Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis
2.2. Imaging of Bee Mandibles
2.3. Development and Characterisation of Bioinspired Substrates
2.4. Adhesion Measurements on Structured/Bioinspired Substrates
2.5. Data Analysis and Statistics
2.6. Image Processing
3. Results
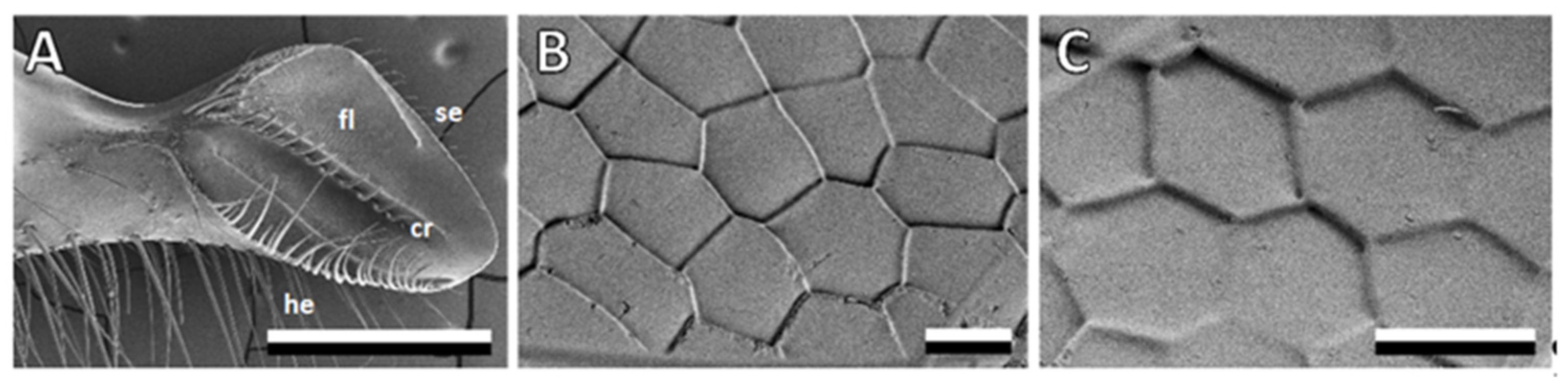
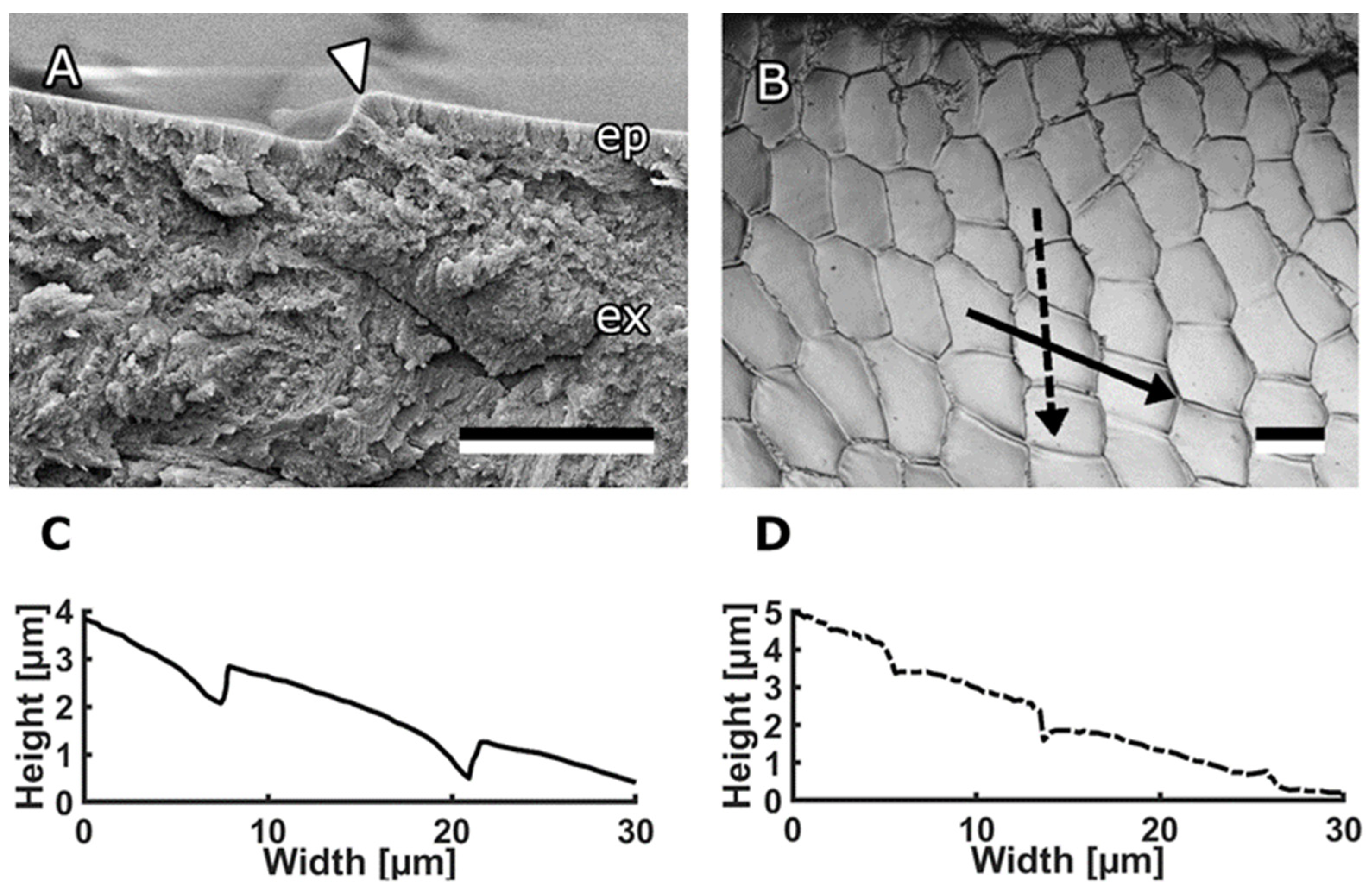
3.1. Bee Mandible Surfaces
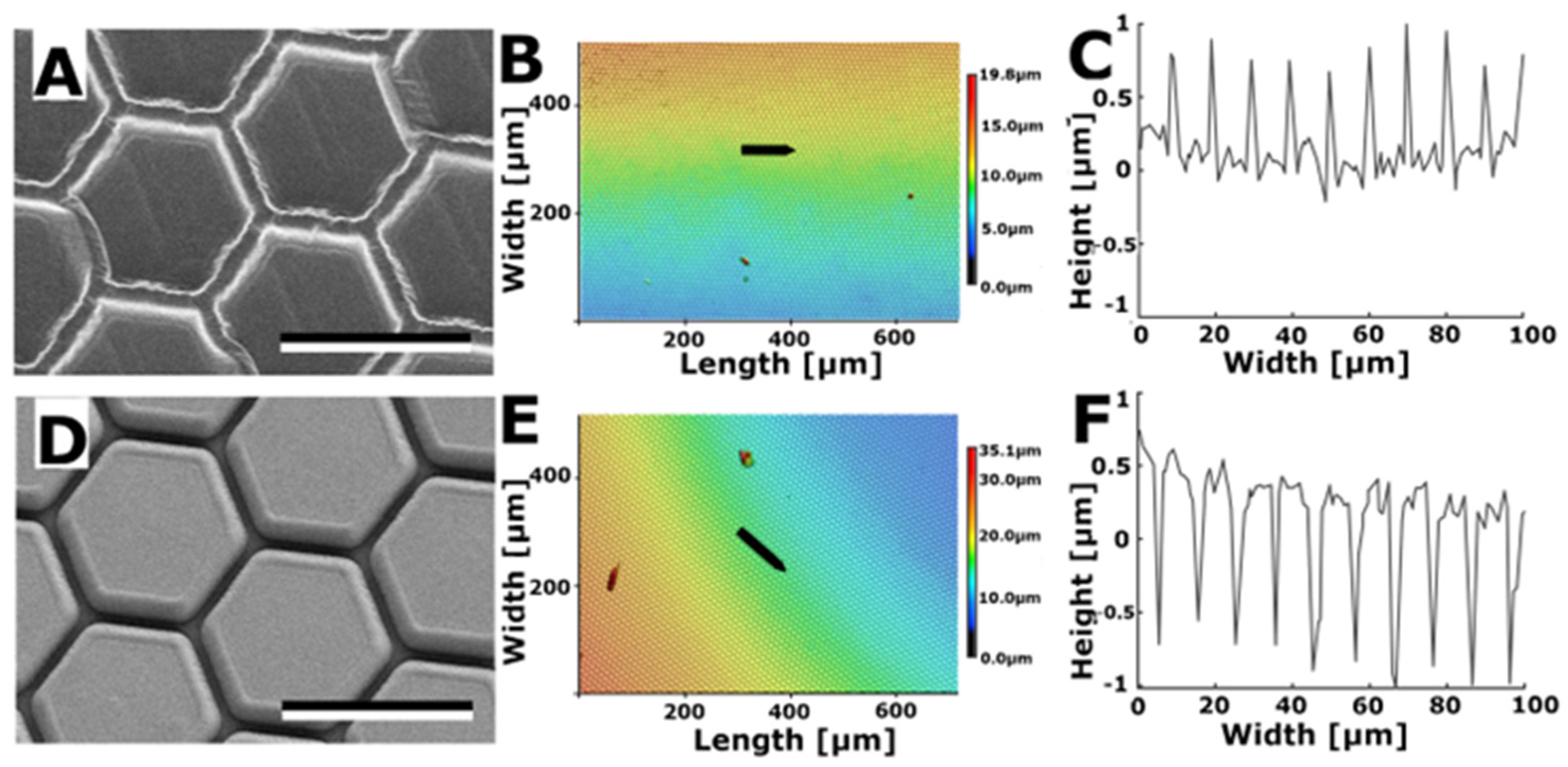
3.2. Characterisation of Bioinspired Surfaces
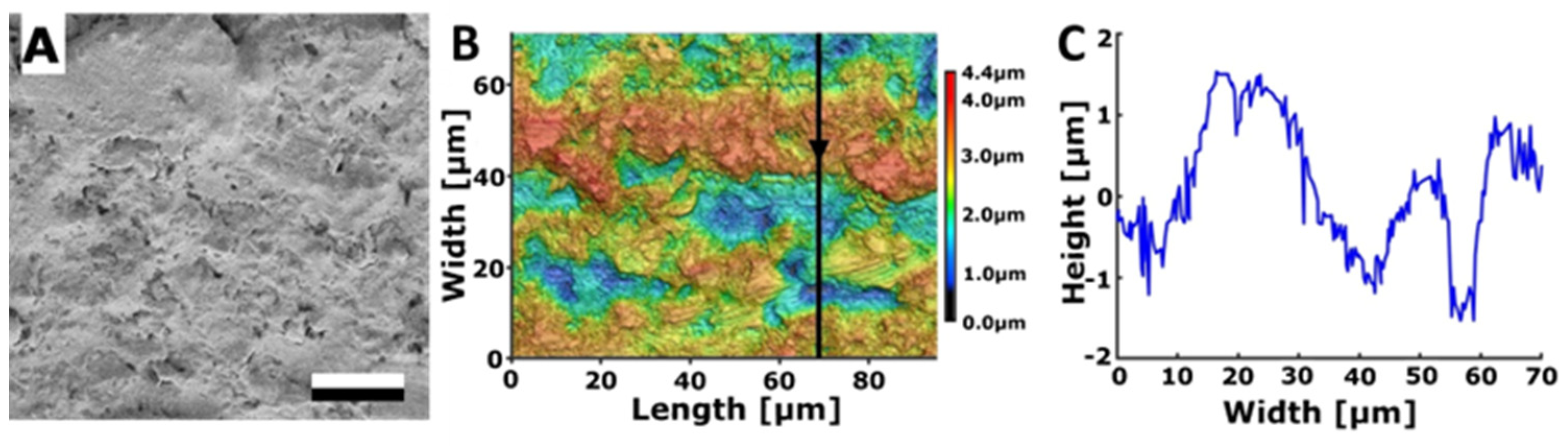
3.2.1. Rough Steel
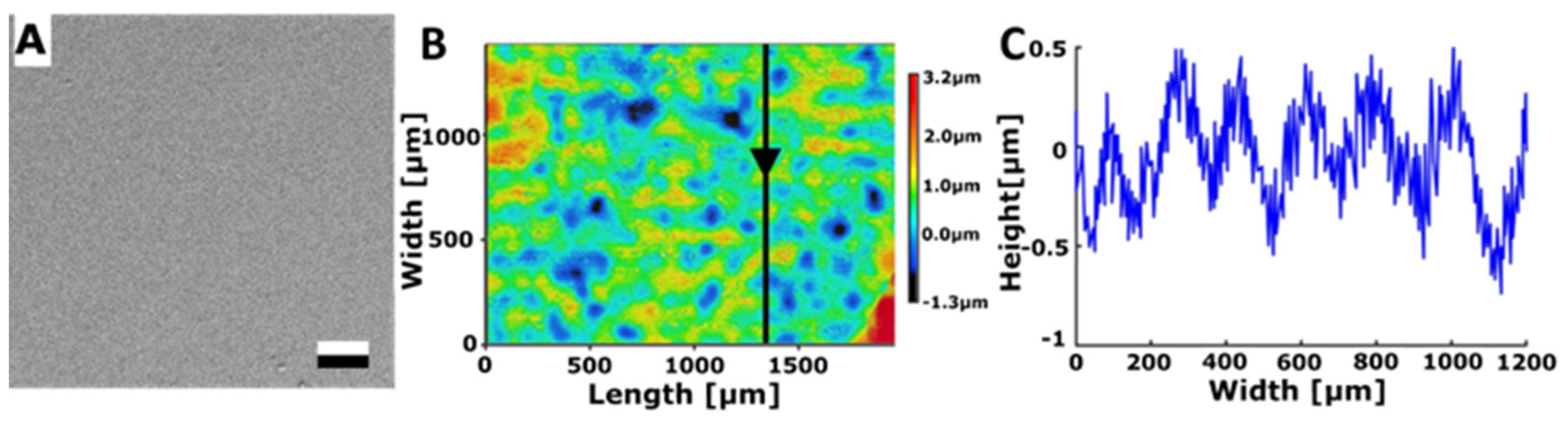
3.2.2. Bénard Structures
3.2.3. Hexagons
3.3. Adhesion on Bioinspired Substrates
4. Discussion
4.1. Development and Characterisation of Bioinspired Surfaces
4.2. Adhesion of Propolis on Bioinspired Surfaces
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Speck, T. Bionik: Faszinierende Lösungen der Natur für die Technik der Zukunft; Lavori Verlag: Freiburg, Germany, 2012. [Google Scholar]
- Nachtigall, W. Bionik: Grundlagen und Beispiele für Ingenieure und Naturwissenschaftler; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Paz-Gómez, G.; del Caño-Ochoa, J.C.; Rodríguez-Alabanda, O.; Romero, P.E.; Cabrerizo-Vílchez, M.; Guerrero-Vaca, G.; Rodríguez-Valverde, M.A. Water-Repellent Fluoropolymer-Based Coatings. Coatings 2019, 9, 293. [Google Scholar] [CrossRef]
- Palumbo, F.; Lo Porto, C.; Favia, P. Plasma Nano-Texturing of Polymers for Wettability Control: Why, What and How. Coatings 2019, 9, 640. [Google Scholar] [CrossRef]
- He, Z.; Wu, C.; Hua, M.; Wu, S.; Wu, D.; Zhu, X.; Wang, J.; He, X. Bioinspired multifunctional anti-icing hydrogel. Matter 2020, 2, 723–734. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Wang, Y.; Chen, J.; Cui, B.; Yin, P.; Chen, J.; Zhang, X.; Zhang, L.; Xin, J.H. Bioinspired Superhydrophobic Surface Constructed from Hydrophilic Building Blocks: A Case Study of Core–Shell Polypyrrole-Coated Copper Nanoneedles. Coatings 2020, 10, 347. [Google Scholar] [CrossRef]
- Volpe, A.; Gaudiuso, C.; Di Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A. Direct Femtosecond Laser Fabrication of Superhydrophobic Aluminum Alloy Surfaces with Anti-icing Properties. Coatings 2020, 10, 587. [Google Scholar] [CrossRef]
- Nachtigall, W. Bionik als Wissenschaft: Erkennen—Abstrahieren—Umsetzen; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; de Castro, S.; Marcucci, M. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Saccardi, L.; Schiebl, J.; Weber, K.; Schwarz, O.; Gorb, S.N.; Kovalev, A. Adhesive Behavior of Propolis on Different Substrates. Front. Mech. Eng. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Richter, A.; Schoeters, E.; Billen, J. Morphology and closing mechanism of the mandibular gland orifice in ants (Hymenoptera: Formicidae). J. Morphol. 2021, 282, 1127–1140. [Google Scholar] [CrossRef]
- Klunk, C.L.; Argenta, M.A.; Casadei-Ferreira, A.; Economo, E.P.; Pie, M.R. Mandibular morphology, task specialization and bite mechanics in Pheidole ants (Hymenoptera: Formicidae). J. R. Soc. Interface 2021, 18, 20210318. [Google Scholar] [CrossRef]
- Silveira, O.T.; dos Santos, J.N.A., Jr. Comparative morphology of the mandibles of female polistine social wasps (Hymenoptera, Vespidae, Polistinae). Rev. Bras. Entomol. 2011, 55, 479–500. [Google Scholar] [CrossRef]
- Saccardi, L.; Brümmer, F.; Schiebl, J.; Schwarz, O.; Kovalev, A.; Gorb, S.N. Interaction between honeybee mandibles and propolis. Beilstein J. Nanotechnol. 2022, 13, 958–974. [Google Scholar] [CrossRef]
- Nakamura, J.; Seeley, T.D. The functional organization of resin work in honeybee colonies. Behav. Ecol. Sociobiol. 2006, 60, 339–349. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Snodgrass, R.E. The Anatomy of the Honey Bee; Washington: Government Printing Office: Washington, DC, USA, 1910.
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; van Englesdorp, D. Standard methods for Apis mellifera anatomy and dissection Métodos estandar para la disección y anatomía de Apis mellifera. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef]
- Voigt, D.; Gorb, S.N. An insect trap as habitat: Cohesion-failure mechanism prevents adhesion of Pameridea roridulae bugs to the sticky surface of the plant Roridula gorgonias. J. Exp. Biol. 2008, 211, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Perez Goodwyn, P.; De Souza, E.; Fujisaki, K.; Gorb, S.N. Moulding technique demonstrates the contribution of surface geometry to the super-hydrophobic properties of the surface of a water strider. Acta Biomater. 2008, 4, 766–770. [Google Scholar] [CrossRef]
- Gorb, E.; Purtov, J.; Gorb, S.N. Adhesion force measurements on the two wax layers of the waxy zone in Nepenthes alata pitchers. Sci. Rep. 2014, 4, 5154. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. A Sticky Affair: Resin Collection by Bornean Stingless Bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Gastauer, M.; Campos, L.A.O.; Wittmann, D. Handling sticky resin by stingless bees (Hymenoptera, Apidae). Rev. Bras. De Entomol. 2011, 55, 234–240. [Google Scholar] [CrossRef]
- Gastauer, M.; Campos, L.A.O.; Wittmann, D. Handling sticky Resin by Stingless Bees: Adhesive Properties of Surface Structures. An. Acad. Bras. Cienc. 2013, 85, 1189–1196. [Google Scholar] [CrossRef][Green Version]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2016, 58, 1–49. [Google Scholar] [CrossRef]
- Bénard, H. Les tourbillons cellulaires dans une nappe liquide. Rev. Générale Sci. Pures Appliquées 1900, 11, 1261–1271. [Google Scholar]
- Rayleigh, L. On convection currents in a horizontal layer of fluid, when the higher temperature is on the under side. Philos. Mag. Ser. 1916, 5, 737–761. [Google Scholar] [CrossRef]
- Seeler, F. Numerische und Experimentelle Untersuchung des Lackfilmverlaufs; Paderborn University: Paderborn, Germany, 2021. [Google Scholar]
- Varenberg, M.; Gorb, S.N. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 2009, 21, 483–486. [Google Scholar] [CrossRef]
- Varenberg, M.; Murarash, B.; Kligerman, Y.; Gorb, S.N. Geometry-controlled adhesion: Revisiting the contact splitting hypothesis. Appl. Phys. A 2011, 103, 933–938. [Google Scholar] [CrossRef]
- Gorb, S.N. Visualisation of native surfaces by two-step moldings. Microsc. Today 2007, 15, 44–47. [Google Scholar] [CrossRef]
- Koch, K.; Schulte, A.J.; Fischer, A.; Gorb, S.N.; Barthlott, W. A fast, precise and low-cost replication technique for nano- and high-aspect-ratio structures of biological and artificial surfaces. Bioinspiration Biomim. 2008, 3, 46002. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.N.; Scherge, M. Biological microtribology: Anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 2000, 267, 1239–1244. [Google Scholar] [CrossRef]
- Jiao, Y.; Gorb, S.N.; Scherge, M. Adhesion measured on the attachment pads of Tettigonia viridissima (Orthoptera, insecta). J. Exp. Biol. 2000, 203, 1887–1895. [Google Scholar] [CrossRef]
- Gorb, E.; Kastner, V.; Peressadko, A.; Arzt, E.; Gaume, L.; Rowe, N.; Gorb, S.N. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J. Exp. Biol. 2004, 207, 2947–2963. [Google Scholar] [CrossRef]
- Wheater, C.P.; Evans, M.E.G. The mandibular forces and pressures of some predacious Coleoptera. J. Insect Physiol. 1989, 35, 815–820. [Google Scholar] [CrossRef]
- Švantner, M.; Kučera, M.; Smazalová, E.; Houdková, Š.; Čerstvý, R. Thermal effects of laser marking on microstructure and corrosion properties of stainless steel. Appl. Opt. 2016, 55, D35–D45. [Google Scholar] [CrossRef] [PubMed]
- Frönd, M.; Ventzke, V.; Riekehr, S.; Kashaev, N.; Klusemann, B.; Enz, J. Microstructure and hardness evolution of laser metal deposited AA5087 wall-structures. Procedia CIRP 2018, 74, 131–135. [Google Scholar] [CrossRef]
- Tang, M.; Shi, Y.; Zhu, W.; Zhang, N.; Zhang, L. A Convenient and High-Efficient Laser Micro-Engraving Treatment for Controllable Preparation of Microstructure on Al Alloy. Materials 2018, 11, 2297. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Niidome, T.; Onoe, H.; Heisterkamp, A.; Terakawa, M. Spatially-targeted laser fabrication of multi-metal microstructures inside a hydrogel. Opt. Express 2019, 27, 14657–14666. [Google Scholar] [CrossRef] [PubMed]
- Ackerl, N.; Fisch, G.; Auerswald, J.; Wegener, K. Evolution of microstructures on stainless steel induced by ultra-short pulsed laser ablation. SN Appl. Sci. 2020, 2, 652. [Google Scholar] [CrossRef]
- Yang, S.-N.; Liu, X.-Q.; Zheng, J.-X.; Lu, Y.-M.; Gao, B.-R. Periodic Microstructures Fabricated by Laser Interference with Subsequent Etching. Nanomaterials 2020, 10, 1313. [Google Scholar] [CrossRef]
- Henriksen, N.G.; Andersen, O.Z.; Jellesen, M.S.; Christiansen, T.L.; Somers, M.A.J. Influence of Laser Marking on Microstructure and Corrosion Performance of Martensitic Stainless Steel Surfaces for Biomedical Applications. HTM J. Heat Treat. Mater. 2022, 77, 177–196. [Google Scholar] [CrossRef]
- Henriksen, N.G.; Poulios, K.; Somers, M.A.J.; Christiansen, T.L. Impact of laser marking on microstructure and fatigue life of medical grade titanium. Mater. Sci. Eng. 2023, 873, 145020. [Google Scholar] [CrossRef]
- Richter, K.; Grunwald, I.; von Byern, J. Bioadhesives. In Handbook of Adhesion Technology; da Silva, L.F.M., Ochsner, A., Adam, R.D., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–45. [Google Scholar] [CrossRef]
- Liang, L.; Lu, L.; Xing, D.; Wan, Z.; Tang, Y. Preparation of superhydrophobic and anti-resin-adhesive surfaces with micro/nanoscale structures on high-speed steel via laser processing. Surf. Coat. Technol. 2019, 357, 57–68. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y. 3D Printing Collembola Cuticle Inspired Superhydrophobic Microstructures for Potential Deicing Application. In Proceedings of the 2020 International Symposium on Flexible Automation, Chicago, IL, USA, 9–10 July 2020; p. V001T07A009. [Google Scholar] [CrossRef]
- Bing, W.; Wang, H.; Tian, L.M.; Zhao, J.; Jin, H.C.; Du, W.B.; Ren, L.Q. Small Structure, Large Effect: Functional Surfaces Inspired by Salvinia Leaves. Small Struct. 2021, 2, 2100079. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef]
- Lu, L.; Yao, W.; Xie, Y.; Li, K.; Wan, Z. Study on the wettability of biomimetic stainless-steel surfaces inspired by Bauhinia Linn. Leaf Surf. Coat. Technol. 2021, 405, 126721. [Google Scholar] [CrossRef]
- Castro-Mora, M.; Vásquez-González, M.; Cordero-Guerrero, J.; Benavides-Acevedo, M.; González, J.; López-Brenes, M.J.; Vega-Baudrit, J.; Corrales-Ureña, Y. Bacterial anti-adhesive films of PDMS coated with microstructures of biogenic silica rosettes extracted from pineapple peels residues. Surf. Interfaces 2022, 30, 101881. [Google Scholar] [CrossRef]
- Oopath, S.V.; Baji, A.; Abtahi, M. Biomimetic Rose Petal Structures Obtained Using UV-Nanoimprint Lithography. Polymers 2022, 14, 3303. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Cheng, X.; Wang, J.; Li, Y.; You, J. Programmable Transition between Adhesive/Anti-Adhesive Performances on Porous PVDF Spheres Supported by Shape Memory PLLA. Polymers 2022, 14, 374. [Google Scholar] [CrossRef]
- Lifka, S.; Stecher, C.; Meyer, M.; Joel, A.-C.; Heitz, J.; Baumgartner, W. Biomimetic, antiadhesive surface structure inspired by the calamistra setae of cribellate spiders for electrospun nanofiber handling. Front. Ecol. Evol. 2023, 11, 99355. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, F.; Gu, H.; Feng, J.; Huang, D.; Zheng, L.; Yao, G.; Chen, Z.; Wang, C. Adhesion failure and anti-adhesion bionic structure optimization of surgical electrodes in soft tissue cutting. J. Manuf. Process. 2023, 89, 444–457. [Google Scholar] [CrossRef]
- Petersen, D.S.; Gorb, S.N.; Heepe, L. The influence of material and roughness on the settlement and the adhesive strength of the barnacle Balanus improvisus in the Baltic Sea. Front. Mar. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Petersen, D.S.; Heepe, L.; Gorb, S.N. Porous silicone substrates inhibit permanent barnacle attachment under natural conditions. Biointerphases 2020, 15, 061013. [Google Scholar] [CrossRef]
- Petersen, D.S.; Schultz, M.; Gorb, S.N.; Heepe, L. A systematic investigation into the effect of fibrillar microstructures on the settlement and attachment strength of the bay barnacle Balanus improvisus under natural conditions. Appl. Phys. A 2020, 126, 1–11. [Google Scholar] [CrossRef]
- Petersen, D.S.; Kleinteich, T.; Gorb, S.N.; and Heepe, L. Competing with barnacle cement: Wetting resistance of a re-entrant surface reduces underwater adhesion of barnacles. J. R. Soc. Interface 2018, 15, 20180396. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
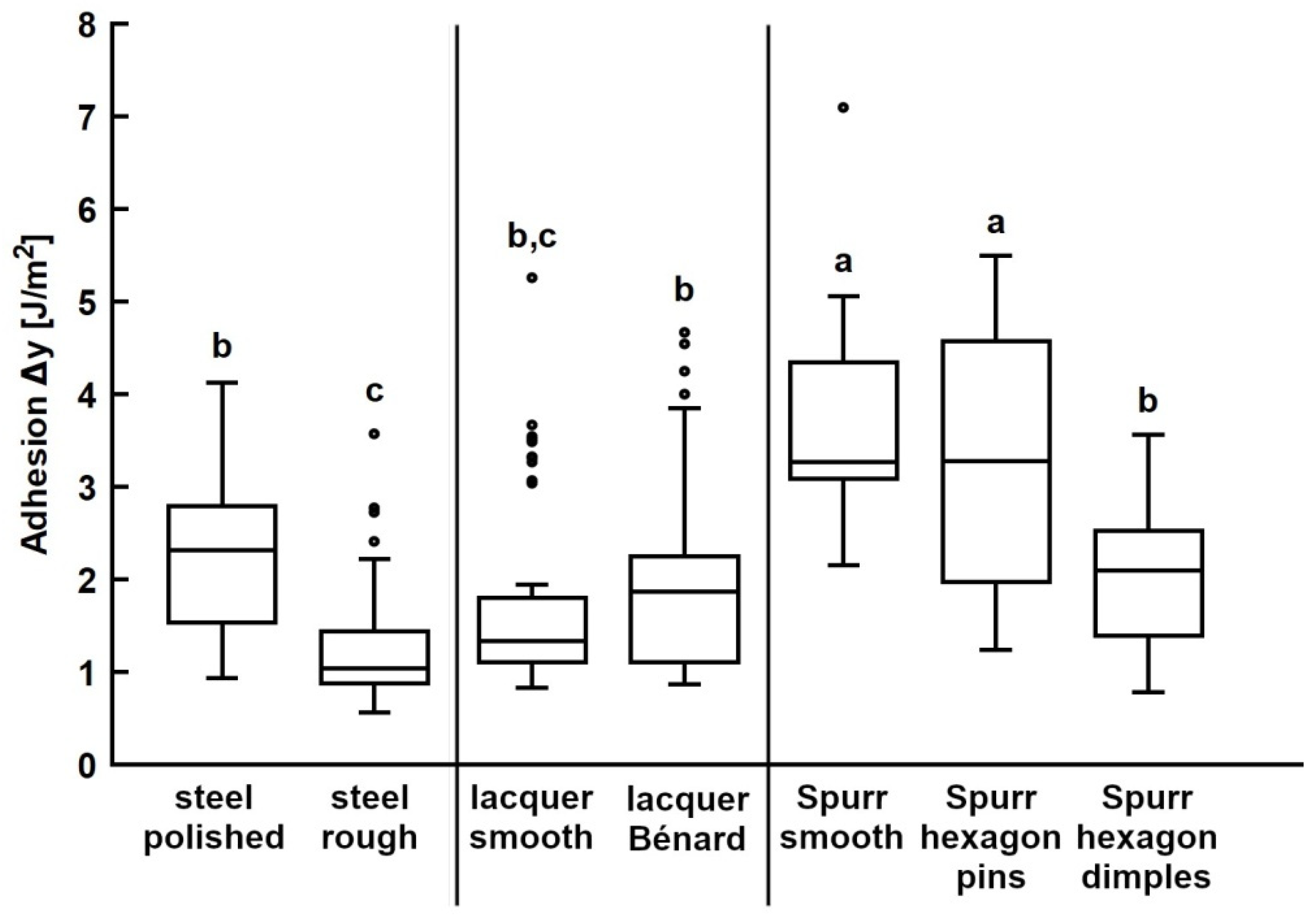
| Substrate | N×n | Work of Adhesion [J/m2] | Pull-off Force [mN] | ||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| Honeybee mandible, unwashed | 150 | 1.01 | 0.21 | 0.71 | 0.33 |
| Steel, polished | 50 | 2.29 | 0.82 | 1.98 | 0.51 |
| Steel, rough | 50 | 1.26 | 0.61 | 1.09 | 0.43 |
| Lacquer, smooth (UV lacquer) | 50 | 1.74 | 0.98 | 4.20 | 0.70 |
| Lacquer, Bénard cells (UV lacquer) | 50 | 1.99 | 1.07 | 3.76 | 0.60 |
| Spurr, smooth | 50 | 3.61 | 0.95 | 3.35 | 0.87 |
| Hexagon dimples (Spurr) | 50 | 1.99 | 0.64 | 1.99 | 0.55 |
| Hexagon pins (Spurr) | 50 | 3.28 | 1.38 | 2.31 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccardi, L.; Schiebl, J.; Balluff, F.; Christ, U.; Gorb, S.N.; Kovalev, A.; Schwarz, O. Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces. Biomimetics 2023, 8, 579. https://doi.org/10.3390/biomimetics8080579
Saccardi L, Schiebl J, Balluff F, Christ U, Gorb SN, Kovalev A, Schwarz O. Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces. Biomimetics. 2023; 8(8):579. https://doi.org/10.3390/biomimetics8080579
Chicago/Turabian StyleSaccardi, Leonie, Jonas Schiebl, Franz Balluff, Ulrich Christ, Stanislav N. Gorb, Alexander Kovalev, and Oliver Schwarz. 2023. "Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces" Biomimetics 8, no. 8: 579. https://doi.org/10.3390/biomimetics8080579
APA StyleSaccardi, L., Schiebl, J., Balluff, F., Christ, U., Gorb, S. N., Kovalev, A., & Schwarz, O. (2023). Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces. Biomimetics, 8(8), 579. https://doi.org/10.3390/biomimetics8080579






