Gallium-Containing Materials and Their Potential within New-Generation Titanium Alloys for Biomedical Applications
Abstract
:1. Introduction
2. β-Type Titanium Alloys with Biocompatible Elements
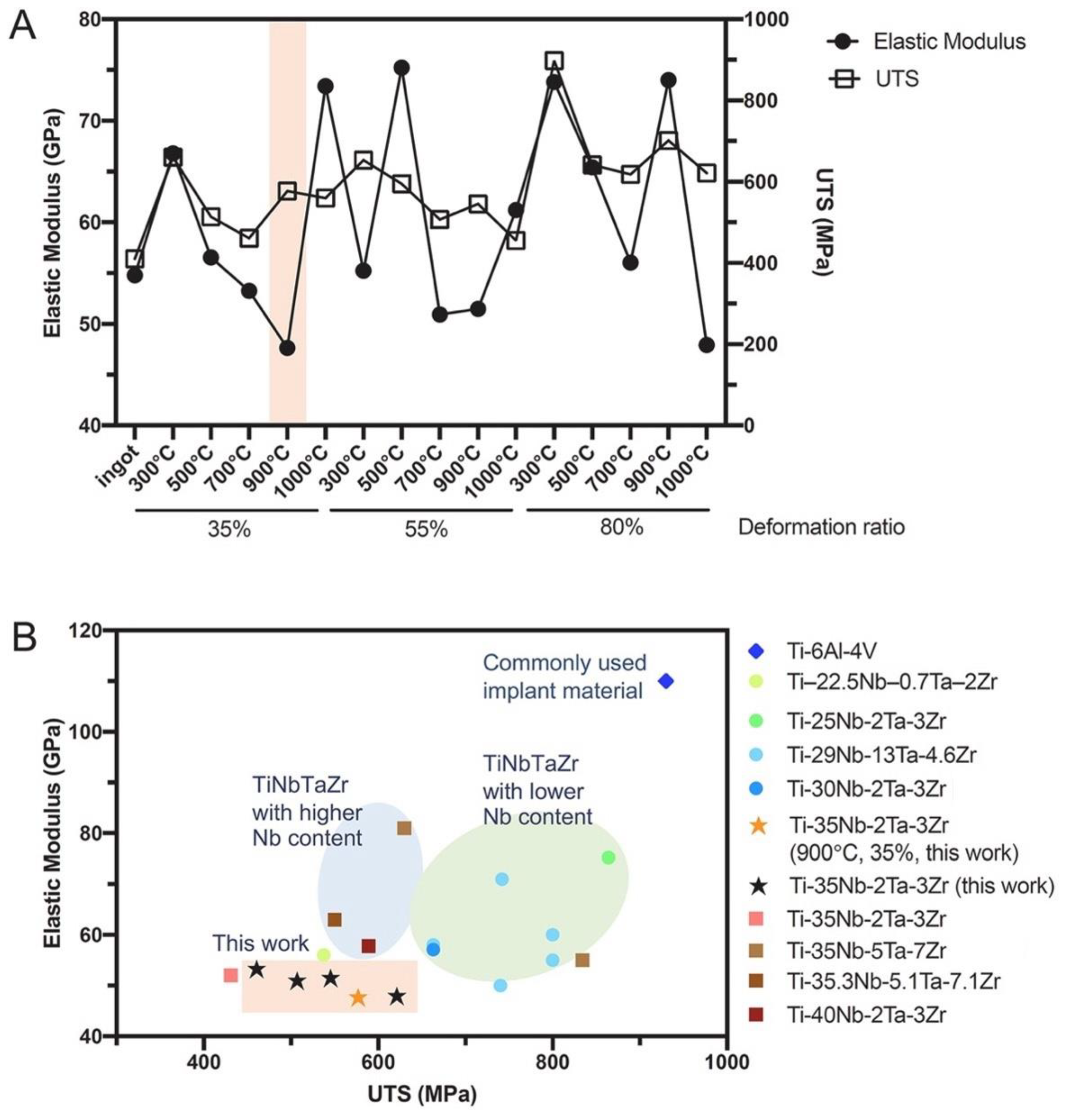
2.1. Influence of Alloying Elements on Microstructure and Mechanical Properties
2.2. Influence of Processing Parameters on Mechanical Properties
2.3. Influence of Alloying Elements on Biocompatibility
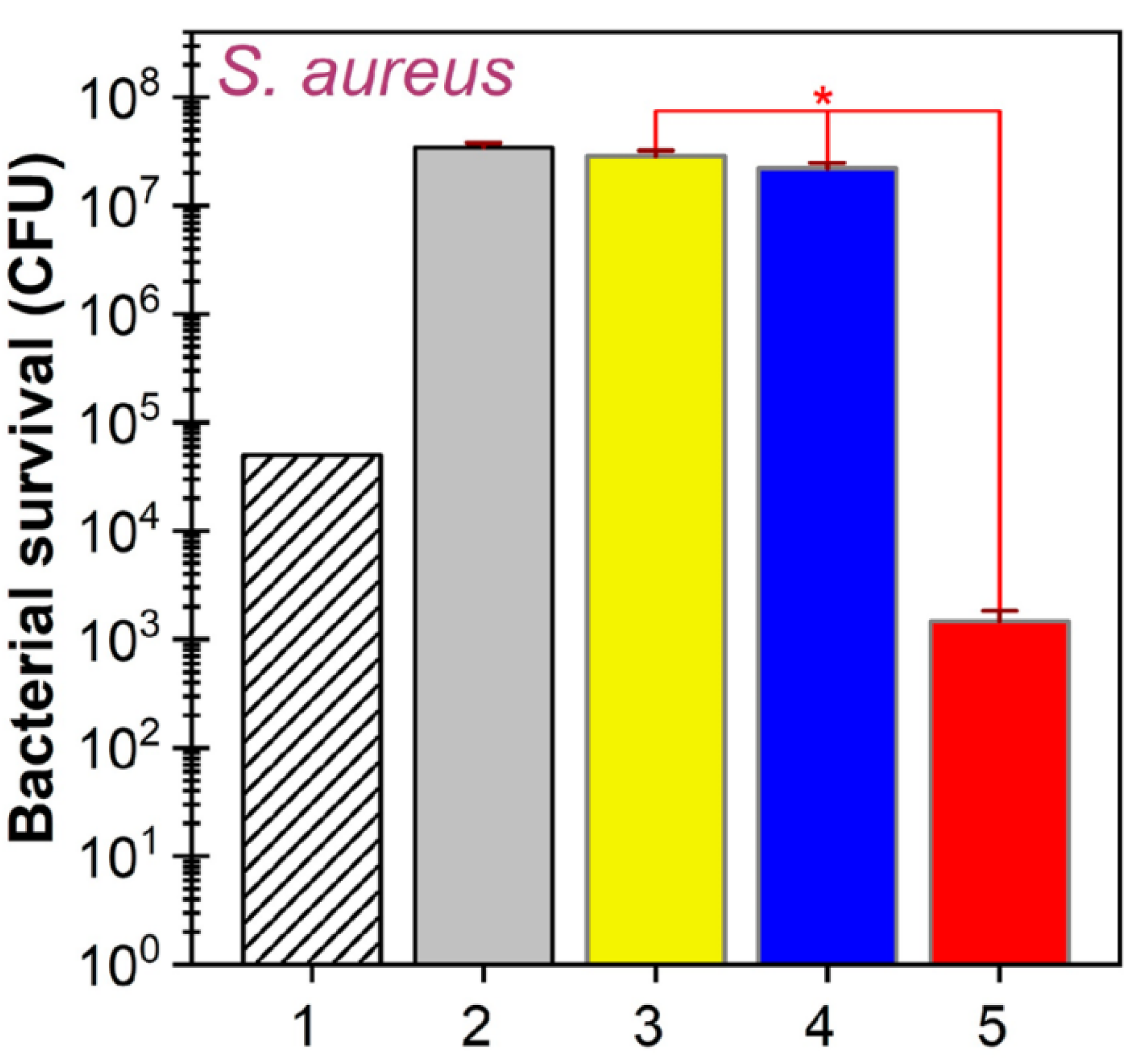
3. Antibacterial Gallium-Based Materials
3.1. Antimicrobial Mechanism of Gallium
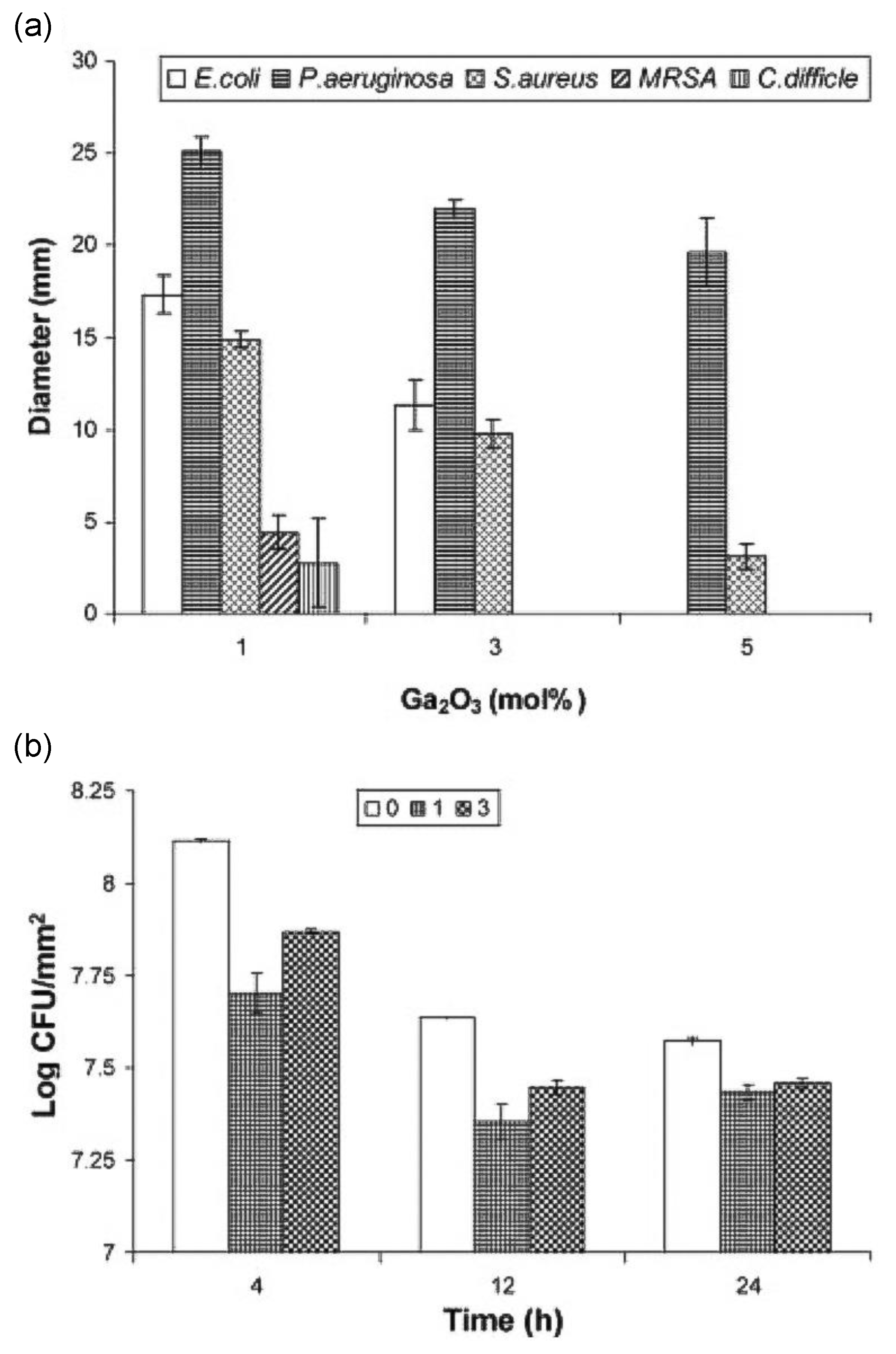
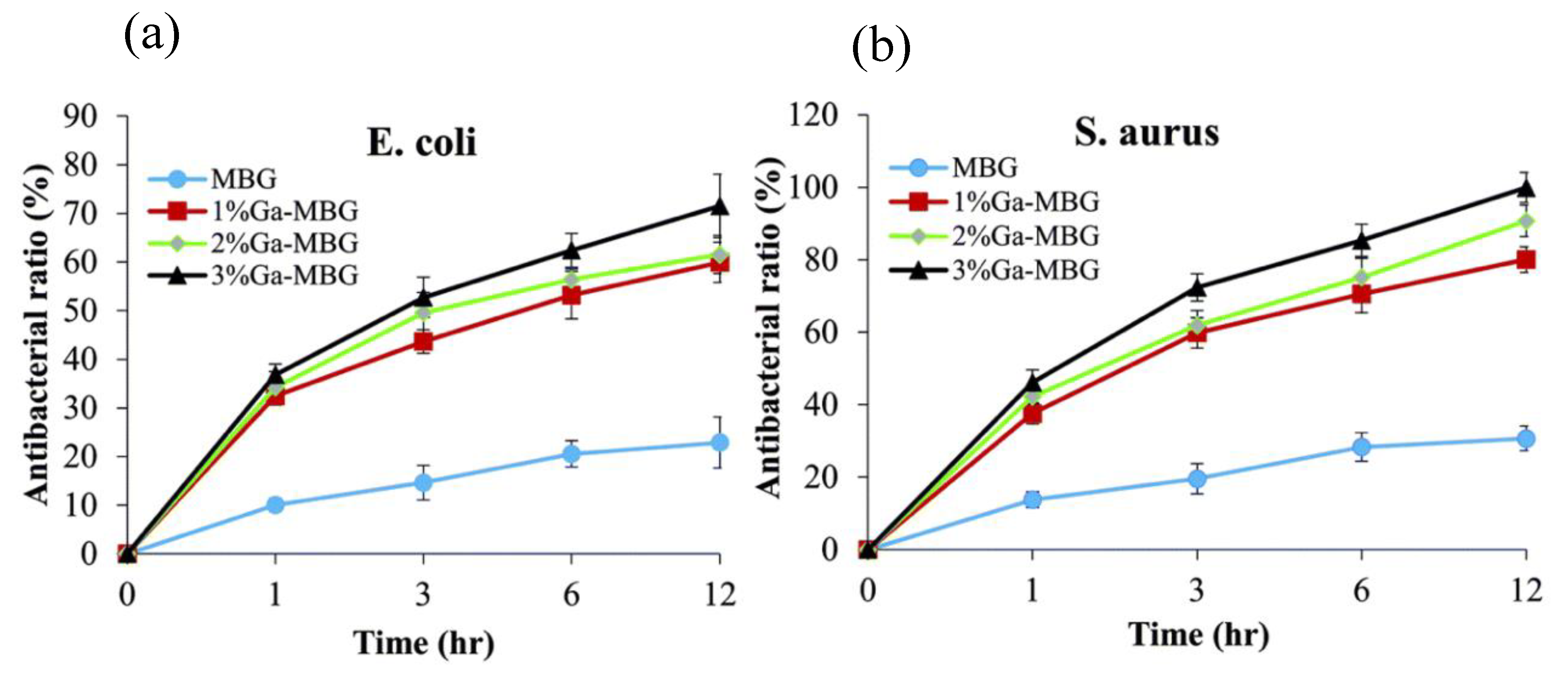
3.2. Gallium in Bioglasses
3.3. Gallium in Liquid Metals
3.4. Gallium in Bioceramic Systems
4. Titanium–Gallium-Based Systems
4.1. Titanium–Gallium Coatings
4.2. Titanium–Gallium-Based Alloys
5. Suggestions for Future Research
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scialla, S.; Martuscelli, G.; Nappi, F.; Singh, S.S.A.; Iervolino, A.; Larobina, D.; Ambrosio, L.; Raucci, M.G. Trends in Managing Cardiac and Orthopaedic Device-Associated Infections by Using Therapeutic Biomaterials. Polymers 2021, 13, 1556. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Yang, R. Effect of Zr and Sn on Young’s modulus and superelasticity of Ti-Nb-based alloys. Mater. Sci. Eng. A—Struct. Mater. Prop. Microstruct. Process. 2006, 441, 112–118. [Google Scholar] [CrossRef]
- Zhang, E.L.; Li, F.B.; Wang, H.Y.; Liu, J.; Wang, C.M.; Li, M.Q.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C—Mater. Biol. Appl. 2013, 33, 4280–4287. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.; Helth, A.; Moreno, J.J.G.; Bonisch, M.; Brackmann, V.; Giebeler, L.; Gemming, T.; Lekka, C.E.; Gebert, A.; Schnettler, R.; et al. Elastic softening of beta-type Ti-Nb alloys by indium (In) additions. J. Mech. Behav. Biomed. Mater. 2014, 39, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the Corrosion Behaviour of Low Modulus Titanium Alloys for Medical Implant Applications: A Review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
- Fallahnezhad, K.; Oskouei, R.H.; Badnava, H.; Taylor, M. The Influence of Assembly Force on the Material Loss at the Metallic Head-Neck Junction of Hip Implants Subjected to Cyclic Fretting Wear. Metals 2019, 9, 422. [Google Scholar] [CrossRef]
- Feyzi, M.; Fallahnezhad, K.; Taylor, M.; Hashemi, R. The mechanics of head-neck taper junctions: What do we know from finite element analysis? J. Mech. Behav. Biomed. Mater. 2021, 116, 104338. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, H.; Fallahnezhad, K.; Oskouei, R.H.; Taylor, M. A finite element study on the mechanical response of the head-neck interface of hip implants under realistic forces and moments of daily activities: Part 1, level walking. J. Mech. Behav. Biomed. Mater. 2017, 75, 470–476. [Google Scholar] [CrossRef]
- Fallahnezhad, K.; Feyzi, M.; Taylor, M.; Hashemi, R. What is the relationship between metal-on-metal and ceramic-on-metal tribocorrosive behaviours? An experimental study on Ti-6Al-4 V/CoCrMo interface. Tribol. Int. 2022, 174, 107720. [Google Scholar] [CrossRef]
- Feyzi, M.; Fallahnezhad, K.; Taylor, M.; Hashemi, R. The Tribocorrosion Behaviour of Ti-6Al-4 V Alloy: The Role of Both Normal Force and Electrochemical Potential. Tribol. Lett. 2022, 70, 83. [Google Scholar] [CrossRef]
- Feyzi, M.; Fallahnezhad, K.; Taylor, M.; Hashemi, R. What role do normal force and frequency play in the tribocorrosion behaviour of Ti-6Al-4 V alloy? Tribol. Int. 2022, 172, 107634. [Google Scholar] [CrossRef]
- Alberta, L.A.; Fortouna, Y.; Vishnu, J.; Pilz, S.; Gebert, A.; Lekka, C.; Nielsch, K.; Calin, M. Effects of Ga on the structural, mechanical and electronic properties of β-Ti-45Nb alloy by experiments and ab initio calculations. J. Mech. Behav. Biomed. 2023, 140, 105728. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Xiao, W.L.; Ren, L.; Fu, Y.; Ma, C.L. The roles of oxygen content on microstructural transformation, mechanical properties and corrosion resistance of Ti-Nb-based biomedical alloys with different beta stabilities. Mater. Charact. 2021, 176, 111122. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A. Is aluminum exposure a risk factor for neurological disorders? J. Res. Med. Sci. 2018, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Matsumoto, H.; Watanabe, S. Mechanical compatibility of titanium implants in hard tissues. Int. Congr. Ser. 2005, 1284, 239–247. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Chiesa, R.; Rimondini, L.; Gasik, M. Metallurgical Gallium Additions to Titanium Alloys Demonstrate a Strong Time-Increasing Antibacterial Activity without any Cellular Toxicity. ACS Biomater. Sci. Eng. 2019, 5, 2815–2820. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.X.; Li, Y.C.; Ipek, R.; Wen, C. Development of Ti-Nb-Zr alloys with high elastic admissible strain for temporary orthopedic devices. Acta Biomater. 2015, 20, 176–187. [Google Scholar] [CrossRef]
- Ning, C.Q.; Ding, D.Y.; Dai, K.R.; Zhai, W.Y.; Chen, L. The effect of Zr content on the microstructure, mechanical properties and cell attachment of Ti-35Nb-xZr alloys. Biomed. Mater. 2010, 5, 45006. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Yang, R. Elastic deformation behaviour of Ti-24Nb-4Zr-7.9Sn for biomedical applications. Acta Biomater. 2007, 3, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Chen, D.S.; Lu, W.J.; Jia, Y.H.; Wang, L.Q.; Zhang, X.L. Corrosion resistance and in vitro response of a novel Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Biomed. Mater. 2013, 8, 55004. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Das, S.; Suwas, S.; Chatterjee, K. Engineering the next-generation tin containing beta titanium alloys with high strength and low modulus for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2018, 78, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Nag, S.; Samuel, S.; Fraser, H.L. Laser-deposited Ti-Nb-Zr-Ta orthopedic alloys. J. Biomed. Mater. Res. A 2006, 78, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Gloriant, T.; Gordin, D.M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I. Synthesis and characterisation of a new superelastic Ti-25Ta-25Nb biomedical alloy. J. Mech. Behav. Biomed. Mater. 2010, 3, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, J.; Xiao, W.; Zhao, X.; Ma, C. Microstructure evolution and mechanical properties of Ti–8Nb–2Fe-0.2O alloy with high elastic admissible strain for orthopedic implant applications. Prog. Nat. Sci. Mater. Int. 2020, 30, 100–105. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, W.; Wang, J.; Ren, L.; Zhao, X.; Ma, C. A novel strategy for developing α + β dual-phase titanium alloys with low Young’s modulus and high yield strength. J. Mater. Sci. Technol. 2021, 76, 122–128. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, W.; Fu, Y.; Ren, L.; Song, B.; Liu, C.; Ma, C. Effects of initial microstructure on the aging behavior and subsequent mechanical properties of Ti–Nb–O titanium alloy. J. Mater. Res. 2022, 37, 2304–2313. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Tan, M.H.C.; Baghi, A.D.; Ghomashchi, R.; Xiao, W.; Oskouei, R.H. Effect of niobium content on the microstructure and Young’s modulus of Ti-xNb-7Zr alloys for medical implants. J. Mech. Behav. Biomed. Mater. 2019, 99, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.Y.; Yu, W.J.; Jin, M.; Wang, Y.C.; Shang, X.Q.; Lin, L.; Zeng, X.Q.; Wang, L.Q.; Lu, E.Y. Mechanobiologically optimized Ti-35Nb-2Ta-3Zr improves load transduction and enhances bone remodeling in tilted dental implant therapy. Bioact. Mater. 2022, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, W.; Ren, L.; Fu, Y.; Ma, C. Effect of oxygen addition and annealing time on microstructure and mechanical properties of Ti–34Nb alloy. J. Iron Steel Res. Int. 2022, 30, 158–164. [Google Scholar] [CrossRef]
- Ramarolahy, A.; Castany, P.; Prima, F.; Laheurte, P.; Peron, I.; Gloriant, T. Microstructure and mechanical behavior of superelastic Ti-24Nb-0.50 and Ti-24Nb-0.5N biomedical alloys. J. Mech. Behav. Biomed. Mater. 2012, 9, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Akahori, T.; Niinomi, M.; Fukui, H.; Ogawa, M.; Toda, H. Improvement in fatigue characteristics of newly developed beta type titanium alloy for biomedical applications by thermo-mechanical treatments. Mater. Sci. Eng. C—Biomim. Supramol. Syst. 2005, 25, 248–254. [Google Scholar] [CrossRef]
- Reck, A.; Pilz, S.; Thormann, U.; Alt, V.; Gebert, A.; Calin, M.; Heiss, C.; Zimmermann, M. Effects of thermomechanical history and environment on the fatigue behavior of (beta)-Ti-Nb implant alloys. In Proceedings of the 12th International Fatigue Congress (Fatigue 2018), Poitiers, France, 27 May–1 June 2018; Volume 165. [Google Scholar] [CrossRef]
- Hao, Y.L.; Niinomi, M.; Kuroda, D.; Fukunaga, K.; Zhou, Y.L.; Yang, R.; Suzuki, A. Young’s modulus and mechanical properties of Ti-29Nb-13Ta-4.6Zr in relation to α′′ martensite. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2002, 33, 3137–3144. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Oneda, T. Improvement in Fatigue Strength of Biomedical beta-type Ti-Nb-Ta-Zr Alloy While Maintaining Low Young’s Modulus Through Optimizing omega-Phase Precipitation. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2012, 43, 294–302. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, W.; Wang, J.; Zhao, X.; Ma, C. Mechanical properties and deformation mechanisms of Ti-15 Nb-5Zr-4Sn-1Fe alloy with varying α phase fraction. J. Alloys Compd. 2022, 898, 162816. [Google Scholar] [CrossRef]
- Xiao, W. Metastability Engineering in Titanium Alloys Enables Advanced Structural/Functional Properties. Mater. Lab 2022, 1, 220024. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, W.; Fu, Y.; Ren, L.; Ma, C. Dependence of mechanical behavior on grain size of metastable Ti–Nb–O titanium alloy. Prog. Nat. Sci. Mater. Int. 2022, 32, 63–71. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Volker, B.; Maier-Kiener, V.; Werbach, K.; Muller, T.; Pilz, S.; Calin, M.; Eckert, J.; Hohenwarter, A. Influence of annealing on microstructure and mechanical properties of ultrafine-grained Ti45Nb. Mater. Des. 2019, 179, 107864. [Google Scholar] [CrossRef]
- Matsumoto, H.; Watanabe, S.; Hanada, S. Microstructures and mechanical properties of metastable beta TiNbSn alloys cold rolled and heat treated. J. Alloys Compd. 2007, 439, 146–155. [Google Scholar] [CrossRef]
- Helth, A.; Pilz, S.; Kirsten, T.; Giebeler, L.; Freudenberger, J.; Calin, M.; Eckert, J.; Gebert, A. Effect of thermomechanical processing on the mechanical biofunctionality of a low modulus Ti-40Nb alloy. J. Mech. Behav. Biomed. Mater. 2017, 65, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Reck, A.; Pilz, S.; Kuczyk, M.; Gebert, A.; Zimmermann, M. Cyclic deformation characteristics of the metastable beta-type Ti-40Nb alloy. Mater. Sci. Eng. A—Struct. Mater. Prop. Microstruct. Process. 2019, 761, 137966. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.S.; Kim, Y.J.; Jang, J.H.; Suh, J.Y.; Park, J.W. Improved pre-osteoblast response and mechanical compatibility of ultrafine-grained Ti-13Nb-13Zr alloy. Clin. Oral Implant. Res. 2011, 22, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Zhang, E.L.; Dong, H.; Liu, J. Biocompatibility of antibacterial Ti-Cu sintered alloy: In Vivo bone response. J. Mater. Sci.-Mater. Med. 2015, 26, 265. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yamamoto, A.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Novel Ti-base superelastic alloys with large recovery strain and excellent biocompatibility. Acta Biomater. 2015, 17, 56–67. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef]
- Muller, A.; Fessele, C.; Zuber, F.; Rottmar, M.; Maniura-Weber, K.; Ren, Q.; Guex, A.G. Gallium Complex-Functionalized P4HB Fibers: A Trojan Horse to Fight Bacterial Infection. ACS Appl. Bio Mater. 2021, 4, 682–691. [Google Scholar] [CrossRef]
- Kurtuldu, F.; Mutlu, N.; Boccaccini, A.R.; Galusek, D. Gallium containing bioactive materials: A review of anticancer, antibacterial, and osteogenic properties. Bioact. Mater. 2022, 17, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Gardner, R.; Kaur, N.; Phanstiel, O. Utilization of Fe3+-acinetoferrin analogs as an iron source by Mycobacterium tuberculosis. Biometals 2008, 21, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kelson, A.B.; Carnevali, M.; Truong-Le, V. Gallium-based anti-infectives: Targeting microbial iron-uptake mechanisms. Curr. Opin. Pharmacol. 2013, 13, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Li, J.F.; Benin, B.M.; Yu, B.; Bunge, S.D.; Abeydeera, N.; Huang, S.D.; Kim, M.H. Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in both Pseudomonas aeruginosa and Staphylococcus aureus. J. Med. Chem. 2021, 64, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Garcia, A.; Angarita-Zapata, V.; Cazares, A.; Jasso-Chavez, R.; Belmont-Diaz, J.; Sanchez-Torres, V.; Lopez-Jacome, L.E.; Coria-Jimenez, R.; Maeda, T.; Garcia-Contreras, R. Characterization of gallium resistance induced in a Pseudomonas aeruginosa cystic fibrosis isolate. Arch. Microbiol. 2020, 202, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.P.; Rahmanto, Y.S.; Chitambar, C.R.; Richardson, D.R. Resistance to the antineoplastic agent gallium nitrate results in marked alterations in intracellular iron and gallium trafficking: Identification of novel intermediates. J. Pharmacol. Exp. Ther. 2006, 317, 153–162. [Google Scholar] [CrossRef]
- Li, F.P.; Liu, F.X.; Huang, K.; Yang, S.B. Advancement of Gallium and Gallium-Based Compounds as Antimicrobial Agents. Front. Bioeng. Biotechnol. 2022, 10, 827960. [Google Scholar] [CrossRef]
- Bento, R.; Gaddam, A.; Ferreira, J.M.F. Sol-Gel Synthesis and Characterization of a Quaternary Bioglass for Bone Regeneration and Tissue Engineering. Materials 2021, 14, 4515. [Google Scholar] [CrossRef]
- Keenan, T.J.; Placek, L.M.; Hall, M.M.; Wren, A.W. Antibacterial and antifungal potential of Ga-bioactive glass and Ga-bioactive glass/polymeric hydrogel composites. J. Biomed. Mater. Res. Part B—Appl. Biomater. 2017, 105, 1102–1113. [Google Scholar] [CrossRef]
- Stan, G.E.; Tite, T.; Popa, A.C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D.; et al. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]
- Yazdi, A.R.; Torkan, L.; Stone, W.; Towler, M.R. The impact of gallium content on degradation, bioactivity, and antibacterial potency of zinc borate bioactive glass. J. Biomed. Mater. Res. Part B—Appl. Biomater. 2018, 106, 367–376. [Google Scholar] [CrossRef]
- Yazdi, A.R.; Torkan, L.; Waldman, S.D.; Towler, M.R. Development of a novel bioactive glass suitable for osteosarcoma-related bone grafts. J. Biomed. Mater. Res. Part B—Appl. Biomater. 2018, 106, 1186–1193. [Google Scholar] [CrossRef]
- Mutlu, N.; Kurtuldu, F.; Unalan, I.; Neščáková, Z.; Kaňková, H.; Galusková, D.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Effect of Zn and Ga doping on bioactivity, degradation, and antibacterial properties of borate 1393-B3 bioactive glass. Ceram. Int. 2022, 48, 16404–16417. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; Chrzanowski, W.; O’Dell, L.A.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Antimicrobial gallium-doped phosphate-based glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef]
- Sahdev, R.; Ansari, T.I.; Higham, S.M.; Valappil, S.P. Potential use of gallium-doped phosphate-based glass material for periodontitis treatment. J. Biomater. Appl. 2015, 30, 85–92. [Google Scholar] [CrossRef]
- Valappil, S.P.; Coombes, M.; Wright, L.; Owens, G.J.; Lynch, R.J.M.; Hope, C.K.; Higham, S.M. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of Porphyromonas gingivalis and Streptococcus gordonii. Acta Biomater. 2012, 8, 1957–1965. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Naveen, S.V.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71–86. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regi, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Solids 2016, 432, 9–14. [Google Scholar] [CrossRef]
- Pham, D.Q.; Gangadoo, S.; Berndt, C.C.; Chapman, J.; Zhai, J.; Vasilev, K.; Truong, V.K.; Ang, A.S.M. Antibacterial Longevity of a Novel Gallium Liquid Metal/Hydroxyapatite Composite Coating Fabricated by Plasma Spray. ACS Appl. Mater. Interfaces 2022, 14, 18974–18988. [Google Scholar] [CrossRef]
- Li, L.; Chang, H.; Yong, N.; Li, M.X.; Hou, Y.; Rao, W. Superior antibacterial activity of gallium based liquid metals due to Ga3+ induced intracellular ROS generation. J. Mater. Chem. B 2021, 9, 85–93. [Google Scholar] [CrossRef]
- Cheeseman, S.; Elbourne, A.; Gangadoo, S.; Shaw, Z.L.; Bryant, S.J.; Syed, N.; Dickey, M.D.; Higgins, M.J.; Vasilev, K.; McConville, C.F.; et al. Interactions between Liquid Metal Droplets and Bacterial, Fungal, and Mammalian Cells. Adv. Mater. Interfaces 2022, 9, 2102113. [Google Scholar] [CrossRef]
- Yang, J.; Nithyanandam, P.; Kanetkar, S.; Kwon, K.Y.; Ma, J.; Im, S.; Oh, J.-H.; Shamsi, M.; Wilkins, M.; Daniele, M.; et al. Liquid Metal Coated Textiles with Autonomous Electrical Healing and Antibacterial Properties. Adv. Mater. Technol. 2023, 8, 2202183. [Google Scholar] [CrossRef]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabeti, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.F.; et al. Antibacterial Liquid Metals: Biofilm Treatment via Magnetic Activation. ACS Nano 2020, 14, 802–817. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, 2104298. [Google Scholar] [CrossRef]
- He, B.L.; Du, Y.X.; Wang, B.W.; Zhao, X.Y.; Liu, S.J.; Ye, Q.; Zhou, F. Self-healing polydimethylsiloxane antifouling coatings based on zwitterionic polyethylenimine-functionalized gallium nanodroplets. Chem. Eng. J. 2022, 427, 131019. [Google Scholar] [CrossRef]
- Mosina, M.; Kovrlija, I.; Stipniece, L.; Locs, J. Gallium containing calcium phosphates: Potential antibacterial agents or fictitious truth. Acta Biomater. 2022, 150, 48–57. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanovic, M.; Kramer, L.; Suvorov, D. Biocompatible nano-gallium/hydroxyapatite nanocomposite with antimicrobial activity. J. Mater. Sci. Mater. Med. 2016, 27, 170. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanovic, M.; Krajnc, A.; Kramer, L.; Turk, B.; Suvorov, D. Designing Ga(III)-containing hydroxyapatite with antibacterial activity. RSC Adv. 2016, 6, 112839–112852. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanovic, M.; Suvorov, D. Antibacterial nanocomposite of functionalized nanogold and gallium-doped hydroxyapatite. Mater. Lett. 2017, 193, 126–129. [Google Scholar] [CrossRef]
- Stuart, B.W.; Grant, C.A.; Stan, G.E.; Popa, A.C.; Titman, J.J.; Grant, D.M. Gallium incorporation into phosphate based glasses: Bulk and thin film properties. J. Mech. Behav. Biomed. 2018, 82, 371–382. [Google Scholar] [CrossRef]
- Stuart, B.W.; Stan, G.E.; Popa, A.C.; Carrington, M.J.; Zgura, I.; Necsulescu, M.; Grant, D.M. New solutions for combatting implant bacterial infection based on silver nano-dispersed and gallium incorporated phosphate bioactive glass sputtered films: A preliminary study. Bioact. Mater. 2022, 8, 325–340. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-One Biointerfaces-Antimicrobial and Bioactive Nanoporous Gallium Titanate Layers for Titanium Implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Torres, D.; Guillem-Marti, J.; Sereno, P.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Ruperez, E. Development of novel dual-action coatings with osteoinductive and antibacterial properties for 3D-printed titanium implants. Surf. Coat. Technol. 2020, 403, 126381. [Google Scholar] [CrossRef]
- Shruti, S.; Andreatta, F.; Furlani, E.; Marin, E.; Maschio, S.; Fedrizzi, L. Cerium, gallium and zinc containing mesoporous bioactive glass coating deposited on titanium alloy. Appl. Surf. Sci. 2016, 378, 216–223. [Google Scholar] [CrossRef]
- Dong, J.J.; Fang, D.; Zhang, L.; Shan, Q.; Huang, Y.C. Gallium-doped titania nanotubes elicit anti-bacterial efficacy in vivo against Escherichia coli and Staphylococcus aureus biofilm. Materialia 2019, 5, 100209. [Google Scholar] [CrossRef]
- Li, K.; Tian, H.C.A.; Guo, A.; Jin, L.G.; Chen, W.Z.; Tao, B.L. Gallium (Ga)-strontium (Sr) layered double hydroxide composite coating on titanium substrates for enhanced osteogenic and antibacterial abilities. J. Biomed. Mater. Res. A 2021, 110, 273–286. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, C.; Dang, X.Y.; Yang, H.; Wang, Y.R.; Chen, Y.L.; Ma, L.; Han, S.G.; Lin, H.; Zhang, X.J.; et al. Gallium loading into a polydopamine-functionalised SrTiO3 nanotube with Combined osteoinductive and antimicrobial activities. Ceram. Int. 2019, 45, 22183–22195. [Google Scholar] [CrossRef]
- Chen, M.W.; Hu, Y.; Hou, Y.H.; Li, M.H.; Tan, L.; Chen, M.H.; Geng, W.B.; Tao, B.L.; Jiang, H.; Luo, Z.; et al. Magnesium/gallium-layered nanosheets on titanium implants mediate osteogenic differentiation of MSCs and osseointegration under osteoporotic condition. Chem. Eng. J. 2022, 427, 130982. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. A 2015, 103, 1176–1187. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Hariharan, A.; Pilz, S.; Gebert, A.; Calin, M. Novel low modulus beta-type Ti-Nb alloys by gallium and copper minor additions for antibacterial implant applications. J. Mater. Res. Technol. 2022, 20, 3306–3322. [Google Scholar] [CrossRef]
- Chen, X.G.; Zhou, J.H.; Qian, Y.; Zhao, L.Z. Antibacterial coatings on orthopedic implants. Mater. Today Bio 2023, 19, 100586. [Google Scholar] [CrossRef]
- Alberta, L.A.; Vishnu, J.; Douest, Y.; Perrin, K.; Trunfio-Sfarghiu, A.M.; Courtois, N.; Gebert, A.; Ter-Ovanessian, B.; Calin, M. Tribocorrosion behavior of β-type Ti-Nb-Ga alloys in a physiological solution. Tribol. Int. 2023, 181, 108325. [Google Scholar] [CrossRef]


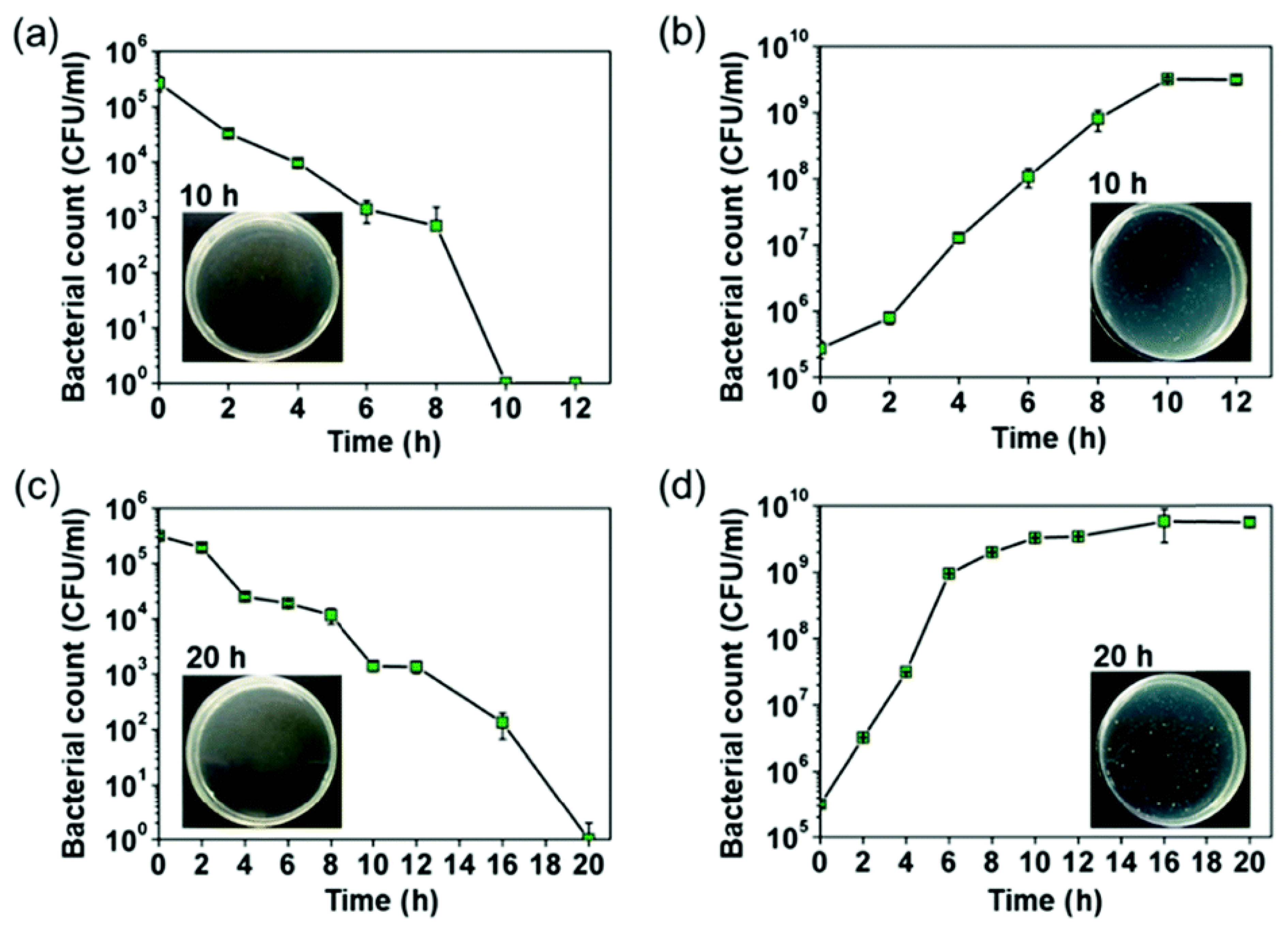
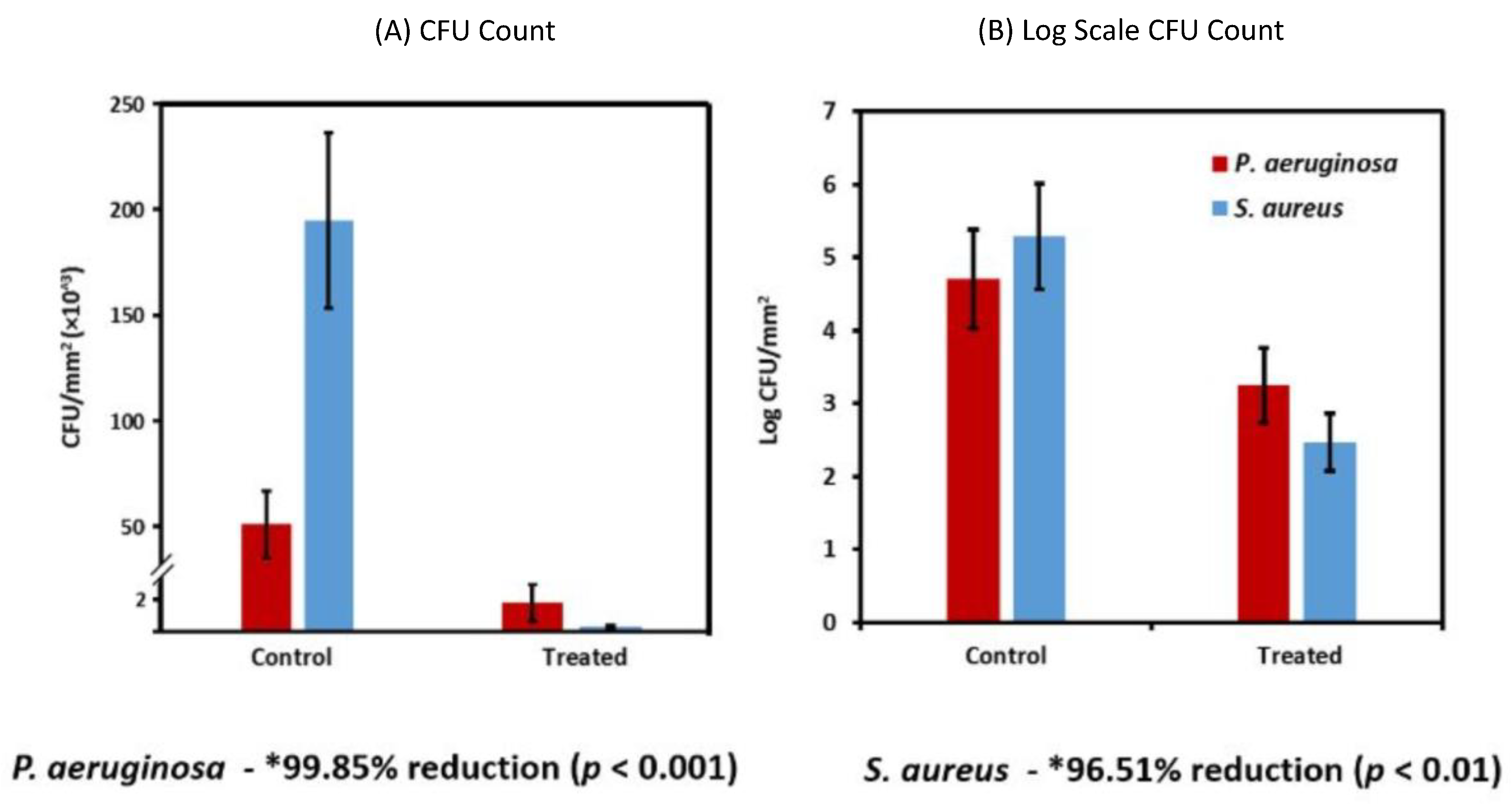
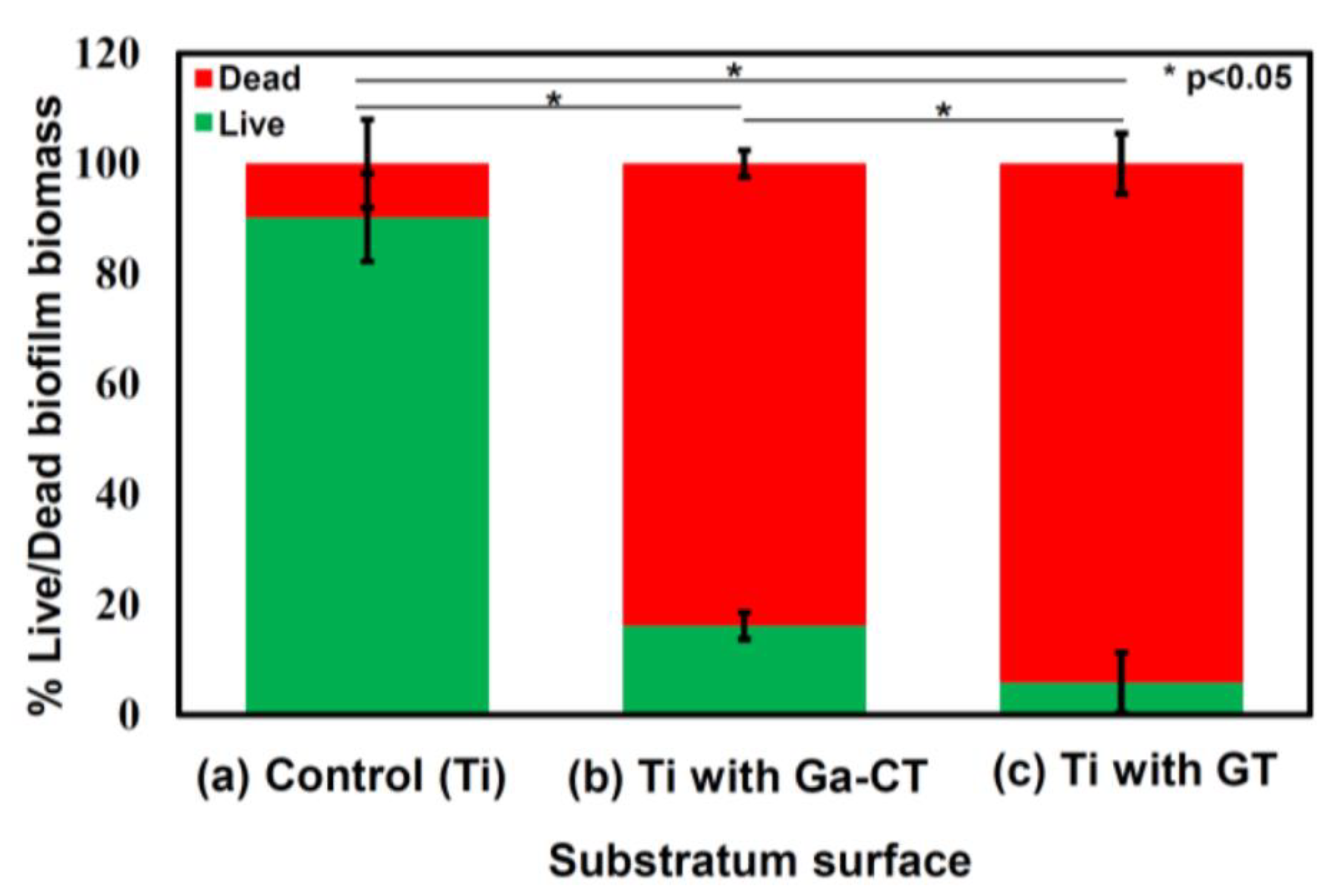
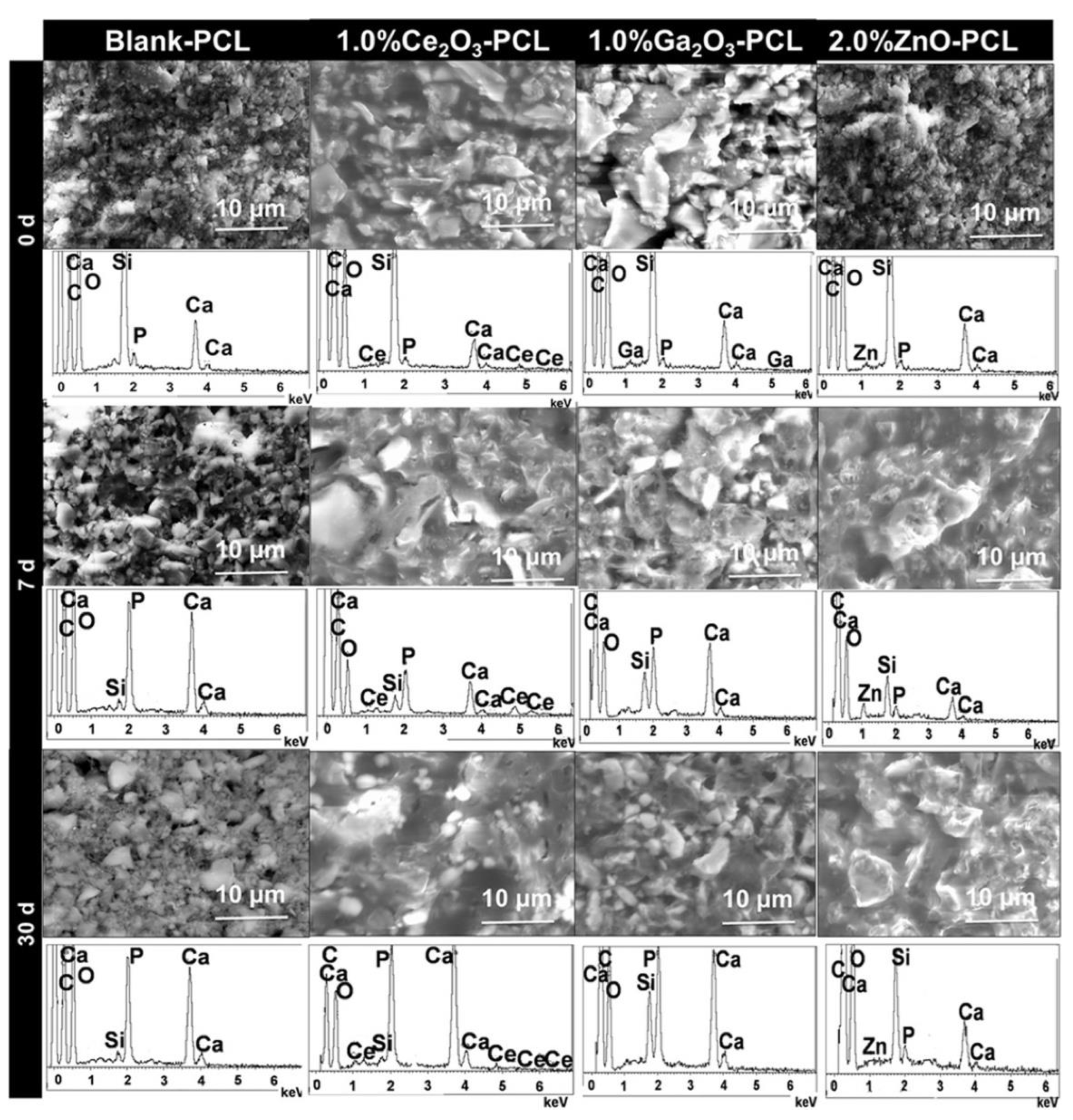

| Periodic Position | Element | Biocompatible | Carcinogenic | Genotoxic | Mutagenic | Cytotoxic | Allyergenic | Prone to Corrosion | Other* |
|---|---|---|---|---|---|---|---|---|---|
| 3d | Ti | Yes | No | No | No | Med | No | No | No |
| V | No | Yes | Yes | Yes | High | Disputed | No | No | |
| Cr | No | Disputed | Yes | Yes | High | Yes | No | No | |
| Mn | No | No | Yes | No | High | No | Yes | No | |
| Fe | No | No | Yes | Disputed | Med | No | Yes | No | |
| Co | No | Yes | Yes | Yes | High | Yes | Yes | Yes | |
| Ni | No | Yes | Yes | Yes | High | Yes | Yes | Yes | |
| Cu | No | No | Yes | Yes | High | Yes | Yes | Yes | |
| 4d | Zr | Yes | No | No | No | Low | No | No | No |
| Nb | Yes | No | No | No | Low | No | No | No | |
| Mo | No | Disputed | Yes | Yes | Low | Yes | Yes | Yes | |
| Tc | No | -Radioactive- | |||||||
| Ru | Yes | No | No | No | Med | No | No | Yes | |
| Rh | No | Yes | Yes | Yes | High | Unknown | No | No | |
| Pd | No | Yes | No | Disputed | Med | Yes | No | No | |
| Ag | No | No | No | No | High | Yes | No | Yes | |
| 5d | Hf | Unknown | Unknown | Unknown | Unknown | Med | No | No | Unknown |
| Ta | Yes | No | No | No | Low | No | No | No | |
| W | No | Yes | Yes | No | Med | No | Yes | No | |
| Re | Unknown | Unknown | Unknown | Unknown | Unknown | No | No | Unknown | |
| Os | No | Unknown | Yes | Yes | High | No | Yes | No | |
| Ir | No | No | No | Yes | High | No | No | Yes | |
| Pt | No | Yes | Yes | Yes | High | Yes | No | No | |
| Au | Yes | No | No | No | High | No | No | No | |
| Other | Al | No | No | Yes | No | Low | No | No | Yes |
| Zn | No | No | No | No | High | No | No | Yes | |
| Sn | Yes | No | No | No | Low | No | No | Yes | |
| Substrate | Coating(s) | Properties Investigated/Methods | Notable Results | Ref. |
|---|---|---|---|---|
| Commercially pure Ti | P2O5-CaO-MgO-Na2O-XGa2O3 (X = 6, 8.6 mol%). | Elemental mapping and microstructural analysis. Surface roughness and surface features. Mechanical properties (reduced moduli, elastic modulus, hardness). Cytocompatibility tests. Antibacterial assays. | All samples cytocompatible. Antibacterial activity effective at 24 h for Gram-positive and Gram-negative bacteria. | [83,84] |
| Ti | GaCl3 | Surface morphology. Ion release. Antibacterial activity. | Notable antibacterial activity against A. baumannii. Up to 94.2% biofilm removal. | [85] |
| Porous Ti | Ga(NO3)3 | Surface characterisation. Ion release. Antibacterial assay. Cytotoxicity and biocompatibility assays. | Effectively inhibited P. aeruginosa. Effective osteogenic differentiation and mineralisation on Saos-2 cells. | [86] |
| Ti-6Al-4V | Mesoporous bioactive glass substituted with Ce, Ga, Zn. | Surface characterisation. Cytotoxicity and biocompatibility assays. | Homogenous and crack-free coatings. Positive cytocompatibilities. | [87] |
| TiO2 nanotubes Pure Ti sheets | Ga(NO3)3-PDLLA | Biofilm characterisation, cytotoxicity, and biocompatibility assays. Antibacterial assay. | Inhibition of E. coli and S. aureus bacteria. | [88] |
| Ti | LDH-Ga and Sr | Biofilm characterisation, cytotoxicity, and biocompatibility assays. Antibacterial assay. | Enhanced differentiation of cells and osteoblasts. Antimicrobial inhibition against E. coli and S. aureus. | [89] |
| SrTiO3 nanotubes on Ti | Ga(NO3)3-PDA | Surface characterisation. Antibacterial assay. Cytotoxicity and biocompatibility assays. | Superior osteoinductive activity. Gradual and constant antibacterial agent release of E. coli and S. aureus. Almost no bacteria after 7 days. | [90] |
| Ti | Ga(NO3)3 | Surface characterisation. Cytotoxicity, osteogenesis and osteoclastic biocompatibility assays. | Promoted osteogenesis, suppressed osteoclast generation. | [91] |
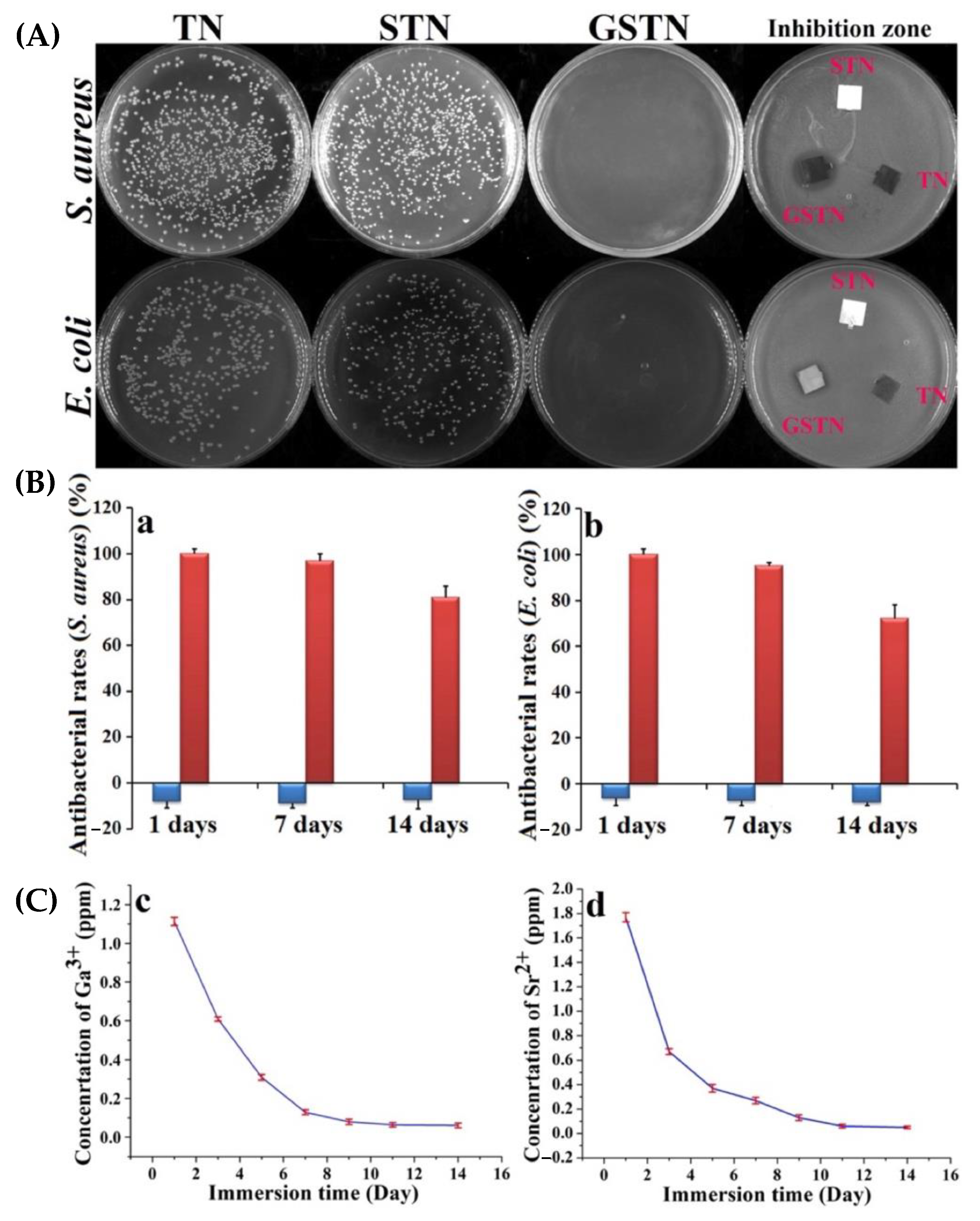
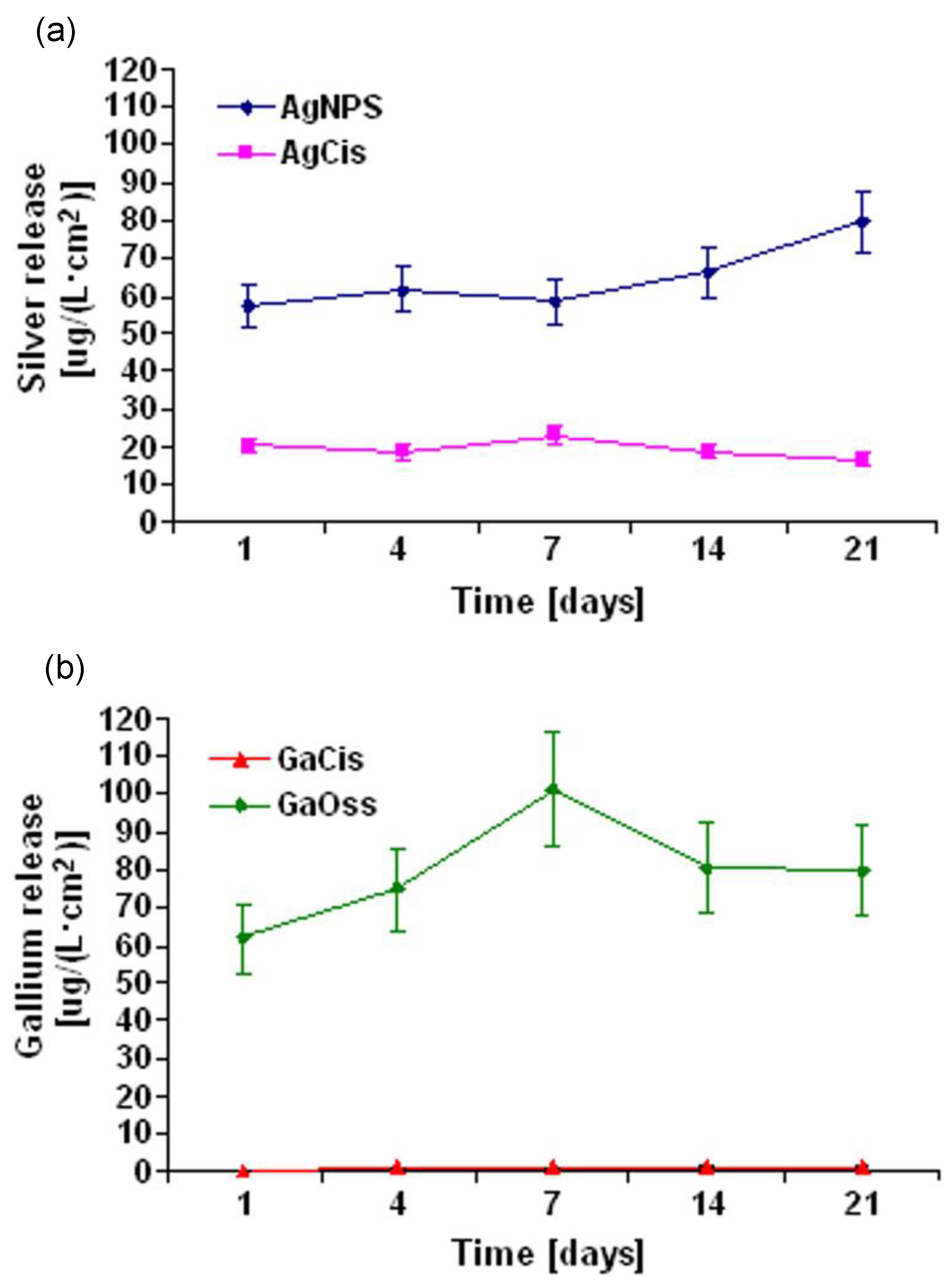
| Grade 2 Ti | GaCis and GaOss (Ga(NO3)3) | Morphological characterisation. Mechanical properties (elastic modulus, hardness). Antibacterial assay. Cytotoxicity and biocompatibility assays. | Strong inhibition of bacteria between 27–35%. Inhibition of A. baumannii. Good cytocompatibilities. | [18,92] |
| Chemical Composition (wt%) | Properties Investigated/Methods | Notable Results | Ref. |
|---|---|---|---|
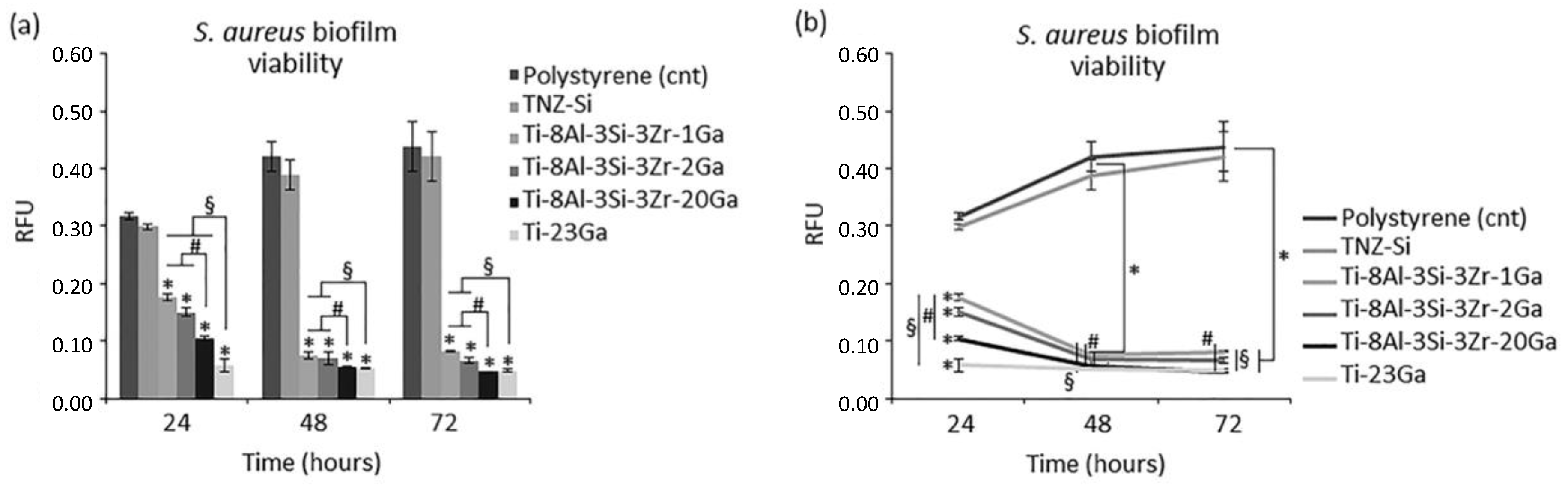
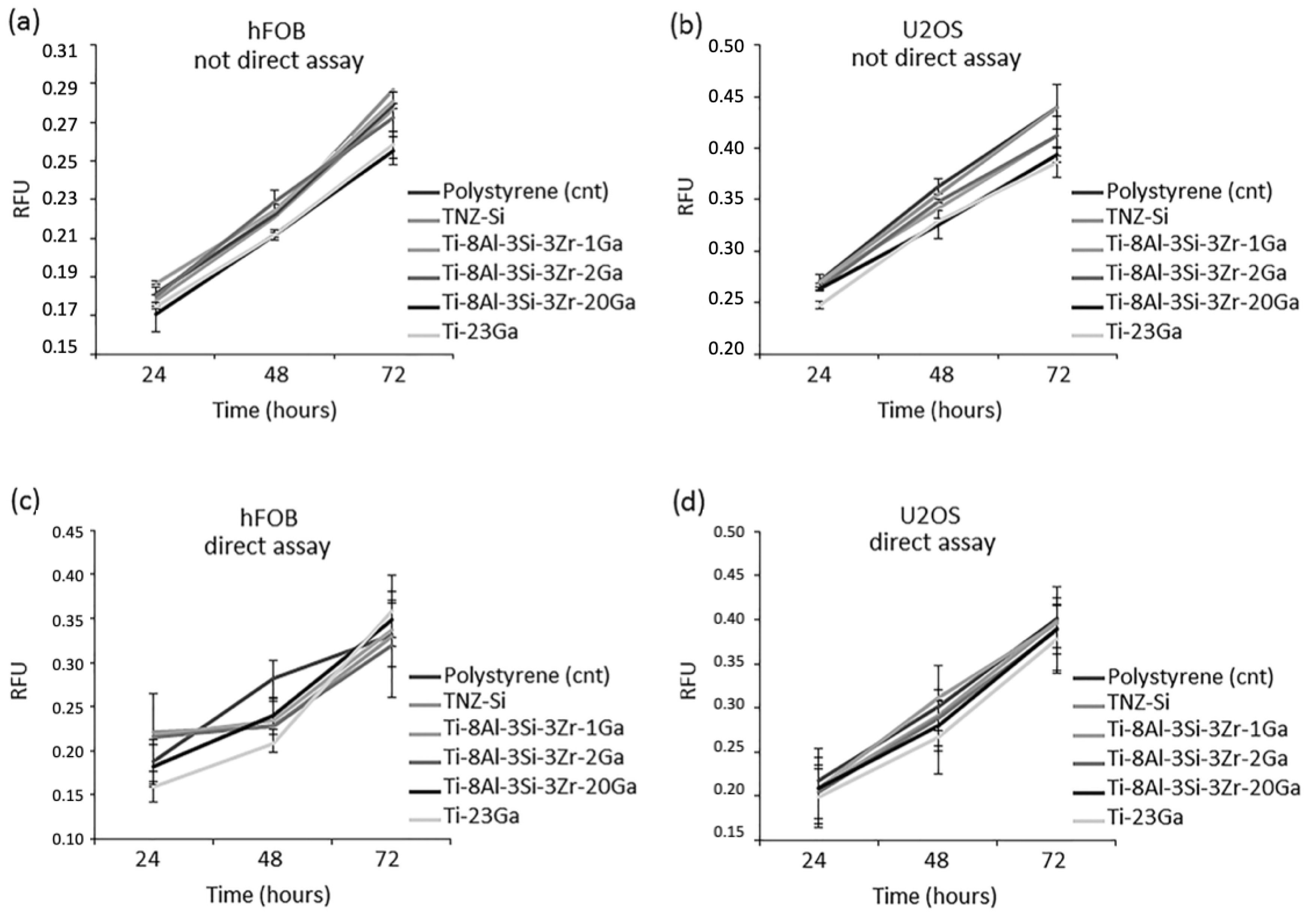
| Ti-8Al-3Si-3Zr-1Ga Ti-8Al-3Si-3Zr-2Ga Ti-8Al-3Si-3Zr-20Ga | Microstructural analysis. Antibacterial assays. Biocompatibility/cytotoxicity assays. | Inhibition of S. aureus, more than 80% reduction in metabolic activity. Potent antibacterial efficiency for all samples, even 1–2 wt% additions of Ga. Great cytocompatibilities. | [19] |
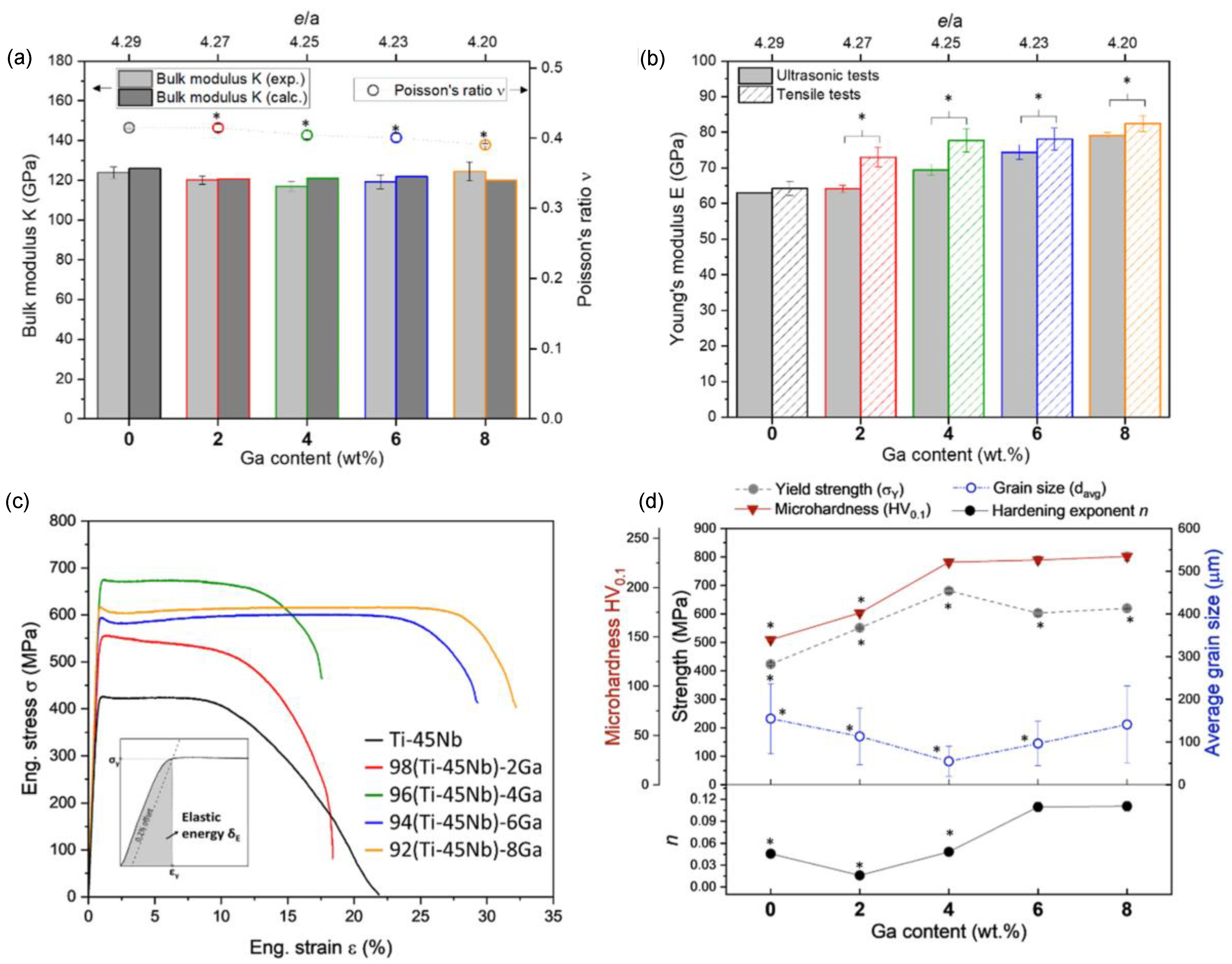
| Ti-45Nb-2Ga Ti-45Nb-4Ga Ti-45Nb-6Ga Ti-45Nb-8Ga | Chemical composition analysis. Mechanical properties (yield strength, Young’s modulus, hardness, ductility). | 4 wt% Ga depicted the best combination of mechanical properties. 40% increase in strength over Ti-45Nb. Maximum yield strength: 620 ± 2 MPa. Microhardness: 232 ± 5 HV. Young’s modulus: 73 ÷ 82 GPa. Maximum ductility: 32% | [12,93] |
| Ti-45Nb-4Ga Ti-45Nb-8Ga | Microstructural analysis. Mechanical properties. Corrosion and tribocorrosion properties. | Single β phase. Ga caused no deleterious effect on the corrosion resistance. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McHendrie, R.; Xiao, W.; Truong, V.K.; Hashemi, R. Gallium-Containing Materials and Their Potential within New-Generation Titanium Alloys for Biomedical Applications. Biomimetics 2023, 8, 573. https://doi.org/10.3390/biomimetics8080573
McHendrie R, Xiao W, Truong VK, Hashemi R. Gallium-Containing Materials and Their Potential within New-Generation Titanium Alloys for Biomedical Applications. Biomimetics. 2023; 8(8):573. https://doi.org/10.3390/biomimetics8080573
Chicago/Turabian StyleMcHendrie, Rhianna, Wenlong Xiao, Vi Khanh Truong, and Reza Hashemi. 2023. "Gallium-Containing Materials and Their Potential within New-Generation Titanium Alloys for Biomedical Applications" Biomimetics 8, no. 8: 573. https://doi.org/10.3390/biomimetics8080573
APA StyleMcHendrie, R., Xiao, W., Truong, V. K., & Hashemi, R. (2023). Gallium-Containing Materials and Their Potential within New-Generation Titanium Alloys for Biomedical Applications. Biomimetics, 8(8), 573. https://doi.org/10.3390/biomimetics8080573








