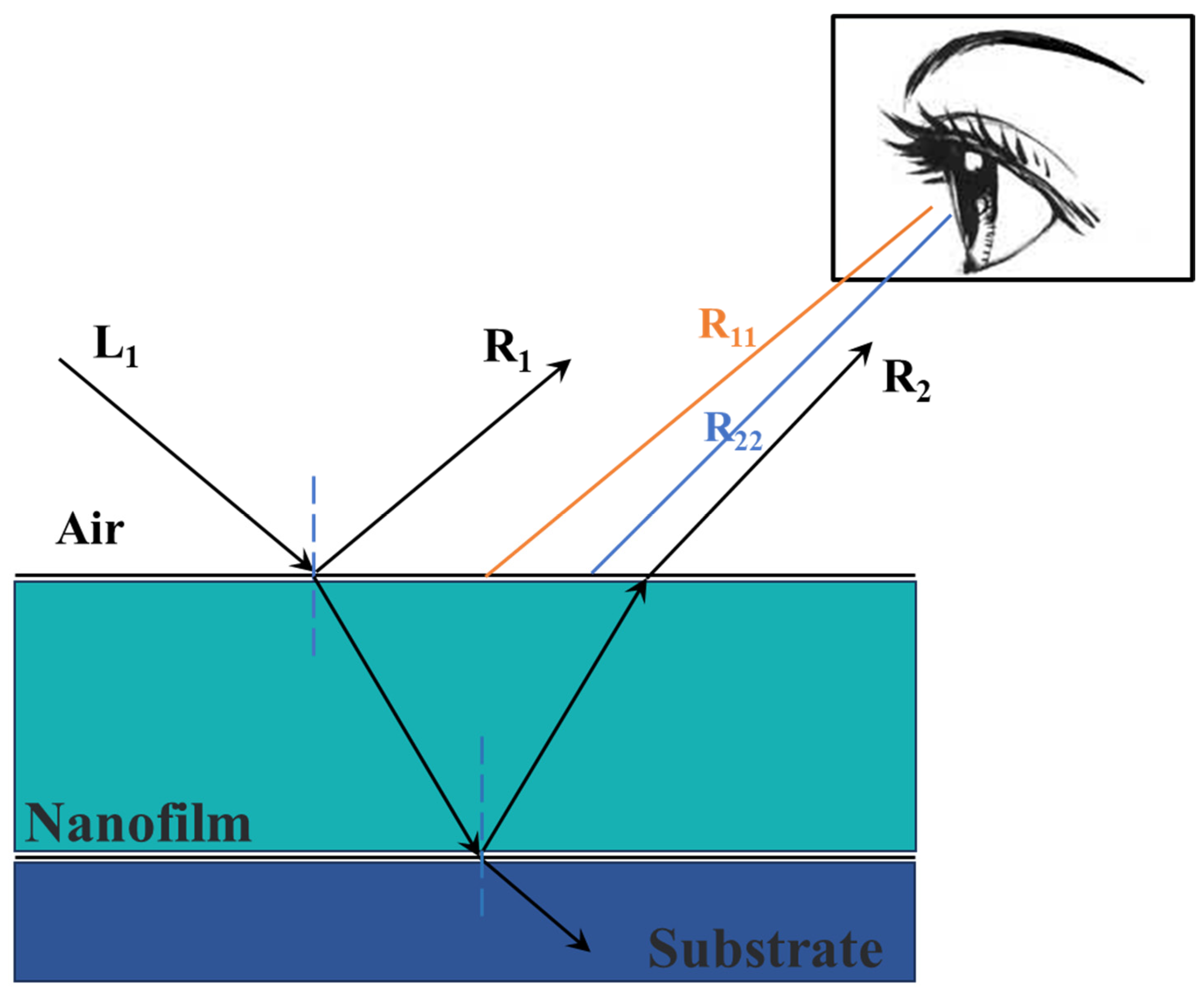
3. Results and Discussion
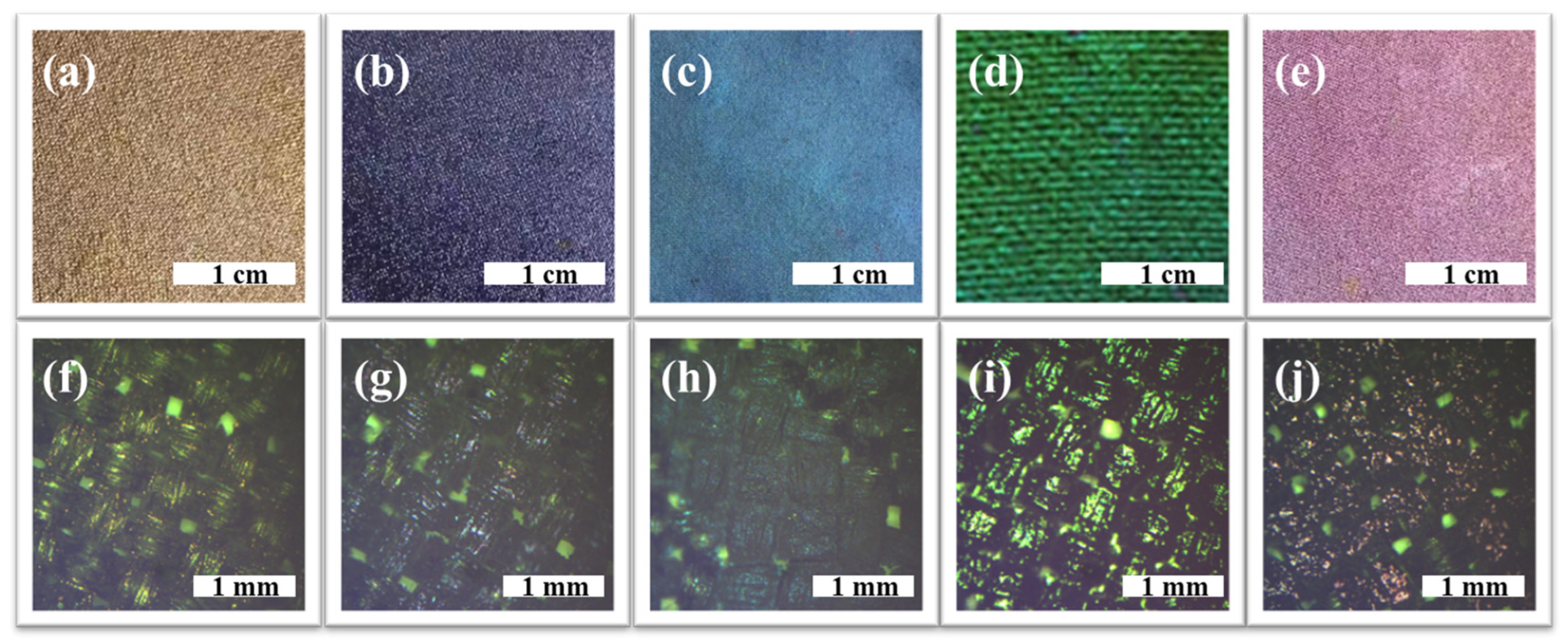
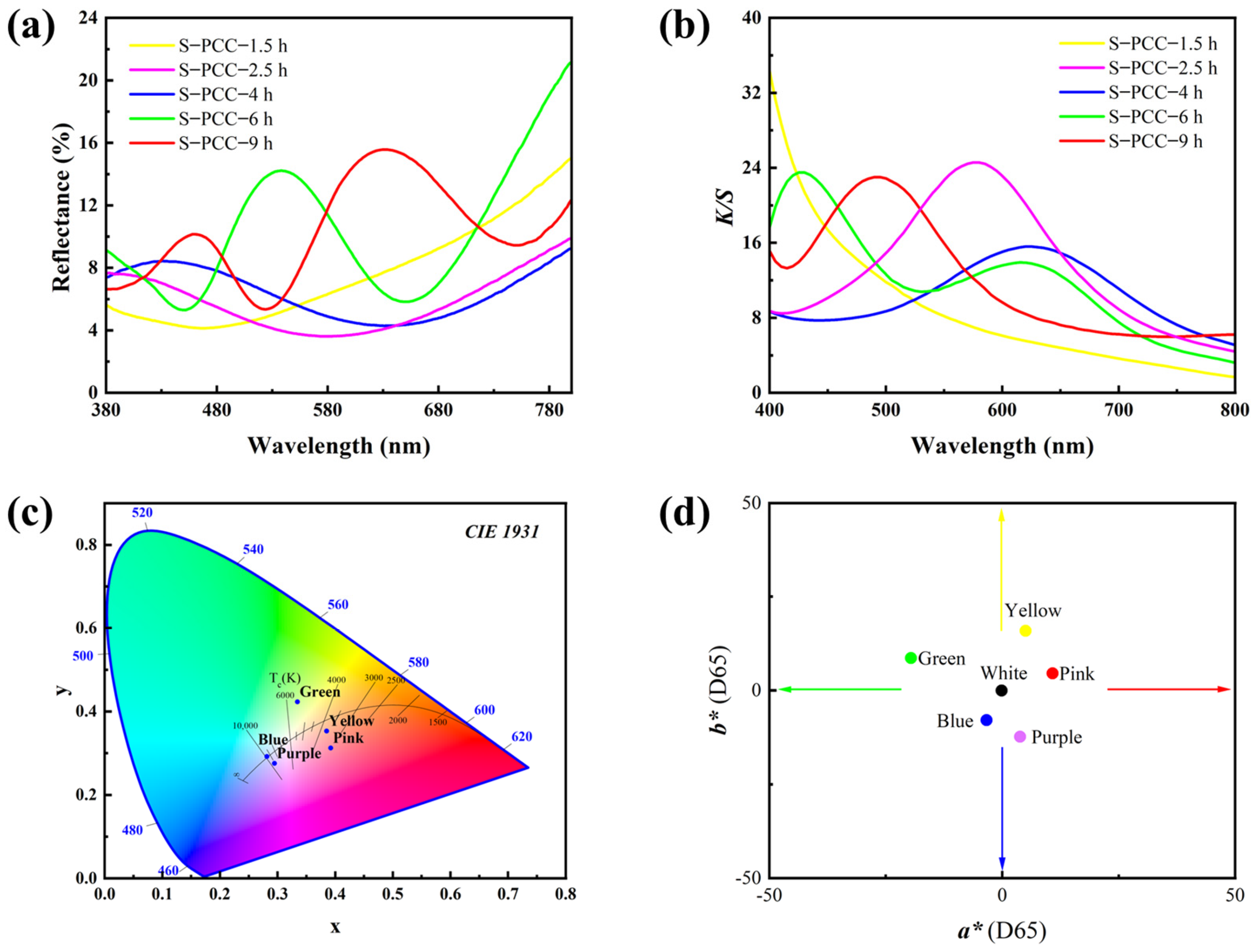
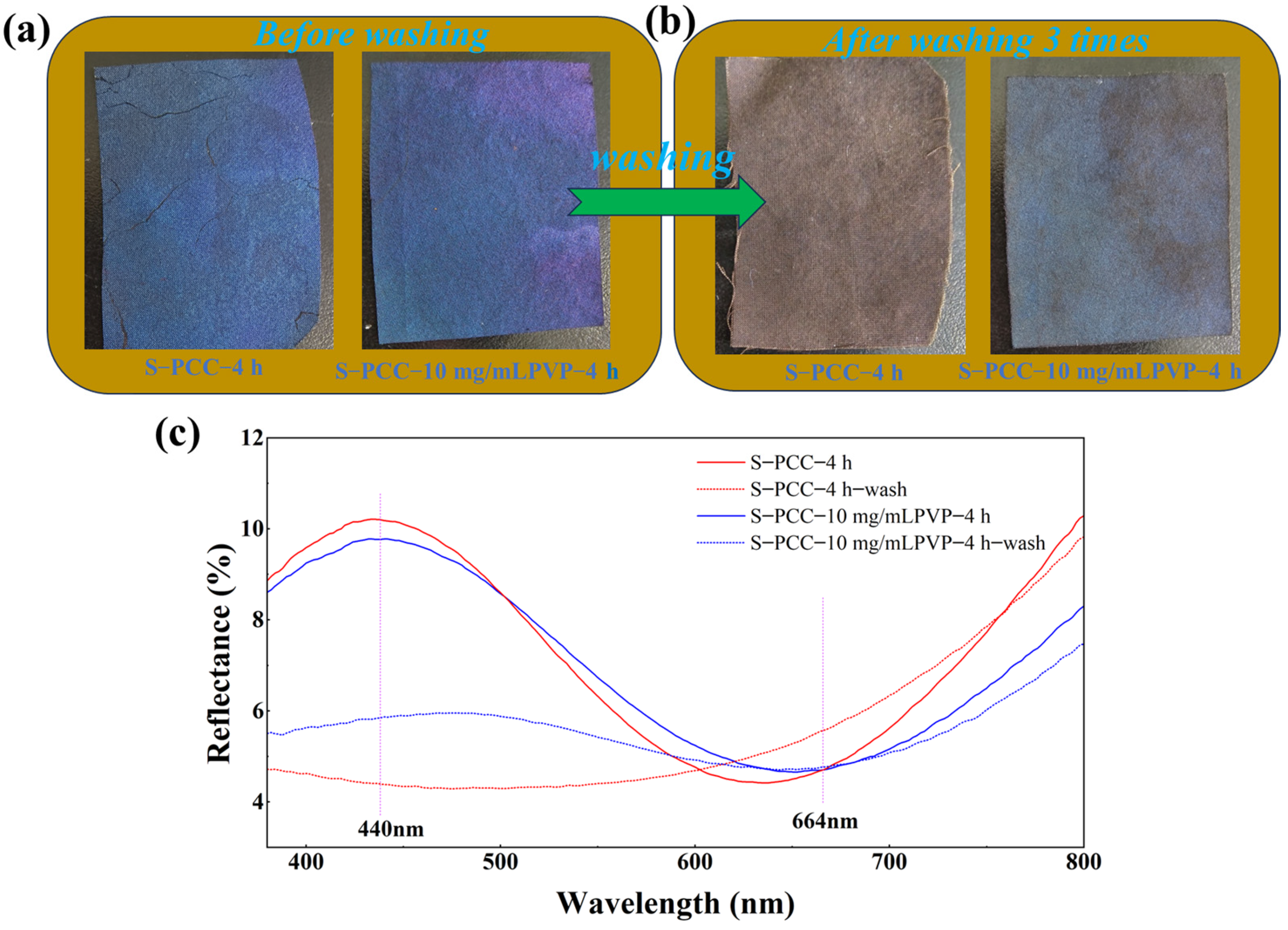
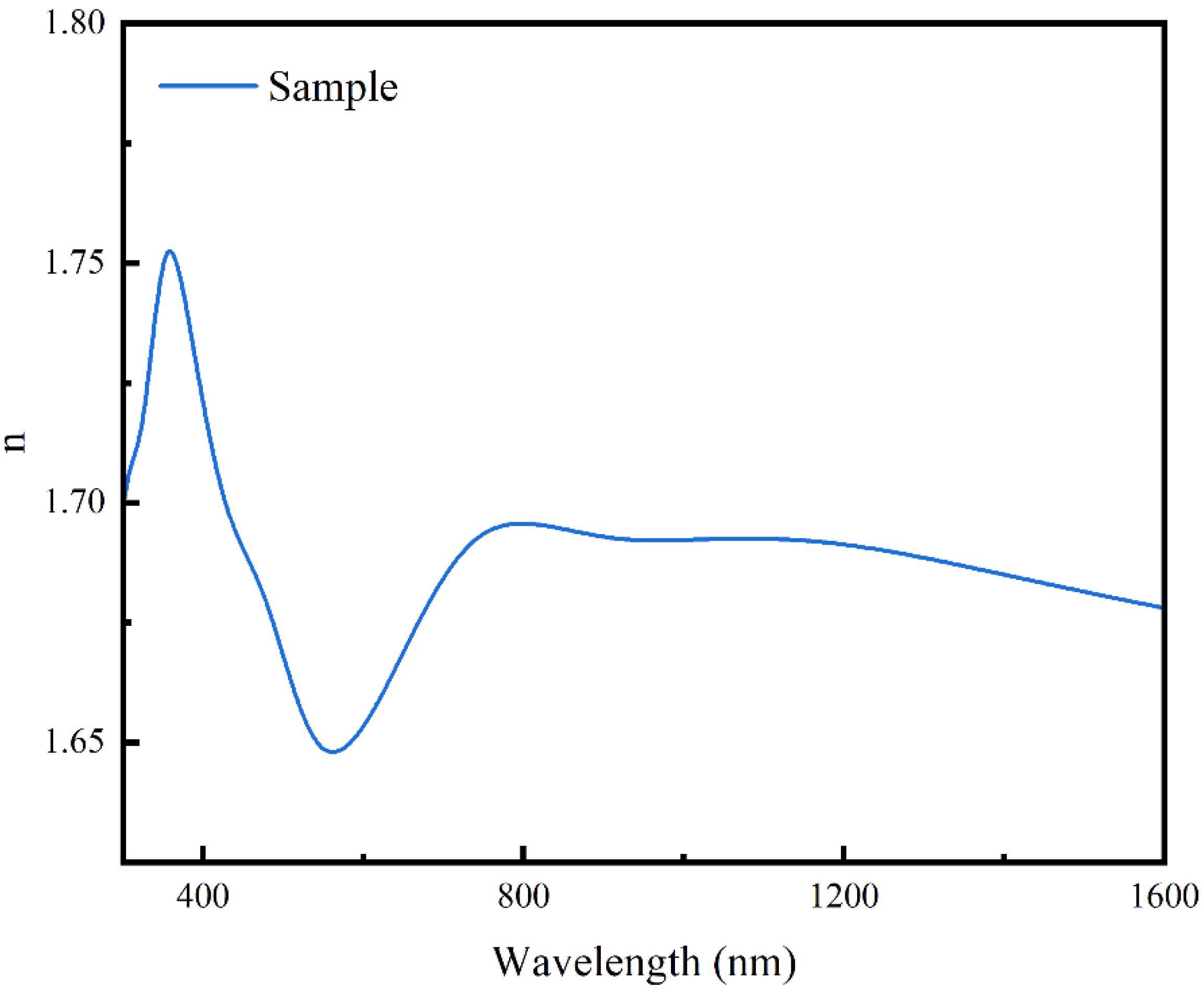
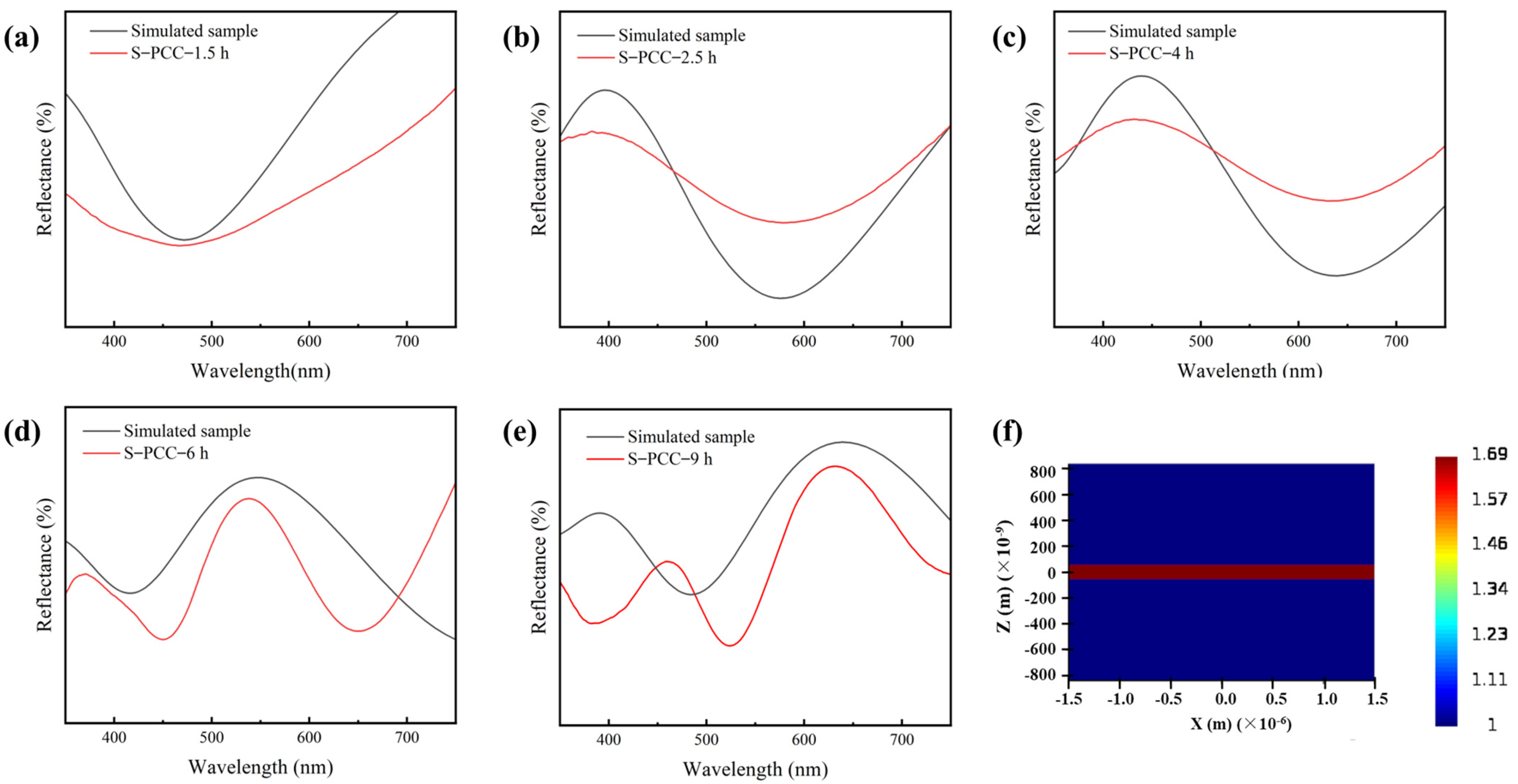
3.1. Color Performance Characterization
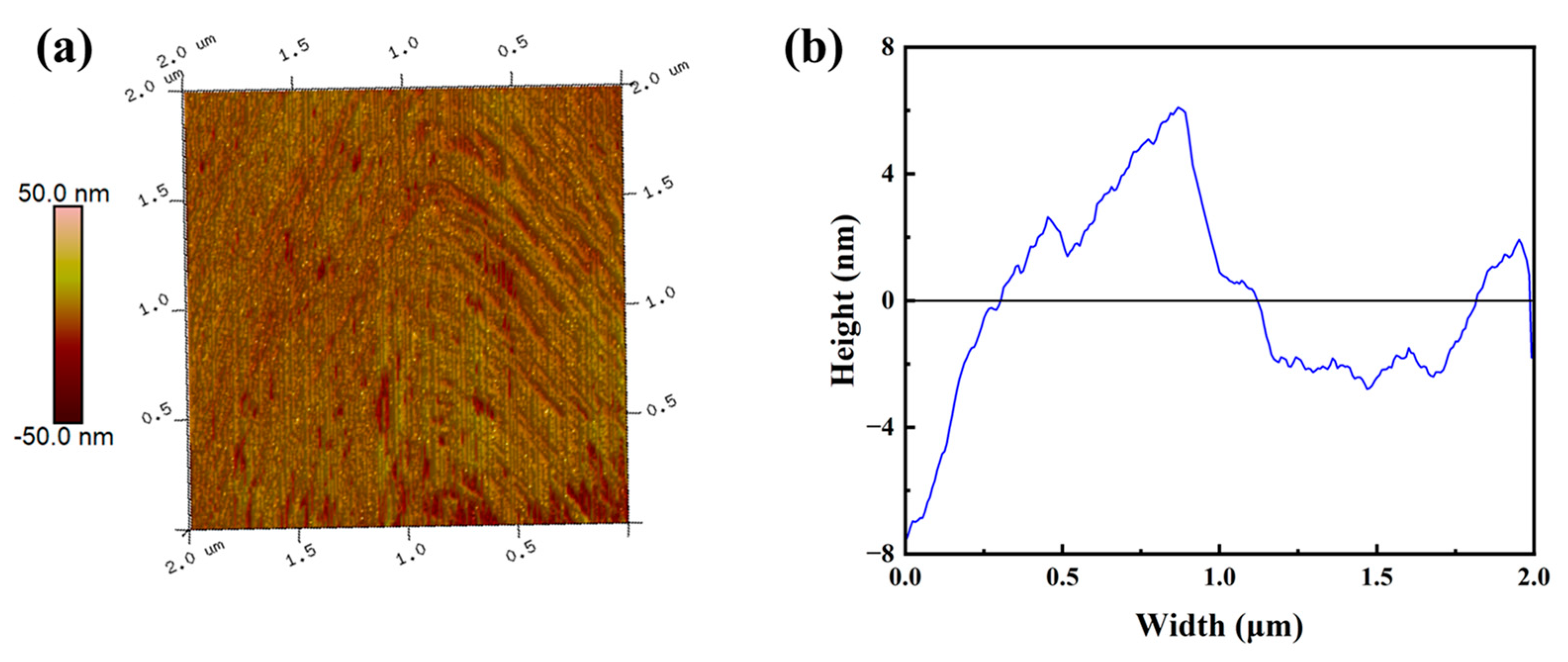
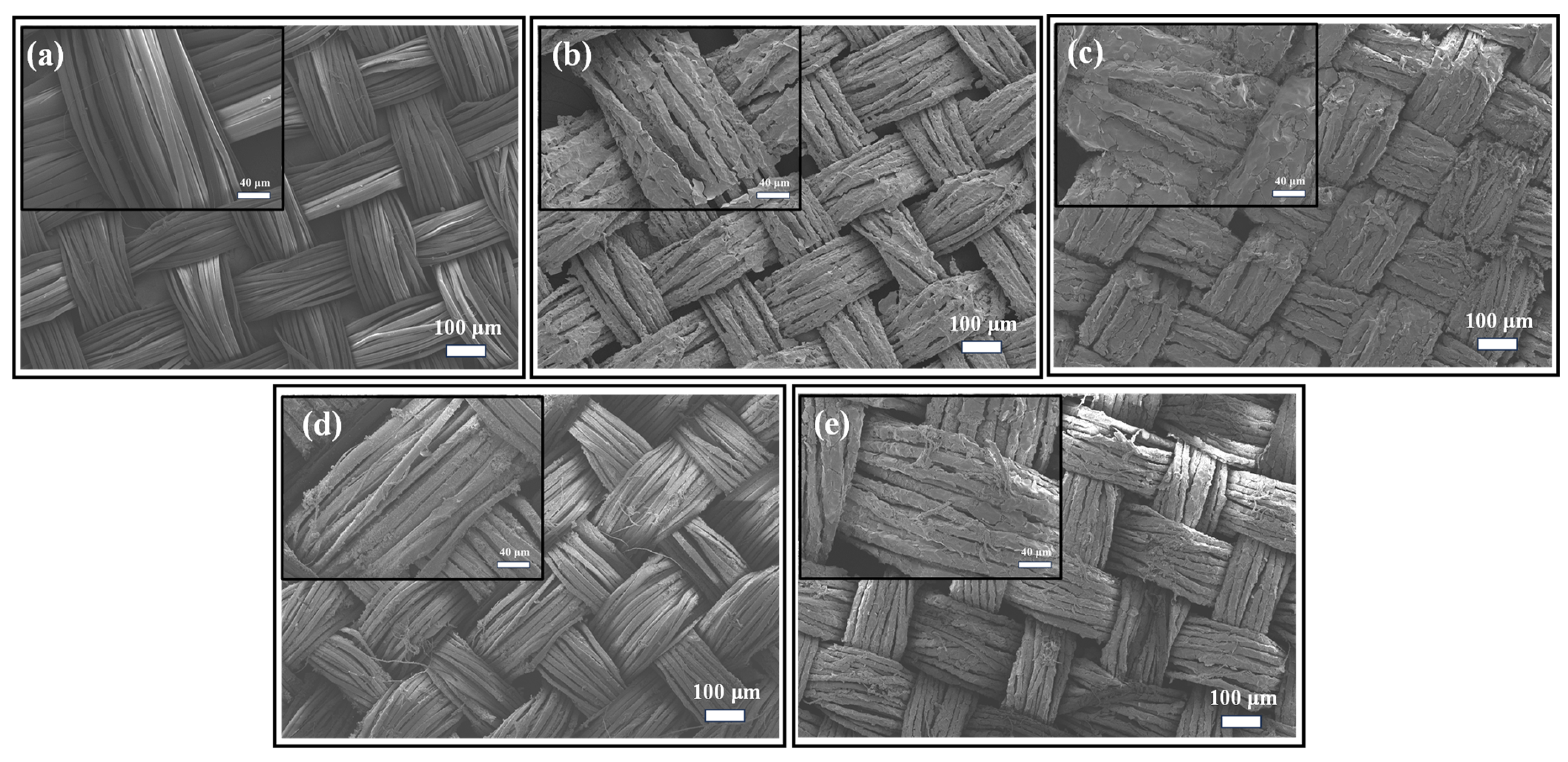
3.2. Microstructure Characterization of PCC Films
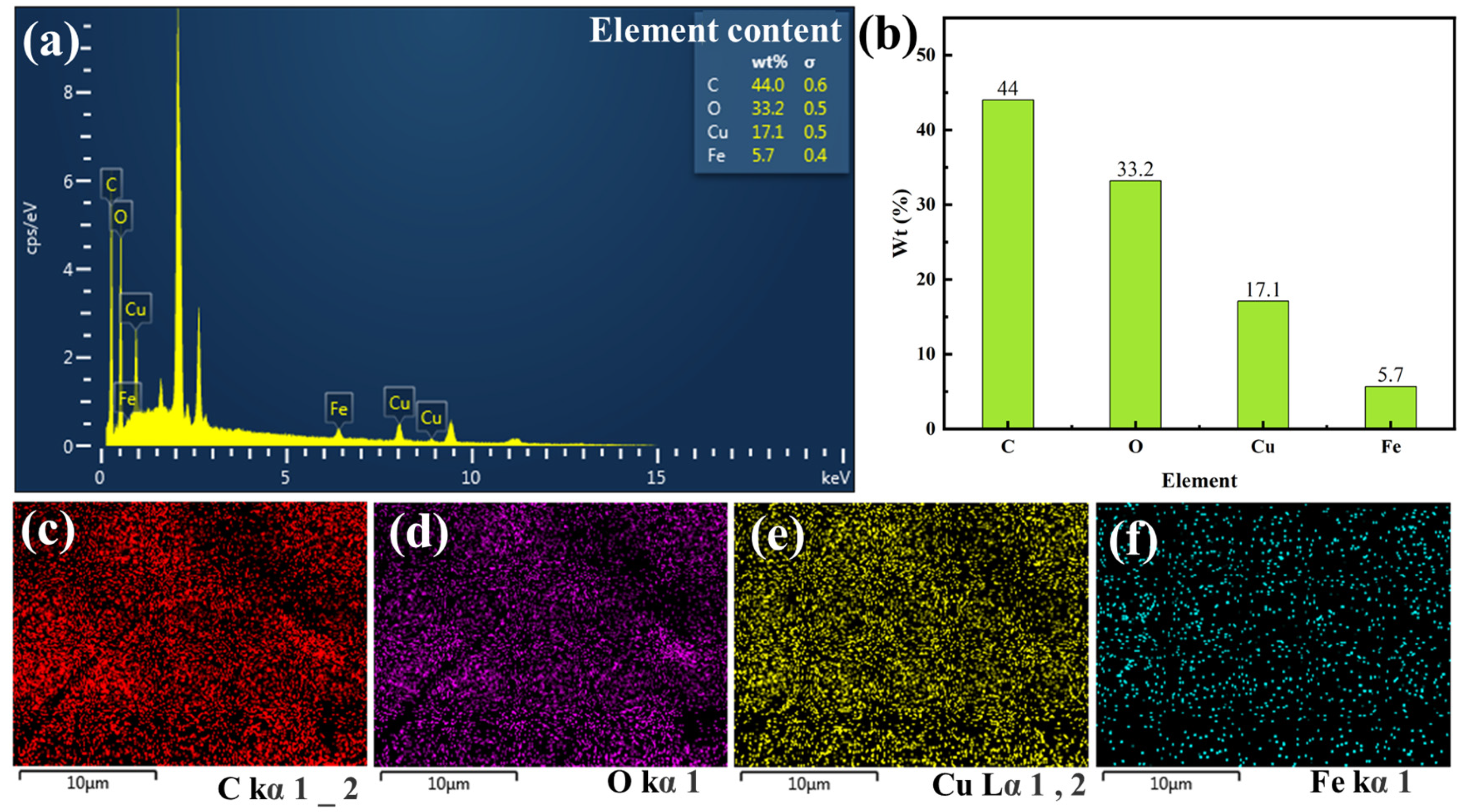
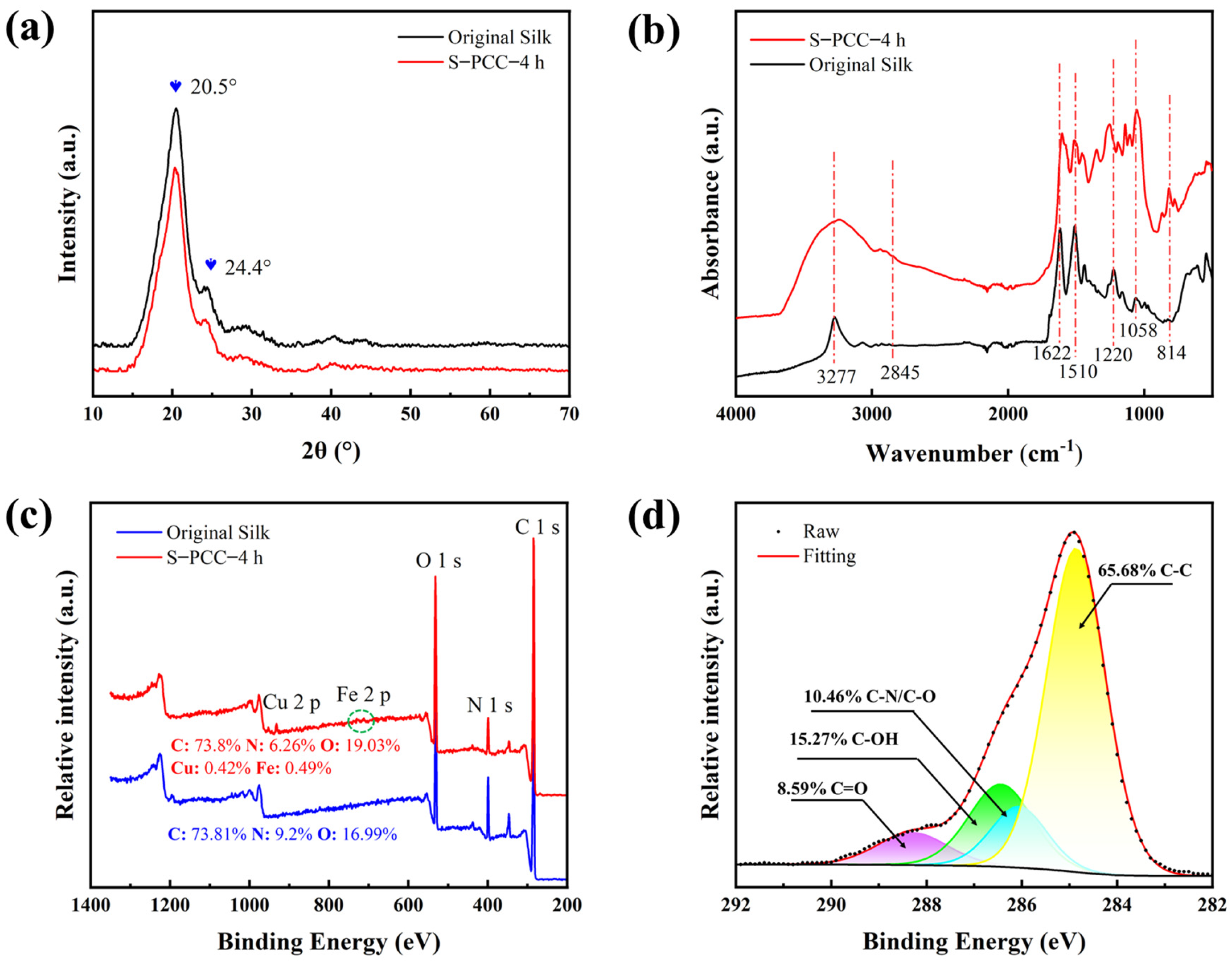
3.3. Surface Composition Analysis of Silk Fabric Modified with PCC
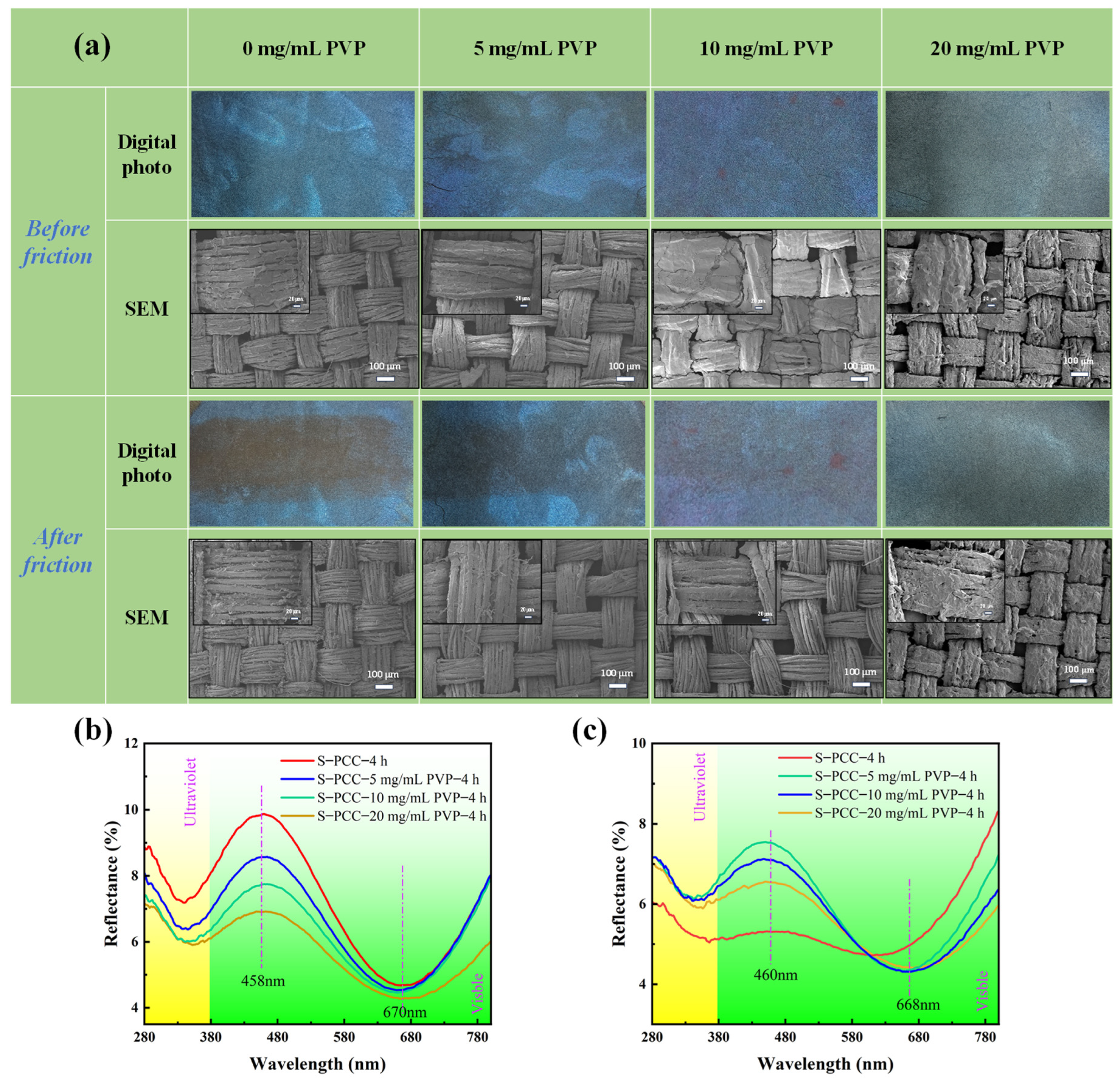
3.4. Mechanical Stability of PCC Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haroun, A.A.; Diab, H.A.; Hakeim, O.A. Cellulosic Fabrics Printing with Multifunctional Encapsulated Phthalocyanine Pigment Blue Using Phase Separation Method. Carbohyd Polym. 2016, 146, 102–108. [Google Scholar] [CrossRef]
- Kinoshita, S.; Yoshioka, S. Structural Colors in Nature: The Role of Regularity and Irregularity in the Structure. ChemPhys Chem. 2005, 6, 1442–1459. [Google Scholar] [CrossRef]
- Fang, K.; Xie, R.; Liu, X.; Zhao, G.; Han, D.; Chen, W.; Shi, Z.; Hao, L.; Cai, Y. Reactive Dye/Poly(Styrene-Co-Butyl Acrylate-Co-Trimethyl (Vinylbenzyl) Ammonium Chloride) Nanospheres with High Coloration Performance for Cleaner Dyeing of Cotton Fabrics. Cellulose 2019, 26, 5807–5819. [Google Scholar] [CrossRef]
- Hirogaki, K.; Nakamura, D.; Sekiguchi, K.; Satake, T.; Tabata, I. The Structural Formation of Closely Packed Colloidal Crystals on Fibre and the Effect of Fibre Surface Functionality on Crystalline Structure. Color. Technol. 2018, 134, 271–274. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Savin, T. Recent Advances in the Biomimicry of Structural Colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Nueangnoraj, K.; Nishihara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of Colored Pigments with Amorphous Arrays of Black and White Colloidal Particles. Angew. Chem.-Int. Edit. 2013, 52, 7261–7265. [Google Scholar] [CrossRef]
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural Colors: From Natural to Artificial Systems. Wires Nanomed. Nanobi 2016, 8, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Beckerlee, H.B. A Study of Thin Film Interference. Philos Photogr. 2018, 9, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Han, S.Y.; Li, Z.; Baac, H.W.; Park, H.J. Flexible High-Color-Purity Structural Color Filters Based on a Higher-Order Optical Resonance Suppression. Sci. Rep. 2019, 9, 14917. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, K.; Liu, W.; Liu, X.; Xu, W.; Deng, B. Development of Structural Colored Cotton Fabric via the Layer-by-Layer Electrostatic Self-Assembling of SiO2 Nanoparticles. Cellulose 2020, 27, 4133–4144. [Google Scholar] [CrossRef]
- Forster, J.D.; Noh, H.; Liew, S.F.; Saranathan, V.; Schreck, C.F.; Yang, L.; Park, J.-G.; Prum, R.O.; Mochrie, S.G.J.; O’Hern, C.S.; et al. Biomimetic Isotropic Nanostructures for Structural Coloration. Adv. Mater. 2010, 22, 2939–2944. [Google Scholar] [CrossRef]
- Xu, B.; Xu, Q.; Hou, M.; Su, J.; Zhang, H.; Lu, X.; Ni, Z. Non-Iridescent Structural Color Patterns with Robust Mechanical Properties Produced by Two-Step Photopolymerization. Dyes Pigments 2023, 218, 111522. [Google Scholar] [CrossRef]
- Cong, H.; Yu, B.; Wang, S.; Qi, L.; Wang, J.; Ma, Y. Preparation of Iridescent Colloidal Crystal Coatings with Variable Structural Colors. Opt. Express 2013, 21, 17831–17838. [Google Scholar] [CrossRef]
- Kohri, M.; Yamazaki, S.; Kawamura, A.; Taniguchi, T.; Kishikawa, K. Bright Structural Color Films Independent of Background Prepared by the Dip-Coating of Biomimetic Melanin-like Particles Having Polydopamine Shell Layers. Colloid. Surf. A-Physicochem. Eng. Asp. 2017, 532, 564–569. [Google Scholar] [CrossRef]
- Lee, G.H.; Han, S.H.; Kim, J.B.; Kim, J.H.; Lee, J.M.; Kim, S.-H. Colloidal Photonic Inks for Mechanochromic Films and Patterns with Structural Colors of High Saturation. Chem. Mat. 2019, 31, 8154–8162. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Wei, T.; Li, Y.; Xing, T.; Shawkey, M.D.; Chen, G. TA-Fe(Iii) Complex Coated PS Nanospheres for Non-Iridescent Structural Coloration of Cotton Fabric. J. Mater. Chem. C 2022, 10, 17472–17480. [Google Scholar] [CrossRef]
- Zhou, J.; Duan, Z.; Lu, B.; Liu, X.; Yang, H.; Deng, B. Preparation of Structural Colors on Cotton Fabrics with Hydrophobicity and High Color Fastness through Chemical Bonds between Polyphenolic Hydroxyl Groups and Polysiloxanes. Cellulose 2023, 30, 6639–6653. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.; Ryu, S.; Kim, S.Y.; Weon, B.M. Crack Formation and Prevention in Colloidal Drops. Sci. Rep. 2015, 5, 13166. [Google Scholar] [CrossRef]
- Xia, T.; Luo, W.; Hu, F.; Qu, W.; Zhang, Z.; Lin, Y.; Liu, X.Y. Fabrication of Crack-Free Photonic Crystal Films on Superhydrophobic Nanopin Surface. ACS Appl. Mater. Interfaces 2017, 9, 22037–22041. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. 2013, 125, 10966–10970. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Zhong, Q.-Z.; Du, Y.; Lv, Y.; Xu, Z.-K. Enzyme-Triggered Coatings of Tea Catechins/Chitosan for Nanofiltration Membranes with High Performance. Green. Chem. 2016, 18, 6205–6208. [Google Scholar] [CrossRef]
- Wu, T.-F.; Hong, J.-D. Dopamine-Melanin Nanofilms for Biomimetic Structural Coloration. Biomacromolecules 2015, 16, 660–666. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.; Zhang, Y.; Zhao, Y.; Chen, L. H2O2-Triggered Rapid Deposition of Poly(Caffeic Acid) Coatings: A Mechanism-Based Entry to Versatile and High-Efficient Molecular Separation. ACS Appl. Mater. Interfaces 2020, 12, 52104–52115. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; Mozos-Pascual, M.D.L.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol Composition and Antioxidant and Metal Chelating Activities of the Solid Residues from the Essential Oil Industry. Ind. Crop. Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Barrett, D.G.; Sileika, T.S.; Messersmith, P.B. Molecular Diversity in Phenolic and Polyphenolic Precursors of Tannin-Inspired Nanocoatings. Chem. Commun. 2014, 50, 7265–7268. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhou, L.; Chai, L.; Fan, Q.; Shao, J. Structural Colouration of Textiles with High Colour Contrast Based on Melanin-like Nanospheres. Dye. Pigment. 2019, 169, 36–44. [Google Scholar] [CrossRef]
- Weiyao, L.; Anvay, P.; Xuhao, Z.; Zhao, W.; Ming, X.; Matthew, D.S.; Nathan, C.G.; Ali, D. Characterization of broadband complex refractive index of synthetic melanin coatings and their changes after ultraviolet irradiation. Appl. Phys. Lett. 2020, 117, 203701. [Google Scholar] [CrossRef]
- Xuan, Z.; Li, J.; Liu, Q.; Yi, F.; Wang, S.; Lu, W. Artificial Structural Colors and Applications. Innovation 2021, 2, 100081. [Google Scholar] [CrossRef]
- Cong, H.; Yu, B.; Zhao, X.S. Imitation of Variable Structural Color in Paracheirodon Innesi Using Colloidal Crystal Films. Opt. Express 2011, 19, 12799. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, L.; Liu, Y.; Xu, Z.; Yin, J.; Ge, D.; Jiang, X. Dynamic Structural Color from Wrinkled Thin Films. Adv. Opt. Mater. 2020, 8, 2000234. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Duan, Z.; Lu, B.; Deng, B.; Xu, W. Preparation of Structural Color on Cotton Fabric with High Color Fastness through Multiple Hydrogen Bonds between Polyphenol Hydroxyl and Lactam. ACS Appl. Mater. Interfaces 2022, 14, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Shao, F. Research and Application of K/S Value in Stain Identification. PRT 2023, 52, 176–182. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and Classification of Silks Using Infrared Spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, Q.; Zhou, J.; Zhang, Y.; Ju, H.; Deng, Z. Chemical Modification of Silk Fibroin through Serine Amino Acid Residues. Materials 2022, 15, 4399. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, F.; Xu, C.; Yuan, F.; Gao, Y. Molecular Interaction between (−)-Epigallocatechin-3-Gallate and Bovine Lactoferrin Using Multi-Spectroscopic Method and Isothermal Titration Calorimetry. Food Res. Int. 2014, 64, 141–149. [Google Scholar] [CrossRef]
- Wang, D.; Kim, D.; Shin, C.-H.; Zhao, Y.; Park, J.-S.; Ryu, M. Evaluation of Epigallocatechin Gallate (EGCG) to Remove Pb(II) Using Spectroscopic and Quantum Chemical Calculation Method. Environ. Earth Sci. 2019, 78, 138. [Google Scholar] [CrossRef]
- Elline; Fibryanto, E.; Amanda, H. Characterization of Nano-Hydroxyapatite–Collagen and Epigallocatechin-3-Gallate (EGCG) Composites by Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDS), X-Ray Diffraction (XRD), and Fourier Transform Infrared (FTIR) Spectroscopy. Sci. Dent. J. 2022, 6, 80. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, Y.; Wang, L.; Li, J.; Li, H.; Shi, Y.; Pan, D. Rapid Adsorption of Phosphorus at Low Concentration from Water Using a Novel Green Organometallic Material EGCG-Fe. J. Environ. Chem. Eng. 2021, 9, 106242. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly (N-Vinyl Pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef]

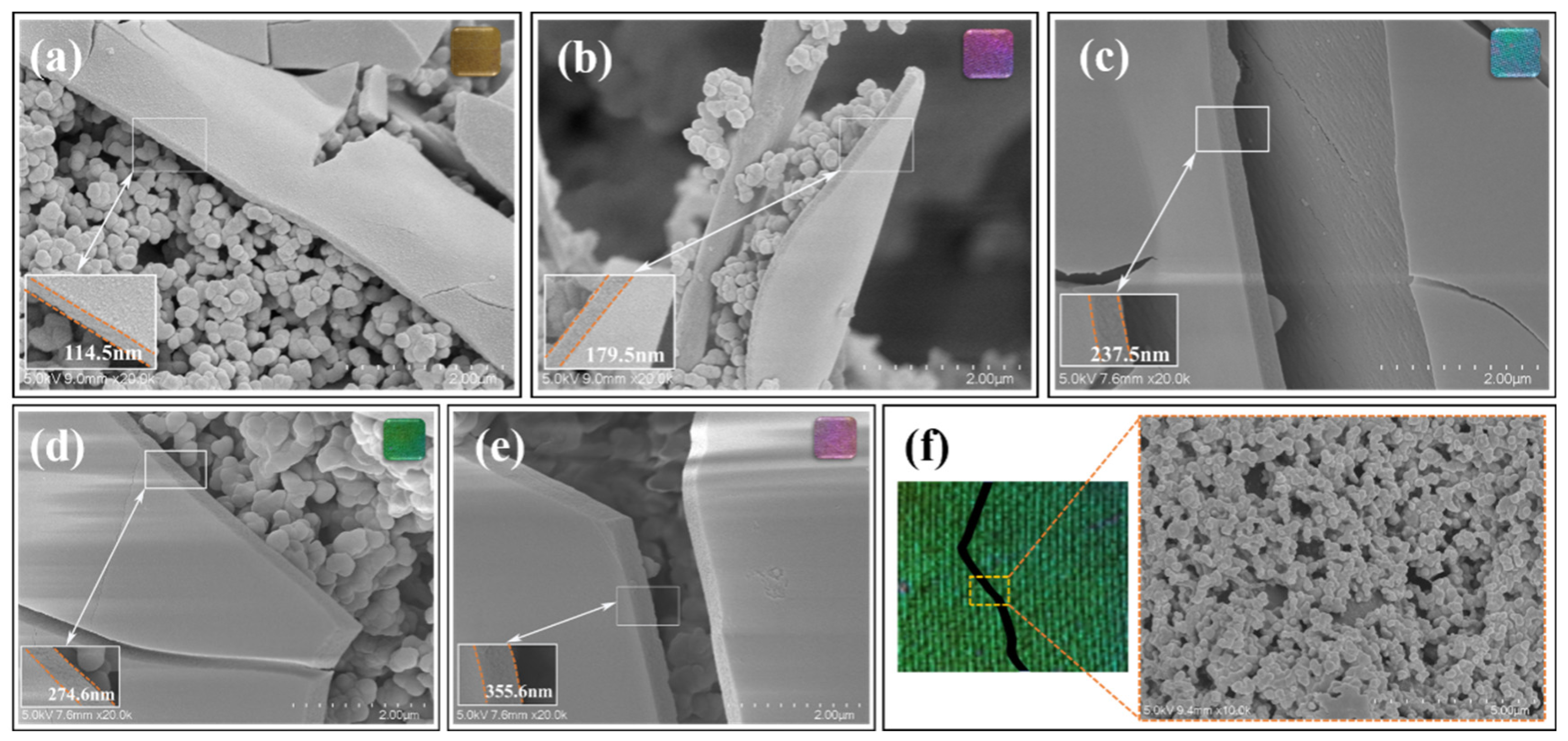

| Reaction time (h) | 1.5 | 2.5 | 4.0 | 6.0 | 9.0 |
| Film thickness (nm) | 114.5 ± 6.91 | 179.5 ± 5.82 | 237.5 ± 5.35 | 274.6 ± 9.82 | 355.6 ± 4.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Sha, D.; Li, Y.; Wang, M.; Zhu, X.; Wang, X.; Chen, G.; Li, Y.; Xing, T. Preparation of Natural Plant Polyphenol Catechin Film for Structural Coloration of Silk Fabrics. Biomimetics 2024, 9, 15. https://doi.org/10.3390/biomimetics9010015
Yang S, Sha D, Li Y, Wang M, Zhu X, Wang X, Chen G, Li Y, Xing T. Preparation of Natural Plant Polyphenol Catechin Film for Structural Coloration of Silk Fabrics. Biomimetics. 2024; 9(1):15. https://doi.org/10.3390/biomimetics9010015
Chicago/Turabian StyleYang, Shuaikang, Desheng Sha, Yijiang Li, Meiqi Wang, Xiaowei Zhu, Xiangrong Wang, Guoqiang Chen, Yichen Li, and Tieling Xing. 2024. "Preparation of Natural Plant Polyphenol Catechin Film for Structural Coloration of Silk Fabrics" Biomimetics 9, no. 1: 15. https://doi.org/10.3390/biomimetics9010015
APA StyleYang, S., Sha, D., Li, Y., Wang, M., Zhu, X., Wang, X., Chen, G., Li, Y., & Xing, T. (2024). Preparation of Natural Plant Polyphenol Catechin Film for Structural Coloration of Silk Fabrics. Biomimetics, 9(1), 15. https://doi.org/10.3390/biomimetics9010015






