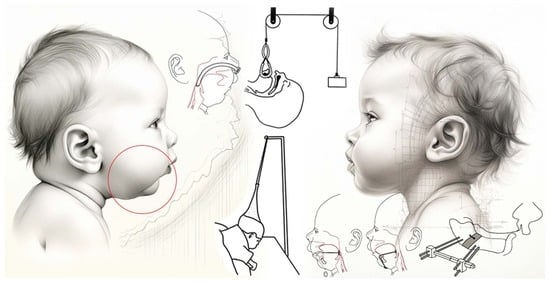
The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review
Abstract
:1. Introduction
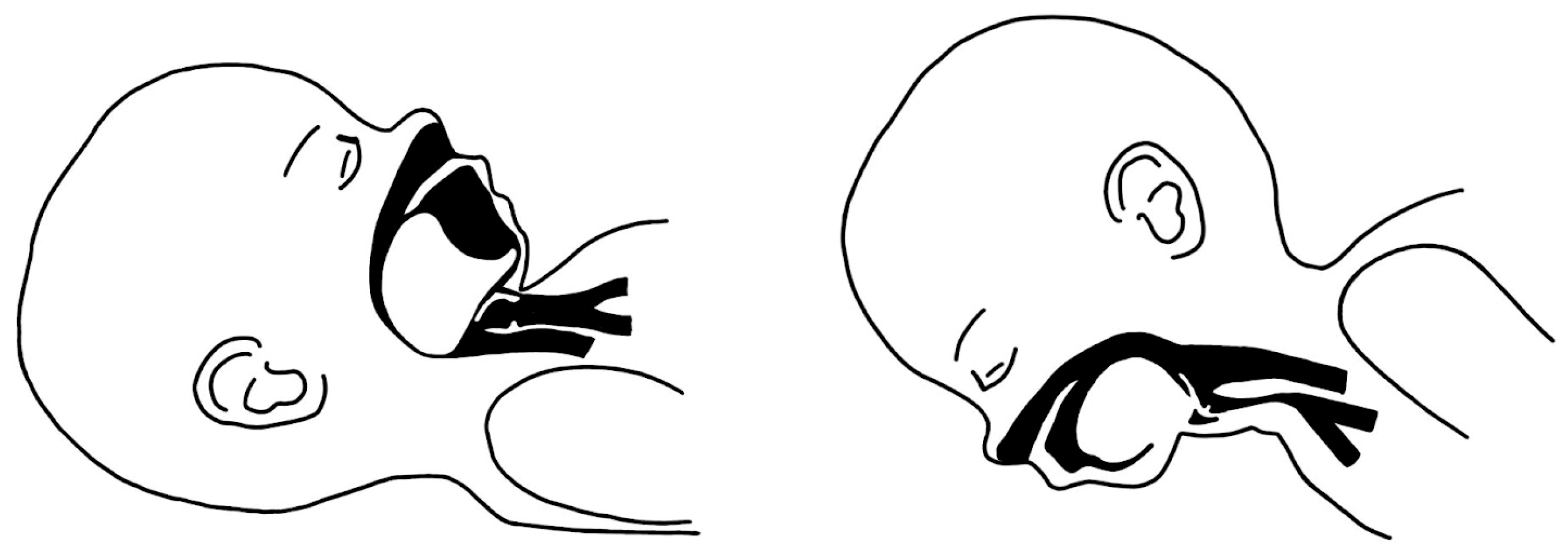
2. Overview of Diagnostic Approaches and Understanding the Pathology
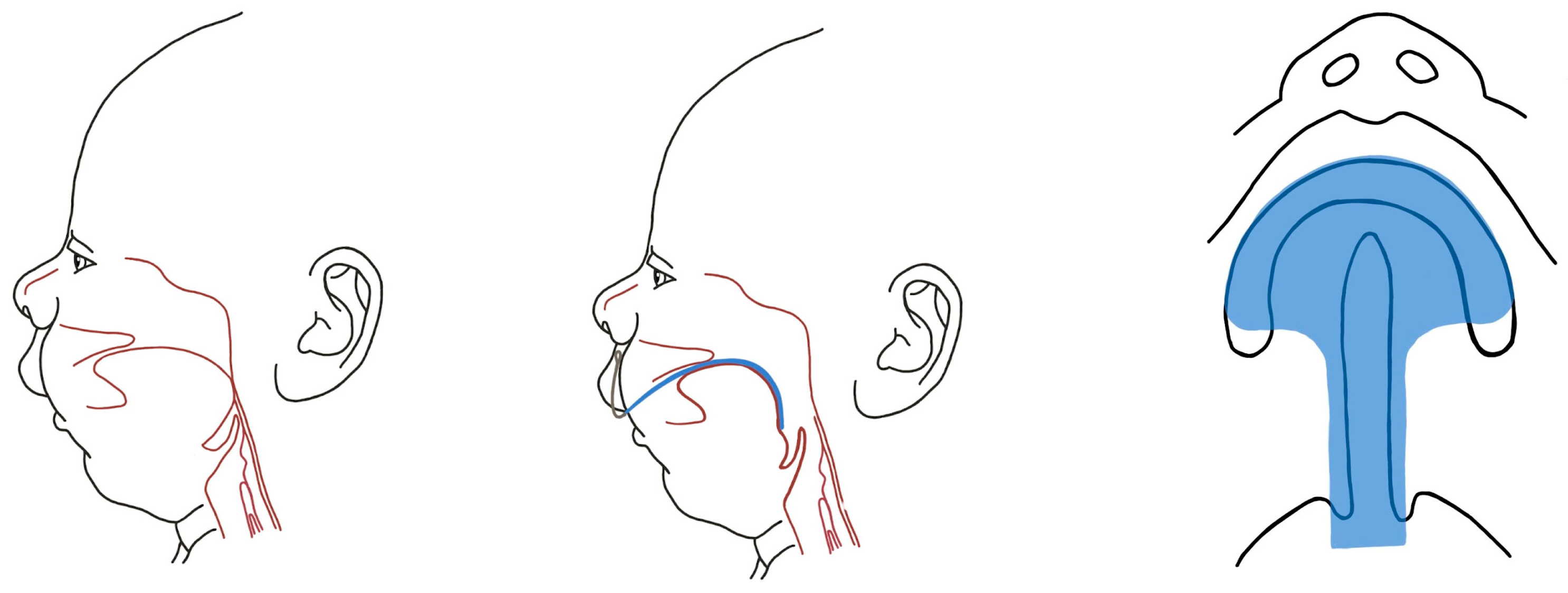
The Embryological Basis and Etiopathogenesis of Robin Sequence
3. Treatment Modalities
3.1. Nonsurgical Treatment
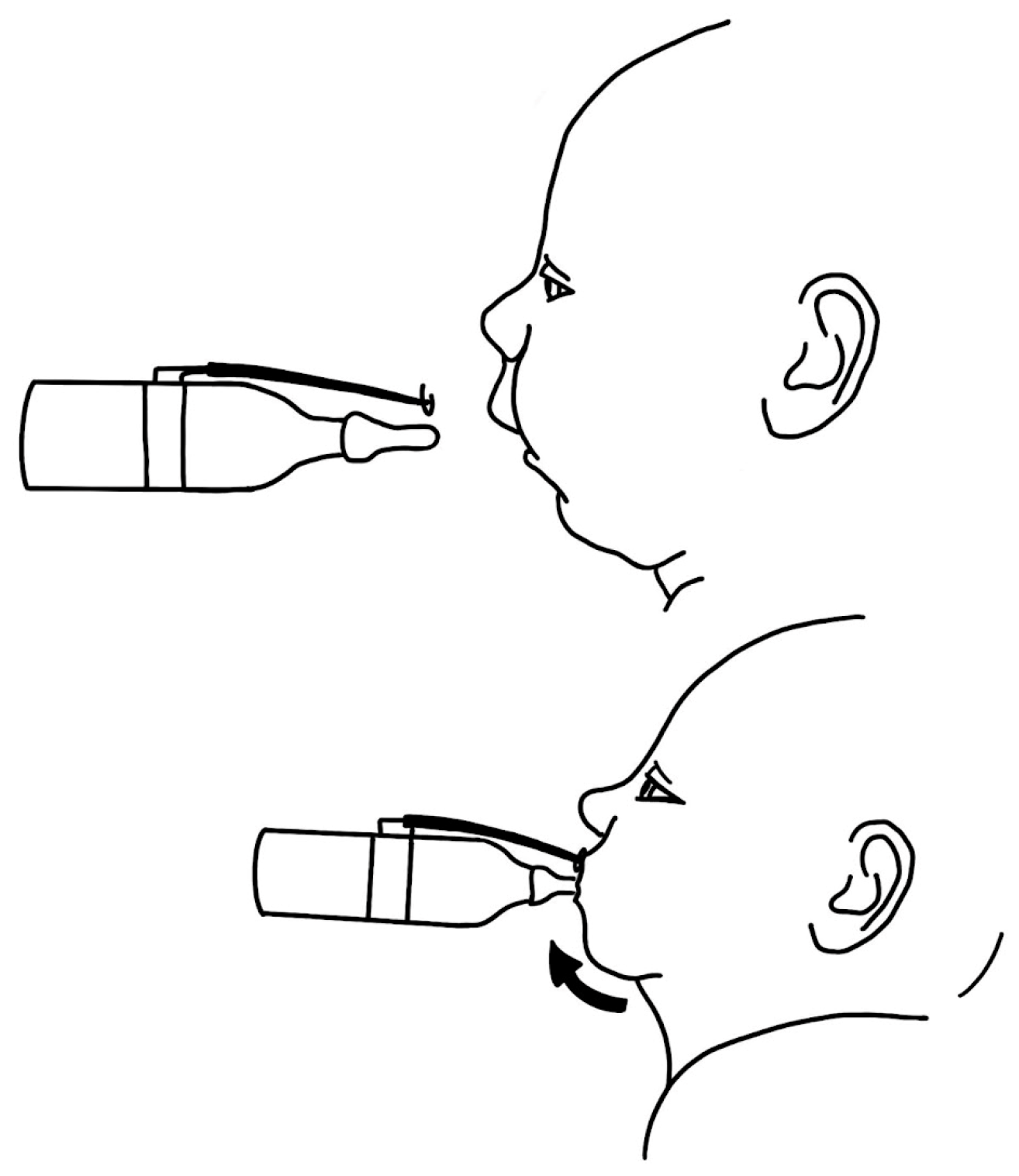
3.1.1. Positioning
3.1.2. Stenting
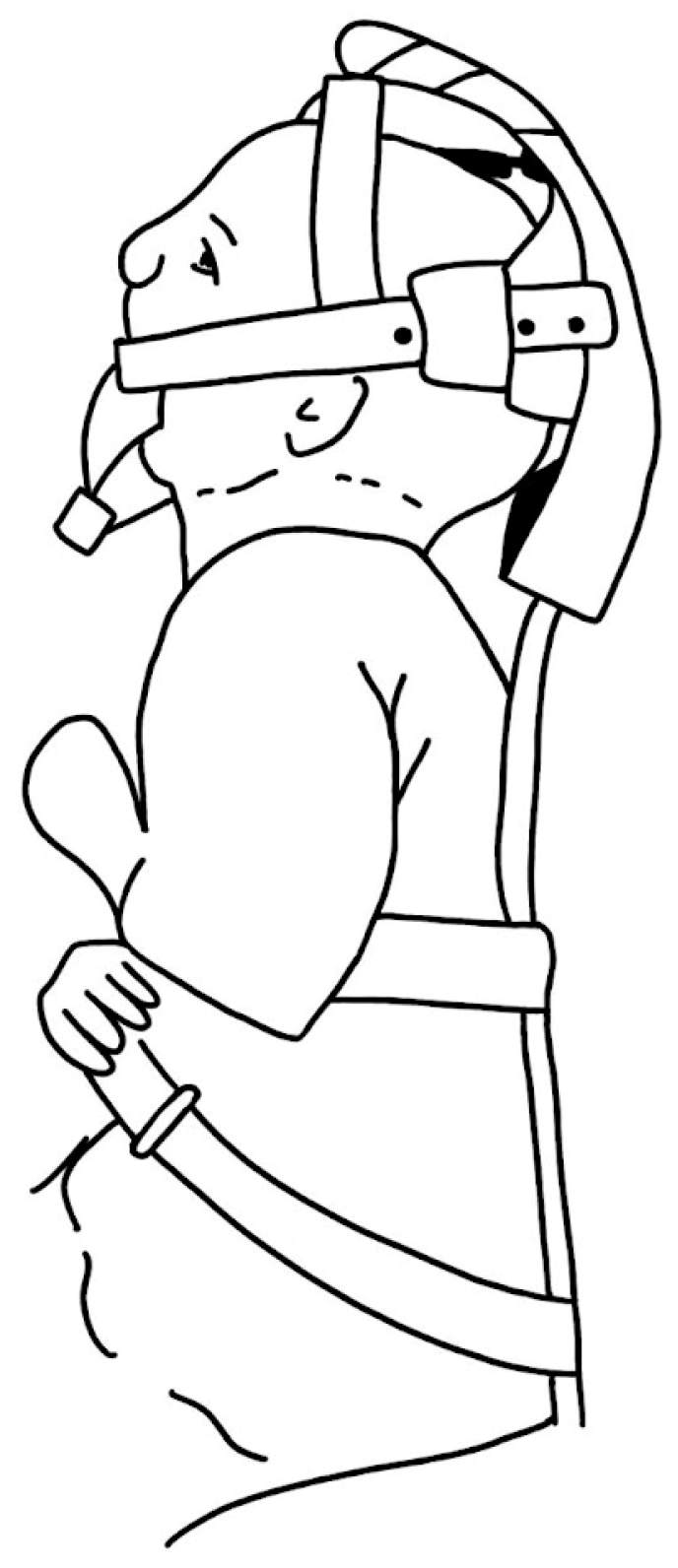
3.1.3. Appliances
3.2. Surgical Treatment
3.2.1. Tracheostomy
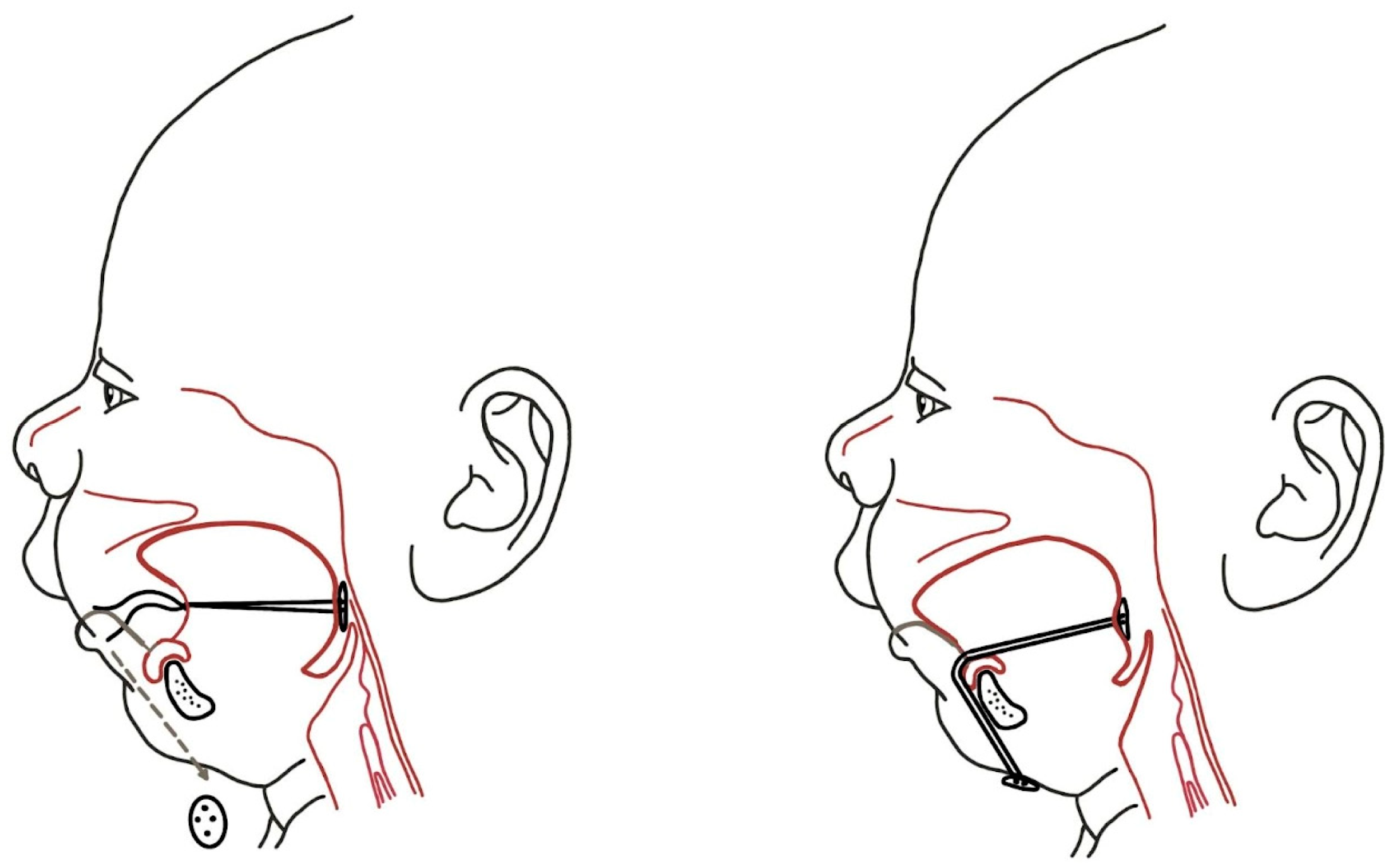
3.2.2. Tongue-Lip Adhesion
3.2.3. Mandibular Distraction Osteosynthesis
3.2.4. Other Surgical Procedures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Robin, P. La Chute de La Base de La Lanque Consideree Comme Une Nouvelle Cause de Gene Dans La Respiraration Naso-Pharyngienne. Bull. Acad. Med. 1923, 89, 37–41. [Google Scholar]
- Maas, C.; Poets, C.F. Initial Treatment and Early Weight Gain of Children with Robin Sequence in Germany: A Prospective Epidemiological Study. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F491–F494. [Google Scholar] [CrossRef]
- Wright, M.F.; Knowles, R.L.; Cortina-Borja, M.; Javadpour, S.; Mehendale, F.V.; Urquhart, D.S. Epidemiology of Robin Sequence in the UK and Ireland: An Active Surveillance Study. Arch. Dis. Child. 2023, 108, 748–753. [Google Scholar] [CrossRef]
- Shprintzen, R.J. Pierre Robin, Micrognathia, and Airway Obstruction: The Dependency of Treatment on Accurate Diagnosis. Int. Anesthesiol. Clin. 1988, 26, 64–71. [Google Scholar] [CrossRef]
- Fairbairn, P. Suffocation in an Infant, from Retraction of the Base of the Tongue. Am. J. Dent. Sci. 1846, 7, 189–192. [Google Scholar]
- Douglas, B. A further report on the treatmentof micrognathia with obstruction by a plastic procedure. Plast. Reconstr. Surg. 1950, 5, 113–122. [Google Scholar] [CrossRef]
- Robin, P. Glossoptosis due to atresia and hypotrophy of the mandible. Arch. Pediatr. Adolesc. Med. 1934, 48, 541–547. [Google Scholar] [CrossRef]
- Kiskadden, W.S.; Dietrich, S.R. Review of the treatment of micrognathia. Plast. Reconstr. Surg. 1953, 12, 364–373. [Google Scholar] [CrossRef]
- Dennison, W.M. The Pierre Robin Syndrome. Pediatrics 1965, 36, 336–341. [Google Scholar] [CrossRef]
- Benjamin, B.; Walker, P. Management of Airway Obstruction in the Pierre Robin Sequence. Int. J. Pediatr. Otorhinolaryngol. 1991, 22, 29–37. [Google Scholar] [CrossRef]
- Wilson, A.C.; Moore, J.; Moore, M.H.; Martin, A.J.; Staugas, E.M.; Kennedy, J.D. Late Presentation of Upper Airway Obstruction in Pierre Robin Sequence. Arch. Dis. Child. 2000, 83, 435–438. [Google Scholar] [CrossRef]
- Breugem, C.C.; Evans, K.N.; Poets, C.F.; Suri, S.; Picard, A.; Filip, C.; Paes, E.C.; Mehendale, F.V.; Saal, H.M.; Basart, H.; et al. Best Practices for the Diagnosis and Evaluation of Infants with Robin Sequence. JAMA Pediatr. 2016, 170, 894–902. [Google Scholar] [CrossRef]
- Freed, G.; Pearlman, M.A.; Brown, A.S.; Barot, L.R. Polysomnographic Indications for Surgical Intervention in Pierre Robin Sequence: Acute Airway Management and Follow-up Studies after Repair and Take-down of Tongue-Lip Adhesion. Cleft Palate J. 1988, 25, 151–155. [Google Scholar]
- Bull, M.J.; Givan, D.C.; Sadove, A.M.; Bixler, D.; Hearn, D. Improved Outcome in Pierre Robin Sequence: Effect of Multidisciplinary Evaluation and Management. Pediatrics 1990, 86, 294–301. [Google Scholar] [CrossRef]
- Gilhooly, J.T.; Smith, J.D.; Howell, L.L.; Deschaine, B.L.; Richey, S.L. Bedside Polysomnography as an Adjunct in the Management of Infants with Robin Sequence. Plast. Reconstr. Surg. 1993, 92, 23–27. [Google Scholar] [CrossRef]
- Bravo, G.; Ysunza, A.; Arrieta, J.; Pamplona, M.C. Videonasopharyngoscopy Is Useful for Identifying Children with Pierre Robin Sequence and Severe Obstructive Sleep Apnea. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 27–33. [Google Scholar] [CrossRef]
- Lee, J.J.; Ford, M.D.; Tobey, A.B.; Jabbour, N. Diagnosing Tongue Base Obstruction in Pierre Robin Sequence Infants. Cleft Palate-Craniofacial J. 2018, 55, 692–696. [Google Scholar] [CrossRef]
- Miller, C. Feeding Issues and Interventions in Infants and Children with Clefts and Craniofacial Syndromes. Semin. Speech Lang. 2011, 32, 115–126. [Google Scholar] [CrossRef]
- de Vries, I.A.C.; Breugem, C.C.; van der Heul, A.M.B.; Eijkemans, M.J.C.; Kon, M.; van der Molen, A.B.M. Prevalence of Feeding Disorders in Children with Cleft Palate Only: A Retrospective Study. Clin. Oral Investig. 2013, 18, 1507–1515. [Google Scholar] [CrossRef]
- Holder-Espinasse, M.; Abadie, V.; Cormier-Daire, V.; Beyler, C.; Manach, Y.; Munnich, A.; Lyonnet, S.; Couly, G.; Amiel, J. Pierre Robin Sequence: A Series of 117 Consecutive Cases. J. Pediatr. 2001, 139, 588–590. [Google Scholar] [CrossRef]
- Izumi, K.; Konczal, L.L.; Mitchell, A.L.; Jones, M.C. Underlying Genetic Diagnosis of Pierre Robin Sequence: Retrospective Chart Review at Two Children’s Hospitals and a Systematic Literature Review. J. Pediatr. 2012, 160, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Alembick, Y.; Roth, M.P. Associated Anomalies in Pierre Robin Sequence. Am. J. Med. Genet. A 2023, 191, 2312–2323. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Javorka, V.; Stanko, P.; Lysy, J.; Suchancova, B.; Lehotska, V.; Valkovic, L.; Makovnik, M. Digital and Manual Cephalometric Analysis. Bratisl. Lek. Listy 2010, 111, 97–100. [Google Scholar] [PubMed]
- Urban, R.; Haluzová, S.; Strunga, M.; Surovková, J.; Lifková, M.; Tomášik, J.; Thurzo, A. AI-Assisted CBCT Data Management in Modern Dental Practice: Benefits, Limitations and Innovations. Electronics 2023, 12, 1710. [Google Scholar] [CrossRef]
- Strunga, M.; Urban, R.; Surovková, J.; Thurzo, A. Artificial Intelligence Systems Assisting in the Assessment of the Course and Retention of Orthodontic Treatment. Healthcare 2023, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, I.A.; Tsolakis, A.I.; Elshebiny, T.; Matthaios, S.; Palomo, J.M. Comparing a Fully Automated Cephalometric Tracing Method to a Manual Tracing Method for Orthodontic Diagnosis. J. Clin. Med. 2022, 11, 6854. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Pospíšilová, V.; Gmitterová, K.; Gálfiová, P.; Polák, Š.; Galbavý, Š. The Phylogenesis and Ontogenesis of the Human Pharyngeal Region Focused on the Thymus, Parathyroid, and Thyroid Glands. Neuro Endocrinol. Lett. 2008, 29, 837–845. [Google Scholar]
- Fabik, J.; Psutkova, V.; Machon, O. The Mandibular and Hyoid Arches-From Molecular Patterning to Shaping Bone and Cartilage. Int. J. Mol. Sci. 2021, 22, 7529. [Google Scholar] [CrossRef]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [CrossRef]
- Vega-Lopez, G.A.; Cerrizuela, S.; Tribulo, C.; Aybar, M.J. Neurocristopathies: New Insights 150 Years after the Neural Crest Discovery. Dev. Biol. 2018, 444 (Suppl. 1), S110–S143. [Google Scholar] [CrossRef]
- Antonaci, M.; Wheeler, G.N. MicroRNAs in Neural Crest Development and Neurocristopathies. Biochem. Soc. Trans. 2022, 50, 965. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.; Han, D.; Grimaldi, A.; Sarrion, P.; Park, S.S.; Pelikan, R.; Sanchez-Lara, P.A.; Chai, Y. Disruption of the ERK/MAPK Pathway in Neural Crest Cells as a Potential Cause of Pierre Robin Sequence. Development 2015, 142, 3734–3745. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Morisseau-Durand, M.-P.; Beyler, C.; Manach, Y.; Couly, G. Brainstem Dysfunction: A Possible Neuroembryological Pathogenesis of Isolated Pierre Robin Sequence. Eur. J. Pediatr. 2002, 161, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Barone, S.; Belhous, K.; Morice, A.; Soupre, V.; Bennardo, F.; Boddaert, N.; Vazquez, M.-P.; Abadie, V.; Picard, A. Pierre Robin Sequence: A Comprehensive Narrative Review of the Literature over Time. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gómez, O.J.; Barón, O.I.; Peñarredonda, M.L. Pierre Robin Sequence: An Evidence-Based Treatment Proposal. J. Craniofacial Surg. 2018, 29, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.B.; Pashayan, H.M. Management of Infants with Roboin Anomaly. Clin. Pediatr. 1980, 19, 519–521. [Google Scholar] [CrossRef]
- Pasyayan, H.M.; Lewis, M.B. Clinical Experience with the Robin Sequence. Cleft Palate J. 1984, 21, 270–276. [Google Scholar]
- Caouette-laberge, L.; Caroline Plamondon, F.; Larocque, Y.; Caouette-Laberge, L.; Sainte-Justine, H. Subperiosteal Release of the Floor of the Mouth in Pierre Robin Sequence: Experience with 12 Cases. Cleft Palate-Craniofacial J. 1996, 33, 468–472. [Google Scholar] [CrossRef]
- Sher, A.E. Mechanisms of Airway Obstruction in Robin Sequence: Implications for Treatment. Cleft Palate-Craniofacial J. 1992, 29, 224–231. [Google Scholar] [CrossRef]
- Coutier, L.; Guyon, A.; Reix, P.; Franco, P. Impact of Prone Positioning in Infants with Pierre Robin Sequence: A Polysomnography Study. Sleep Med. 2019, 54, 257–261. [Google Scholar] [CrossRef]
- Poets, C.F.; Bacher, M. Treatment of Upper Airway Obstruction and Feeding Problems in Robin-Like Phenotype. J. Pediatr. 2011, 159, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Axtrup, S. Treatment of Micrognathia. Acta Paediatr. 1963, 52, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Argamaso, R.V. Glossopexy for Upper Airway Obstruction in Robin Sequence. Cleft Palate Craniofac. J. 1992, 29, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Masters, I.B.; Chang, A.B.; Harris, M.; O’Neil, M.C. Modified Nasopharyngeal Tube for Upper Airway Obstruction. Arch. Dis. Child. 1999, 80, 186–187. [Google Scholar] [CrossRef]
- Wagener, S.; Rayatt, S.S.; Tatman, A.J.; Gornall, P.; Slator, R. Management of Infants with Pierre Robin Sequence. Cleft Palate-Craniofacial J. 2003, 40, 180–185. [Google Scholar] [CrossRef]
- Drago Marquezini Salmen, I.C.; Lazarini Marques, I. In Situ and Home Care Nasopharyngeal Intubation Improves Respiratory Condition and Prevents Surgical Procedures in Early Infancy of Severe Cases of Robin Sequence. BioMed Res. Int. 2015, 2015, 608905. [Google Scholar] [CrossRef]
- Eley, R.C.; Farber, S. Hypoplasia of the mandible (micrognathy): As a cause of cyanotic attacks in the newly born infant: Report of four cases. JAMA Pediatr. 1930, 39, 1167–1175. [Google Scholar] [CrossRef]
- Nisenson, A. Receding Chin and Glossoptosis. J. Pediatr. 1948, 32, 397–403. [Google Scholar] [CrossRef]
- Hotz, M.; Gnoinski, W. Clefts of the Secondary Palate Associated with the “Pierre Robin Syndrome”. Management by Early Maxillary Orthopaedics. Swed. Dent. J. Suppl. 1982, 15, 89–98. [Google Scholar]
- Pielou, W.D. Non-Surgical Management of Pierre Robin Syndrome. Arch. Dis. Child. 1967, 42, 20–23. [Google Scholar] [CrossRef]
- Poets, C.F.; Koos, B.; Reinert, S.; Wiechers, C. The Tübingen Palatal Plate Approach to Robin Sequence: Summary of Current Evidence. J. Cranio-Maxillofac. Surg. 2019, 47, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Knechtel, P.; Weismann, C.; Poets, C.F. Caring for Infants with Robin Sequence Treated with the Tübingen Palatal Plate: A Review of Personal Practice. Children 2023, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Fracture Load of an Orthodontic Appliance for Robin Sequence Treatment in a Digital Workflow. Materials 2021, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Müller-Hagedorn, S.; Arand, J.; Scholz, T.; Poets, C.F.; Wiechers, C. An Innovative Method for Manufacturing the Tuebingen Palatal Plate for Infants with Robin Sequence. BMC Pediatr. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Buchenau, W.; Wenzel, S.; Bacher, M.; Möller-Hagedorn, S.; Arand, J.; Poets, C.F. Functional Treatment of Airway Obstruction and Feeding Problems in Infants with Robin Sequence. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F142–F146. [Google Scholar] [CrossRef] [PubMed]
- Bockstedte, M.; Xepapadeas, A.B.; Spintzyk, S.; Poets, C.F.; Koos, B.; Aretxabaleta, M. Development of Personalized Non-Invasive Ventilation Interfaces for Neonatal and Pediatric Application Using Additive Manufacturing. J. Personal. Med. 2022, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.F.; Wiechers, C.; Arand, J.; Koos, B.; Xepapadeas, A.B. Technical Note on Introducing a Digital Workflow for Newborns with Craniofacial Anomalies Based on Intraoral Scans—Part II: 3D Printed Tübingen Palatal Plate Prototype for Newborns with Robin Sequence. BMC Oral Health 2020, 20, 171. [Google Scholar] [CrossRef]
- Tayebi, L.; Masaeli, R.; Zandsalimi, K. 3D Printing in Oral & Maxillofacial Surgery; Springer: Berlin/Heidelberg, Germany, 2021; 217p. [Google Scholar] [CrossRef]
- Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. Recent Advancements in Regenerative Dentistry: A Review. Mater. Sci. Eng. C 2016, 69, 1383–1390. [Google Scholar] [CrossRef]
- Schmidt, G.; Hirschfelder, A.; Heiland, M.; Matuschek, C. Customized Pre-Epiglottic Baton Plate—A Practical Guide for Successful, Patient-Specific, Noninvasive Treatment of Neonates with Robin Sequence. Cleft Palate-Craniofacial J. 2021, 58, 1063–1069. [Google Scholar] [CrossRef]
- Poets, C.F.; Maas, C.; Buchenau, W.; Arand, J.; Vierzig, A.; Braumann, B.; Müller-Hagedorn, S. Multicenter Study on the Effectiveness of the Pre-Epiglottic Baton Plate for Airway Obstruction and Feeding Problems in Robin Sequence. Orphanet. J. Rare Dis. 2017, 12, 46. [Google Scholar] [CrossRef]
- Effert, J.; Uhlig, S.; Wiechers, C.; Quante, M.; Poets, C.F.; Schulz, M.C.; Reinert, S.; Krimmel, M.; Koos, B.; Weise, C. Prospective Evaluation of Children with Robin Sequence Following Tübingen Palatal Plate Therapy. J. Clin. Med. 2023, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.; Kim, S.-H.; Ahn, H.-W.; Poets, C.F.; Chung, K.-R. Split Orthodontic Airway Plate: An Innovation to the Utilization Method of Conventional Orthodontic Airway Plate for Neonates with Robin Sequence. Korean J. Orthod. 2022, 52, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Šufliarsky, B.; Urbanová, W.; Čverha, M.; Strunga, M.; Varga, I. Pierre Robin Sequence and 3D Printed Personalized Composite Appliances in Interdisciplinary Approach. Polymers 2022, 14, 3858. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Neuschlová, I.; Paouris, D.; Čverha, M. Use of Optical Scanning and 3D Printing to Fabricate Customized Appliances for Patients with Craniofacial Disorders. Semin. Orthod. 2022, 28, 92–99. [Google Scholar] [CrossRef]
- Thurzo, A.; Strunga, M.; Havlínová, R.; Reháková, K.; Urban, R.; Surovková, J.; Kurilová, V. Smartphone-Based Facial Scanning as a Viable Tool for Facially Driven Orthodontics? Sensors 2022, 22, 7752. [Google Scholar] [CrossRef] [PubMed]
- Moyson, F. A Plea against Tracheostomy in the Pierre-Robin Syndrome. Br. J. Plast. Surg. 1961, 14, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.; Sidoti, E.J. Pediatric Management of Robin Sequence. Cleft Palate-Craniofacial J. 1992, 29, 220–223. [Google Scholar] [CrossRef]
- Lubianca Neto, J.F.; Castagno, O.C.; Schuster, A.K. Complications of Tracheostomy in Children: A Systematic Review. Braz. J. Otorhinolaryngol. 2022, 88, 882–890. [Google Scholar] [CrossRef]
- Randall, P.; Krogman, W.M.; Jahins, S. Pierre robin and the syndrome that bears his name. Cleft Palate J. 1965, 36, 237–246. [Google Scholar]
- Routledge, R.T. The Pierre-Robin Syndrome: A Surgical Emergency in the Neonatal Period. Br. J. Plast. Surg. 1960, 13, 204–218. [Google Scholar] [CrossRef]
- Minervini, F. The duhamel procedure for treatment of the pierre robin syndrome. Plast. Reconstr. Surg. 1973, 51, 686. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.W.; Smith, D.J. A Modified Tongue-Lip Adhesion Fo Pierre Robin Anomalad. Cleft Palate J. 1980, 17, 144–147. [Google Scholar] [PubMed]
- Denny, A.D.; Talisman, R.; Hanson, P.R.; Recinos, R.F. Mandibular Distraction Osteogenesis in Very Young Patients to Correct Airway Obstruction. Plast. Reconstr. Surg. 2001, 108, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.L.; Tholpady, S.S.; Sati, S.; Fairbanks, G.; Socas, J.; Choi, M.; Havlik, R.J. The Surgical Correction of Pierre Robin Sequence: Mandibular Distraction Osteogenesis versus Tongue-Lip Adhesion. Plast. Reconstr. Surg. 2014, 133, 1433–1439. [Google Scholar] [CrossRef]
- Cohen, S.R.; Simms, C.; Burstein, F.D. Mandibular Distraction Osteogenesis in the Treatment of Upper Airway Obstruction in Children with Craniofacial Deformities. Plast. Reconstr. Surg. 1998, 101, 312–318. [Google Scholar] [CrossRef]
- Monasterio, F.O.; Drucker, M.; Molina, F.; Ysunza, A. Distraction Osteogenesis in Pierre Robin Sequence and Related Respiratory Problems in Children. J. Craniofacial Surg. 2002, 13, 79–83. [Google Scholar] [CrossRef]
- Scott, A.R.; Tibesar, R.J.; Sidman, J.D. Pierre Robin Sequence. Otolaryngol. Clin. N. Am. 2012, 45, 695–710. [Google Scholar] [CrossRef]
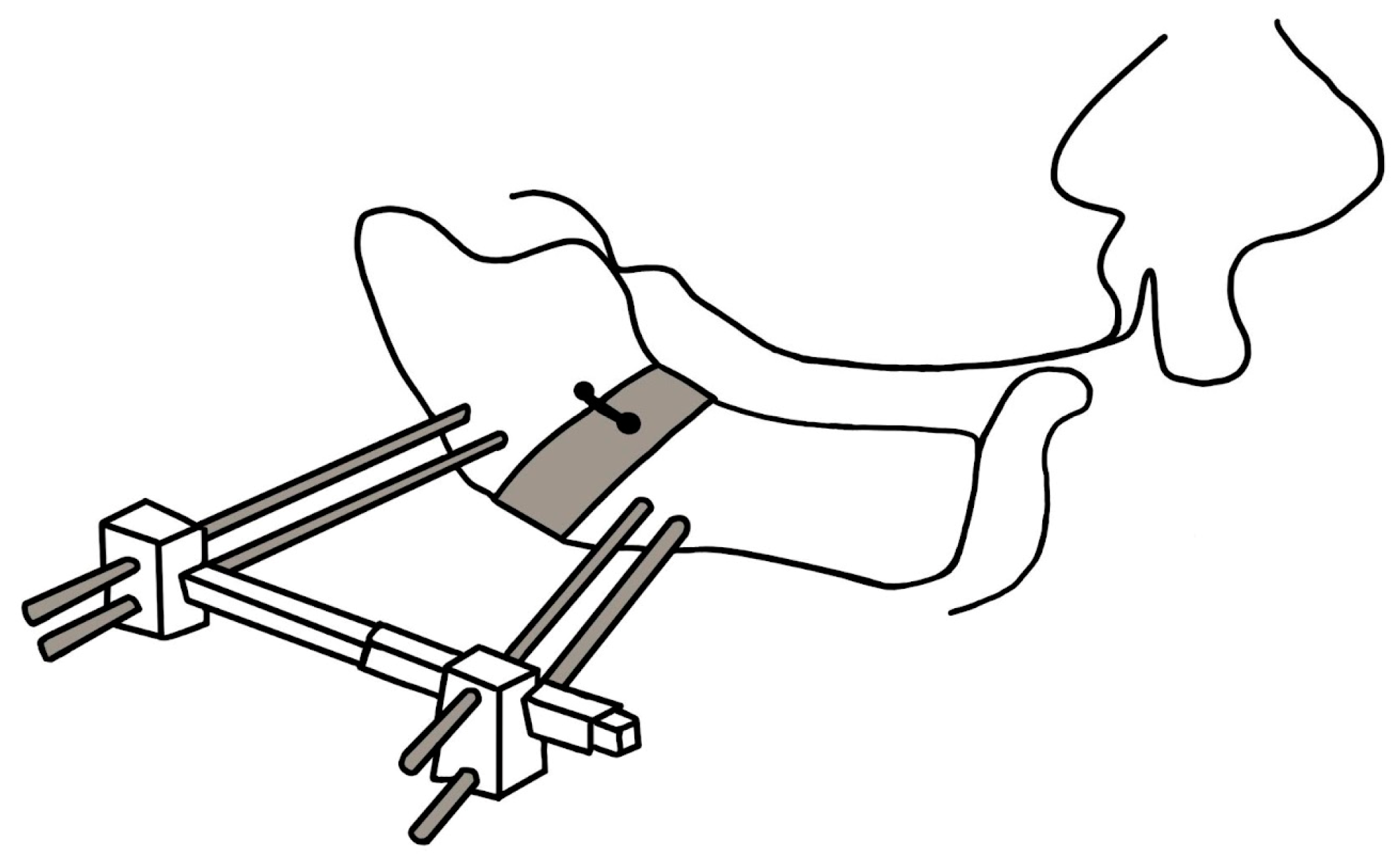
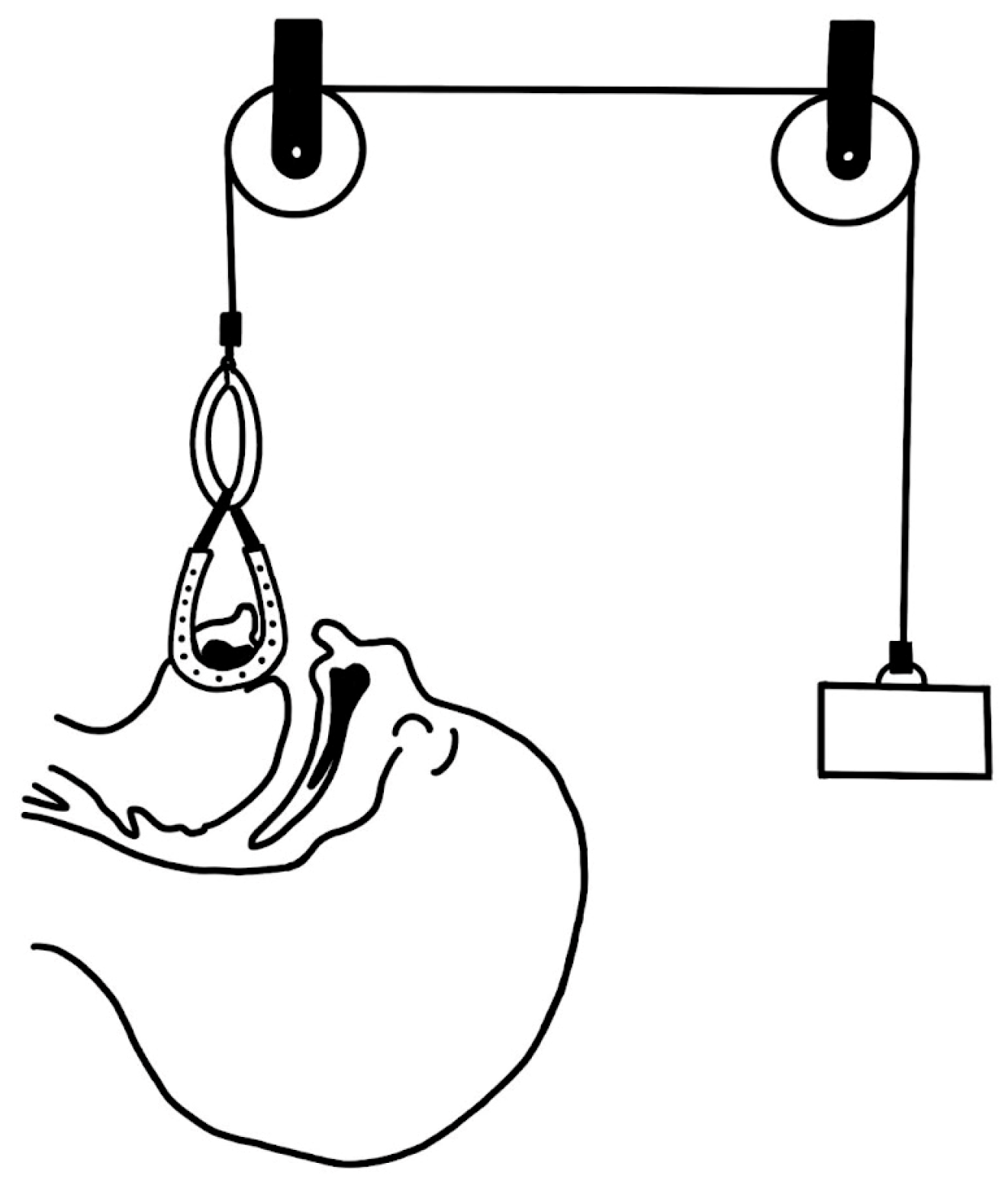
- Callister, A.C. Hypoplasia of the Mandible (Micrognathy) with Cleft Palate: Treatment in Early Infancy by Skeletal Traction. Am. J. Dis. Child. 1937, 53, 1057–1059. [Google Scholar] [CrossRef]
- Champion, R. Treatment of Cleft Palate Associated with Micrognathia. Plast. Reconstr. Surg. 1956, 17, 491–492. [Google Scholar] [CrossRef]
- Hadley, R.C.; Johnson, J.B. Utilization of the Kirschner Wire in Pierre Robin Syndrome: With Case Report. Plast. Reconstr. Surg. 1963, 31, 587–596. [Google Scholar] [CrossRef]
- Lewis, S.R.; Lynch, J.B.; Blocker, T.G. Fascial slings for tongue stabilization in the pierre robin syndrome. Plast. Reconstr. Surg. 1968, 42, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, A.; Rezvani, F.; Terrefe, D.; Ben-Hur, N. A new functional approach to the surgical management of pierre robin syndrome: Experimental and clinical report. Laryngoscope 1976, 86, 979–983. [Google Scholar] [CrossRef]
- Wada, T.; Ishi, T.; Sugai, T.; Molla, M.R.; Matsuya, T.; Miyazaki, T.; Koh, Y. Mandibular Traction for Relieving Respiratory Distress in the Pierre Robin Anomaly A Case Report. J. Maxillofac. Surg. 1983, 11, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Pradel, W.; Lauer, G.; Dinger, J.; Eckelt, U. Mandibular Traction—An Alternative Treatment in Infants with Pierre Robin Sequence. J. Oral Maxillofac. Surg. 2009, 67, 2232–2237. [Google Scholar] [CrossRef]
- Baciliero, U.; Spanio di Spilimbergo, S.; Riga, M.; Padula, E. Respiratory Distress in Pierre Robin Sequence: An Experience with Mandible Traction by Wires. Int. J. Oral Maxillofac. Surg. 2011, 40, 464–470. [Google Scholar] [CrossRef]
- Bekisz, J.M.; Fryml, E.; Flores, R.L. A Review of Randomized Controlled Trials in Cleft and Craniofacial Surgery. J. Craniofacial Surg. 2018, 29, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Viezel-Mathieu, A.; Safran, T.; Gilardino, M.S. A Systematic Review of the Effectiveness of Tongue Lip Adhesion in Improving Airway Obstruction in Children with Pierre Robin Sequence. J. Craniofacial Surg. 2016, 27, 1453–1456. [Google Scholar] [CrossRef]
- Smyth, A.G. A Simple Nasal Splint to Assist the Stability of Nasopharyngeal Tubes in the Pierre Robin Sequence Associated Airway Obstruction: Technical Innovation. J. Cranio-Maxillofac. Surg. 1998, 26, 411–414. [Google Scholar] [CrossRef]
- Resnick, C.M.; LeVine, J.; Calabrese, C.E.; Padwa, B.L.; Hansen, A.; Katwa, U. Early Management of Infants with Robin Sequence: An International Survey and Algorithm. J. Oral Maxillofac. Surg. 2019, 77, 136–156. [Google Scholar] [CrossRef]
- Working Group on Writing a European Guideline on Robin Sequence. European Guideline Robin Sequence An Initiative From the European Reference Network for Rare Craniofacial Anomalies and Ear, Nose and Throat Disorders (ERN-CRANIO). J. Craniofacial Surg. 2023. [Google Scholar] [CrossRef]
- Glynn, F.; Fitzgerald, D.; Earley, M.J.; Rowley, H. Pierre Robin Sequence: An Institutional Experience in the Multidisciplinary Management of Airway, Feeding and Serous Otitis Media Challenges. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhao, J.; Esmeryan, K.D.; Lu, X.; Li, Z.; Wang, K.; Ren, F.; Wang, Q.; Wang, M.; Qian, B. Cicada-Inspired Fluoridated Hydroxyapatite Nanostructured Surfaces Synthesized by Electrochemical Additive Manufacturing. Mater. Des. 2020, 193, 108790. [Google Scholar] [CrossRef]
- Florea, A.-D.; Pop, L.C.; Benea, H.-R.-C.; Tomoaia, G.; Racz, C.-P.; Mocanu, A.; Dobrota, C.-T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]
- Danišovič, L.; Boháč, M.; Zamborský, R.; Oravcová, L.; Provazníková, Z.; Csöbönyeiová, M.; Varga, I. Comparative Analysis of Mesenchymal Stromal Cells from Different Tissue Sources in Respect to Articular Cartilage Tissue Engineering. Gen. Physiol. Biophys. 2016, 35, 207–214. [Google Scholar] [CrossRef]
- Thurzo, A.; Stanko, P.; Urbanova, W.; Lysy, J.; Suchancova, B.; Makovnik, M.; Javorka, V. The WEB 2.0 Induced Paradigm Shift in the e-Learning and the Role of Crowdsourcing in Dental Education. Bratisl. Lek. Listy 2010, 111, 168–175. [Google Scholar]
- Tsolakis, I.A.; Gizani, S.; Tsolakis, A.I.; Panayi, N. Three-Dimensional-Printed Customized Orthodontic and Pedodontic Appliances: A Critical Review of a New Era for Treatment. Children 2022, 9, 1107. [Google Scholar] [CrossRef]
- Faruqi, F.; Katary, A.; Abdel-Rahman, A.; Rahman, N.; Tejedor, L.; Leake, M.; Hofmann, M.; Mueller, S.; Hasic, T. Style2Fab: Functionality-Aware Segmentation for Fabricating Personalized 3D Models with Generative AI. arXiv 2023, arXiv:2309.06379. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Verzunova, K.N.; Akimchenko, I.O.; Frueh, J.; Petrov, V.I.; Slepchenko, G.B.; Bakina, O.V.; Lerner, M.I.; Brizhan, L.K.; Davydov, D.V.; et al. Antibacterial Calcium Phosphate Coatings for Biomedical Applications Fabricated via Micro-Arc Oxidation. Biomimetics 2023, 8, 444. [Google Scholar] [CrossRef]
- Ge, X. Antimicrobial Biomaterials with Non-Antibiotic Strategy. Biosurf. Biotribol. 2019, 5, 71–82. [Google Scholar] [CrossRef]
- Cipollina, A.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Tumedei, M.; Romasco, T.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite Element Analysis (FEA) of a Premaxillary Device: A New Type of Subperiosteal Implant to Treat Severe Atrophy of the Maxilla. Biomimetics 2023, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Kočiš, F.; Novák, B.; Czako, L.; Varga, I. Three-Dimensional Modeling and 3D Printing of Biocompatible Orthodontic Power-Arm Design with Clinical Application. Appl. Sci. 2021, 11, 9693. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čverha, M.; Varga, I.; Trenčanská, T.; Šufliarsky, B.; Thurzo, A. The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review. Biomimetics 2023, 8, 536. https://doi.org/10.3390/biomimetics8070536
Čverha M, Varga I, Trenčanská T, Šufliarsky B, Thurzo A. The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review. Biomimetics. 2023; 8(7):536. https://doi.org/10.3390/biomimetics8070536
Chicago/Turabian StyleČverha, Martin, Ivan Varga, Tereza Trenčanská, Barbora Šufliarsky, and Andrej Thurzo. 2023. "The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review" Biomimetics 8, no. 7: 536. https://doi.org/10.3390/biomimetics8070536
APA StyleČverha, M., Varga, I., Trenčanská, T., Šufliarsky, B., & Thurzo, A. (2023). The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review. Biomimetics, 8(7), 536. https://doi.org/10.3390/biomimetics8070536