Development of Bioactive Scaffolds for Orthopedic Applications by Designing Additively Manufactured Titanium Porous Structures: A Critical Review
Abstract
1. Preface
2. AM-Aided Engineering of “Ideal” Orthopedic Bioactive Implants
- (i)
- Pore design with feedback from (i1) experimental producing of the designed structure; (i2) simulation to estimate strength, anisotropy, stiffness, and fatigue resistance depending on the pore geometry; (i3) experimental validation of numerical results;
- (ii)
- Appropriate choice and thoughtful adjustment of 3D printing equipment, powders, and processing parameters depending on the pore design and product configuration;
- (iii)
- Account for AM-induced defects and post-treatment procedures such as annealing, etching, loading with bioactive compounds, sterilization, trapped air and powder removal, and so on.
3. AM Approaches to Print Porous Structures
3.1. AM Techniques to Print Porous Biocompatible Products of Ti Alloys and the Features of As-Printed Materials
3.2. Computational Techniques for AM-Aimed Cell Design and Virtual Testing of Porous Structures
3.3. Virtual Optimization of Porous Structures for Biomedical Applications
4. Porous Scaffolds for Bone Tissue Engineering: Biomedical Issues
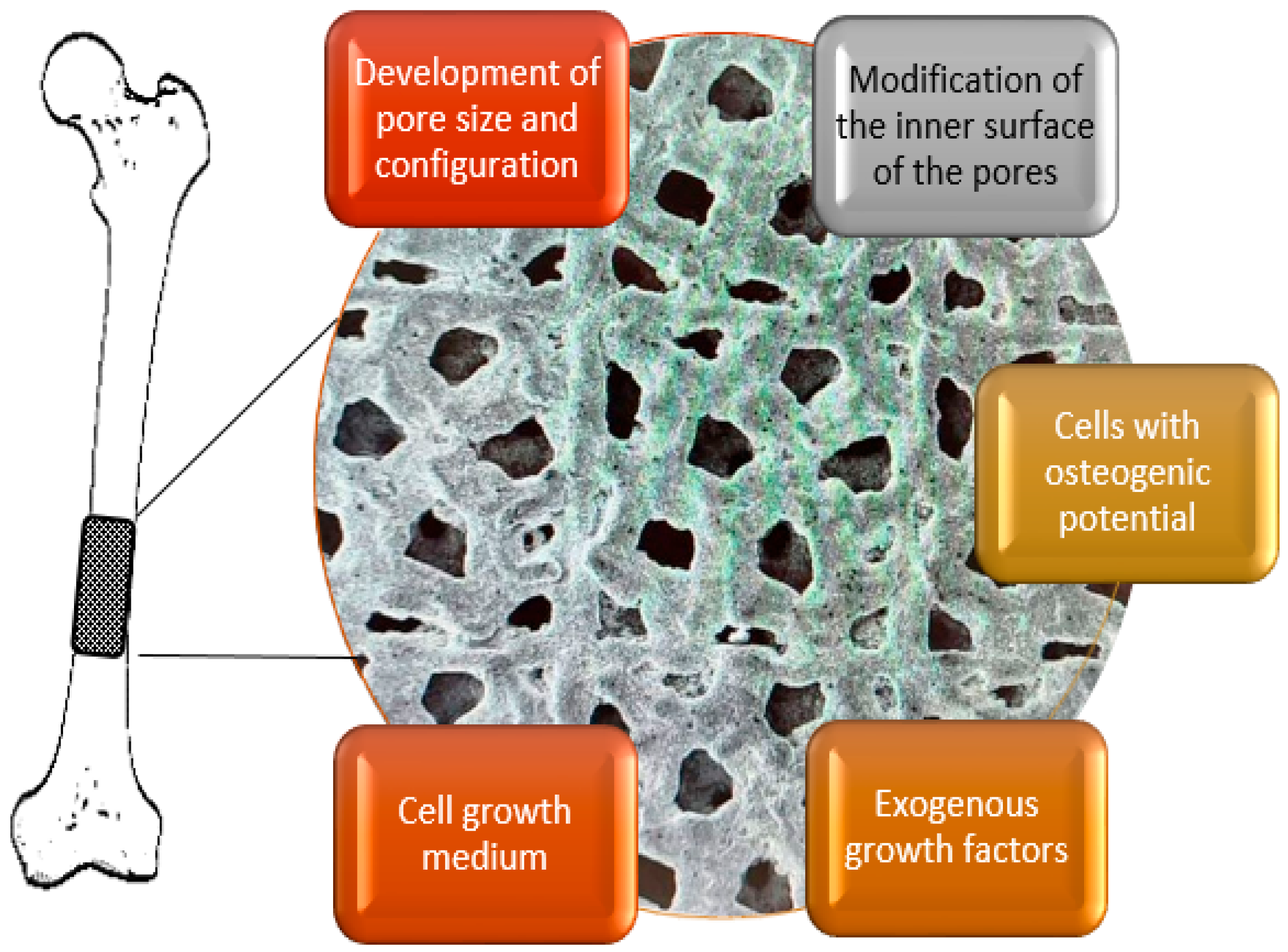
4.1. Porous Matrices
4.2. Cell Geometry
4.3. Biocoatings of Porous Structures
4.4. Cell Colonization
4.5. Clinical Studies of Porous Ti-Based Materials
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.S.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.I.; Esmaeilizadeh, R.; Sarikhanig, E.; Toorandaz, S.; Sarabi, S.A.; et al. Additively manufactured metallic biomaterials. Bioact. Mater. 2022, 15, 214–249. [Google Scholar] [CrossRef]
- Vesvoranan, O.; Anup, A.; Hixon, K.R. Current Concepts and Methods in Tissue Interface Scaffold Fabrication. Biomimetics 2022, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef] [PubMed]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Du Plessis, A.; Razavi, S.M.J.; Benedetti, M.; Murchio, S.; Leary, M.; Watson, M.; Bhate, D.; Berto, F. Properties and applications of additively manufactured metallic cellular materials: A review. Prog. Mater. Sci. 2022, 125, 100918. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- The Orthopaedic Industry Annual Report 2023. Available online: https://www.orthoworld.com/the-orthopaedic-industry-annual-report/ (accessed on 23 August 2023).
- Darvell, B.W. Bioactivity—Symphony or Cacophony? A Personal View of a Tangled Field. Prosthesis 2021, 3, 75–84. [Google Scholar] [CrossRef]
- ISO 23317; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Standards Organization: Geneva, Switzerland, 2014.
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2022, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Yun, B.M.; White, J.B. New biomaterials for orthopedic implants. Orthop. Res. Rev. 2015, 7, 107–130. [Google Scholar] [CrossRef]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, D.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef]
- Imran, R.; Al Rashid, A.; Koç, M. Review on computational modeling for the property, process, product and performance (PPPP) characteristics of additively manufactured porous magnesium implants. Bioprinting 2022, 28, e00236. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, A.; Fan, J.; Wang, M.; Dai, J.; Jin, X.; Deng, H.; Wang, X.; Liang, Y.; Li, H.; et al. A drug-loaded composite coating to improve osteogenic and antibacterial properties of Zn–1Mg porous scaffolds as biodegradable bone implants. Bioact. Mater. 2023, 27, 488–504. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; RamaKrishna, S.; Chen, X. Additive manufacturing of biodegradable magnesium-based materials: Design strategies, properties, and biomedical applications. J. Magnes. Alloys 2023, 11, 801–839. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, P.; Wang, N.; Peng, L.; Kang, B.; Zeng, H.; Yuan, G.; Ding, W. Challenges and Solutions for the Additive Manufacturing of Biodegradable Magnesium Implants. Engineering 2020, 6, 1267–1275. [Google Scholar] [CrossRef]
- Dutta, B.; Sam Froes, F.H. The Additive Manufacturing (AM) of Titanium Alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Antunes, L.H.M.; de Lima, C.R.P. Cobalt-chromium alloys—Properties and applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Goharian, A. (Ed.) Further development of trauma plating fixation. In Trauma Plating Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 361–381. [Google Scholar] [CrossRef]
- Zysset, P.K.; Edward Guo, X.; Edward Hoffler, C.; Moore, K.E.; Goldstein, S.A. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Bagno, A. When the total hip replacement fails: A review on the stress-shielding effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Zhang, L.; Song, B.; Choi, S.-K.; Shi, Y. A topology strategy to reduce stress shielding of additively manufactured porous metallic biomaterials. Int. J. Mech. Sci. 2021, 197, 106331. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Rahbari, A.; Montazerian, H.; Davoodi, E.; Homayoonfar, S. Predicting permeability of regular tissue engineering scaffolds: Scaling analysis of pore architecture, scaffold length, and fluid flow rate effects. Comput. Methods Biomech. Biomed. Eng. 2016, 20, 231–241. [Google Scholar] [CrossRef]
- Zhianmanesh, M.; Varmazyar, M.; Montazerian, H. Fluid permeability of graded porosity scaffolds architectured with minimal surfaces. ACS Biomater. Sci. Eng. 2019, 5, 1228–1237. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Soro, N.; Kent, D.; Dargusch, M.S. Additive manufacturing of low-cost porous titanium-based composites for biomedical applications: Advantages, challenges and opinion for future development. J. Alloys Compd. 2020, 827, 154263. [Google Scholar] [CrossRef]
- Barba, D.; Alabort, E.; Reed, R.C. Synthetic bone: Design by additive manufacturing. Acta Biomater. 2019, 97, 637–656. [Google Scholar] [CrossRef]
- Kim, F.H.; Moylan, S.P. Literature Review of Metal Additive Manufacturing Defects; Advanced Manufacturing Series (NIST AMS); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar] [CrossRef]
- Li, X.; Jia, X.; Yang, Q.; Lee, J. Quality Analysis in metal additive manufacturing with Deep Learning. J. Intell. Manuf. 2020, 31, 2003–2017. [Google Scholar] [CrossRef]
- Mellor, S.; Hao, L.; Zhang, D. Additive manufacturing: A framework for implementation. Int. J. Prod. Econ. 2014, 149, 194–201. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive Manufacturing of Metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Da Silva, L.R.R.; Sales, W.F.; Campos, F.d.A.R.; de Sousa, J.A.G.; Davis, R.; Singh, A.; Coelho, R.T.; Borgohain, B. A Comprehensive Review on Additive Manufacturing of Medical Devices. Prog. Addit. Manuf. 2021, 6, 517–553. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Wen, C. A comparative study on the nanoindentation behavior, wear resistance and in vitro biocompatibility of SLM manufactured CP–Ti and EBM manufactured Ti64 gyroid scaffolds. Acta Biomater. 2019, 97, 587–596. [Google Scholar] [CrossRef]
- Murr, L.E. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J. Mater. Res. Technol. 2020, 9, 1087–1103. [Google Scholar] [CrossRef]
- Gu, D.; Shi, X.; Poprawe, R.; Bourell, D.L.; Setchi, R.; Zhu, J. Material-structure-performance integrated laser-metal additive manufacturing. Science 2021, 372, eabg1487. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in Personalized Drug Delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Tshephe, T.S.; Akinwamide, S.O.; Olevsky, E.; Olubambi, P.A. Additive manufacturing of titanium-based alloys—A review of methods, properties, challenges, and prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef]
- Farazin, A.; Zhang, C.; Gheisizadeh, A.; Shahbazi, A. 3D bio-printing for use as bone replacement tissues: A review of Biomedical Application. Biomed. Eng. Adv. 2023, 5, 100075. [Google Scholar] [CrossRef]
- Weaver, J.S.; Heigel, J.C.; Lane, B.M. Laser spot size and scaling laws for laser beam additive manufacturing. J. Manuf. Process. 2022, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Keaveney, S.; Shmeliov, A.; Nicolosi, V.; Dowling, D.P. Investigation of process by-products during the selective laser melting of Ti6Al4V powder. Addit. Manuf. 2020, 36, 101514. [Google Scholar] [CrossRef]
- Dowling, L.; Kennedy, J.; O’Shaughnessy, S.; Trimble, D. A review of critical repeatability and reproducibility issues in powder bed fusion. Mater. Des. 2020, 186, 108346. [Google Scholar] [CrossRef]
- Hwang, S.; An, S.; Robles, U.; Rumpf, R.C. Process parameter optimization for removable partial denture frameworks manufactured by Selective Laser Melting. J. Prosthet. Dent. 2023, 129, 191–198. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Barbas, A.; Bonnet, A.-S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. J. Mech. Behav. Biomed. Mater. 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Pauzon, C.; Mishurova, T.; Evsevleev, S.; Dubiez-Le Goff, S.; Murugesan, S.; Bruno, G.; Hryha, E. Residual stresses and porosity in Ti-6Al-4V produced by laser powder bed fusion as a function of process atmosphere and component design. Addit. Manuf. 2021, 47, 102340. [Google Scholar] [CrossRef]
- Gloaguen, D.; Girault, B.; Courant, B.; Dubos, P.-A.; Moya, M.-J.; Edy, F.; Kornmeier, J.R. Study of Residual Stresses in Additively Manufactured Ti-6Al-4V by Neutron Diffraction Measurements. Metall. Mater. Trans. A 2020, 51, 951–961. [Google Scholar] [CrossRef]
- Dejene, N.D.; Lemu, H.G. Current Status and Challenges of Powder Bed Fusion-Based Metal Additive Manufacturing: Literature Review. Metals 2023, 13, 424. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Qian, M. Additive manufacturing of strong and ductile Ti–6Al–4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti–6Al–4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and Selective Laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Jin, B.; Wang, Q.; Zhao, L.; Pan, A.; Ding, X.; Gao, W.; Song, Y.; Zhang, X. A Review of Additive Manufacturing Techniques and Post-Processing for High-Temperature Titanium Alloys. Metals 2023, 13, 1327. [Google Scholar] [CrossRef]
- Wei, J.; Sun, H.; Zhang, D.; Gong, L.; Lin, J.; Wen, C. Influence of heat treatments on microstructure and mechanical properties of Ti–26Nb alloy elaborated in situ by laser additive manufacturing with Ti and Nb mixed powder. Materials 2018, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Heat treatment of ti6al4v produced by selective laser melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Barriobero-Vila, P.; Gussone, J.; Stark, A.; Requena, G.; Schell, N.; Haubrich, J. Peritectic Titanium Alloys for 3D Printing. Nat. Commun. 2018, 9, 3426. [Google Scholar] [CrossRef] [PubMed]
- Soro, N.; Saintier, N.; Attar, H.; Dargusch, M.S. Surface and morphological modification of selectively laser melted titanium lattices using a chemical post treatment. Surf. Coat. Technol. 2020, 393, 125794. [Google Scholar] [CrossRef]
- Phani Babu, V.V.; GB, V.K. A review on 3D printing process on metals and their surface roughness and dimensional accuracy. Mater. Today Proc. 2022, 64, 523–530. [Google Scholar] [CrossRef]
- Khrapov, D.; Paveleva, A.; Kozadayeva, M.; Evsevleev, S.; Mishurova, T.; Bruno, G.; Surmenev, R.; Koptyug, A.; Surmeneva, M. Trapped powder removal from sheet-based porous structures based on triply periodic minimal surfaces fabricated by electron beam powder bed fusion. Mater. Sci. Eng. A 2023, 862, 144479. [Google Scholar] [CrossRef]
- Wang, S.; Ning, J.; Zhu, L.; Yang, Z.; Yan, W.; Dun, Y.; Xue, P.; Xu, P.; Bose, S.; Bandyopadhyay, A. Role of porosity defects in metal 3D printing: Formation Mechanisms, impacts on properties and mitigation strategies. Mater. Today 2022, 59, 133–160. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F.; Harley, B.A. Cellular Materials in Nature and Medicine; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Olivares, A.L.; Lacroix, D. Simulation of cell seeding within a three-dimensional porous scaffold: A fluid-particle analysis. Tissue Eng. Part C Methods 2012, 18, 624–631. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Ndreu Halili, A.; Engin Vrana, N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Castro, A.P.; Ruben, R.B.; Gonçalves, S.B.; Pinheiro, J.; Guedes, J.M.; Fernandes, P.R. Numerical and experimental evaluation of TPMS gyroid scaffolds for bone tissue engineering. Comp. Meth. Biomech. Biomed. Eng. 2019, 22, 567–573. [Google Scholar] [CrossRef]
- Castilho, M.; Pires, I.; Gouveia, B.; Rodrigues, J. Structural evaluation of scaffolds prototypes produced by three-dimensional printing. Int. J. Adv. Manuf. Technol. 2011, 56, 561–569. [Google Scholar] [CrossRef]
- Papantoniou, I.; Guyot, Y.; Sonnaert, M.; Kerckhofs, G.; Luyten, F.P.; Geris, L.; Schrooten, J. Spatial optimization in perfusion bioreactors improves bone tissue-engineered construct quality attributes. Biotechnol. Bioeng. 2014, 111, 2560–2570. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Coelho, P.G. Engineering Mineralized and Load Bearing Tissues; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
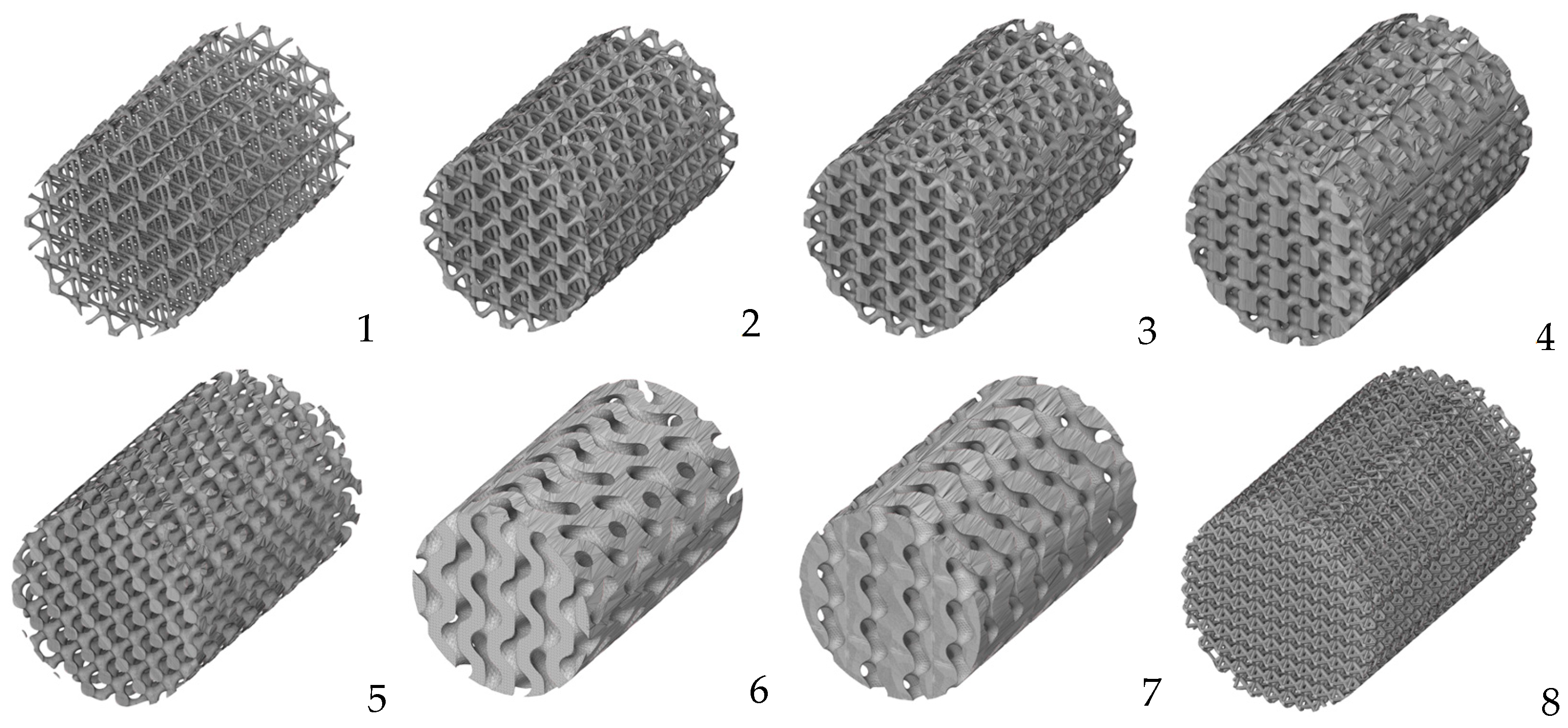
- Alomar, Z.; Concli, F. A Review of the Selective Laser Melting Lattice Structures and Their Numerical Models. Adv. Eng. Mater. 2020, 22, 200611. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.; Qian, M.; Faruque, O.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Des. 2019, 183, 108137. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Yao, D.; Wei, Y. Mechanical and permeability properties of porous scaffolds developed by a Voronoi tessellation for bone tissue engineering. J. Mater. Chem. B 2022, 10, 9699–9712. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Liu, M.; Shi, D.; Hu, N.; Feng, W. Mechanical Properties and in Vivo Assessment of Electron Beam Melted Porous Structures for Orthopedic Applications. Metals 2023, 13, 1034. [Google Scholar] [CrossRef]
- Alomar, Z.; Concli, F. Compressive behavior assessment of a newly developed circular cell-based lattice structure. Mater. Des. 2021, 205, 109716. [Google Scholar] [CrossRef]
- Yoo, D.-J. Advanced porous scaffold design using multi-void triply periodic minimal surface models with high surface area to volume ratios. Int. J. Precis. Eng. Manuf. 2014, 15, 1657–1666. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wei, F.; Chouraki, B.; Sun, X.; Wei, J.; Zhu, L. Study on Performance Simulation of Vascular-like Flow Channel Model Based on TPMS Structure. Biomimetics 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Z.; Zhang, P.; Zhang, D.Z.; Jiang, J.; Ma, Z. Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Mater. Des. 2018, 160, 849–860. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R.K. MSLattice: A free software for generating uniform and graded lattices based on triply periodic minimal surfaces. Mater. Des. Process. Commun. 2020, 3, 205. [Google Scholar] [CrossRef]
- Du Plessis, A.; Kouprianoff, D.-P.; Yadroitsava, I.; Yadroitsev, I. Mechanical Properties and In Situ Deformation Imaging of Microlattices Manufactured by Laser Based Powder Bed Fusion. Materials 2018, 11, 1663. [Google Scholar] [CrossRef]
- Guo, A.X.Y.; Cheng, L.; Zhan, S.; Zhang, S.; Xiong, W.; Wang, Z.; Wang, G.; Cao, S.C. Biomedical applications of the powder-based 3D Printed Titanium Alloys: A Review. J. Mater. Sci. Technol. 2022, 125, 252–264. [Google Scholar] [CrossRef]
- Soro, N.; Brassart, L.; Chen, Y.; Veidt, M.; Attar, H.; Dargusch, M.S. Finite Element Analysis of Porous Commercially Pure Titanium for Biomedical Implant Application. Mater. Sci. Eng. A 2018, 725, 43–50. [Google Scholar] [CrossRef]
- Shen, H.; Brinson, L. Finite element modeling of porous titanium. Int. J. Solids Struct. 2007, 44, 320–335. [Google Scholar] [CrossRef]
- Campoli, G.; Borleffs, M.S.; Amin Yavari, S.; Wauthle, R.; Weinans, H.; Zadpoor, A.A. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing. Mater. Des. 2013, 49, 957–965. [Google Scholar] [CrossRef]
- Mattila, K.; Puurtinen, T.; Hyväluoma, J.; Surmas, R.; Myllys, M.; Turpeinen, T.; Robertsén, F.; Westerholm, J.; Timonen, J. A Prospect for Computing in Porous Materials Research: Very Large Fluid Flow Simulations. J. Comput. Sci. 2016, 12, 62–76. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial Intelligence Aided Design of Tissue Engineering Scaffolds Employing Virtual Tomography and 3D Convolutional Neural Networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef]
- Javaid, S.; Gorji, H.T.; Soulami, K.B.; Kaabouch, N. Identification and ranking biomaterials for bone scaffolds using machine learning and PROMETHEE. Res. Biomed. Eng. 2023, 39, 129–138. [Google Scholar] [CrossRef]
- Jafari Chashmi, M.; Fathi, A.; Shirzad, M.; Jafari-Talookolaei, R.-A.; Bodaghi, M.; Rabiee, S.M. Design and analysis of porous functionally graded femoral prostheses with improved stress shielding. Designs 2020, 4, 12. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in pharmaceutical technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Conev, A.; Litsa, E.E.; Perez, M.R.; Diba, M.; Mikos, A.G.; Kavraki, L.E. Machine learning-guided three-dimensional printing of tissue engineering scaffolds. Tissue Eng. Part A 2020, 26, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Siegkas, P. Generating 3D porous structures using machine learning and additive manufacturing. Mater. Des. 2022, 220, 110858. [Google Scholar] [CrossRef]
- Sujeeun, L.Y.; Goonoo, N.; Ramphul, H.; Chummun, I.; Gimié, F.; Baichoo, S.; Bhaw-Luximon, A. Correlating in vitro performance with physico-chemical characteristics of nanofibrous scaffolds for skin tissue engineering using supervised machine learning algorithms. R. Soc. Open Sci. 2020, 7, 201293. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Januszyk, M.; Longaker, M.T. Machine Learning in Tissue Engineering. Tissue Eng. Part A 2023, 29, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Timercan, A.; Sheremetyev, V.; Brailovski, V. Mechanical properties and fluid permeability of gyroid and diamond lattice structures for intervertebral devices: Functional requirements and comparative analysis. Sci. Technol. Adv. Mater. 2021, 22, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tao, C.; Wu, S.; Chen, B. Influence of Modified Microstructures and Characterized Defects on Tensile Properties and Anisotropy of Selective Laser Melting-Produced Ti6Al4V Alloys. J. Mater. Eng. Perform. 2022, 31, 7705–7718. [Google Scholar] [CrossRef]
- Timercan, A.; Terriault, P.; Brailovski, V. Axial tension/compression and torsional loading of diamond and gyroid lattice structures for biomedical implants: Simulation and experiment. Mater. Des. 2023, 225, 111585. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Lyu, Y.; Cheng, L. On the Various Numerical Techniques for the Optimization of Bone Scaffold. Materials 2023, 16, 974. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’Yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Zhao, M.; Bosiakov, S.; Li, L. A Critical Review of Additive Manufacturing Techniques and Associated Biomaterials Used in Bone Tissue Engineering. Polymers 2022, 14, 2117. [Google Scholar] [CrossRef]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S.; Shuai, C. A combined strategy to enhance the properties of Zn by Laser Rapid Solidification and laser alloying. J. Mech. Behav. Biomed. Mater. 2018, 82, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
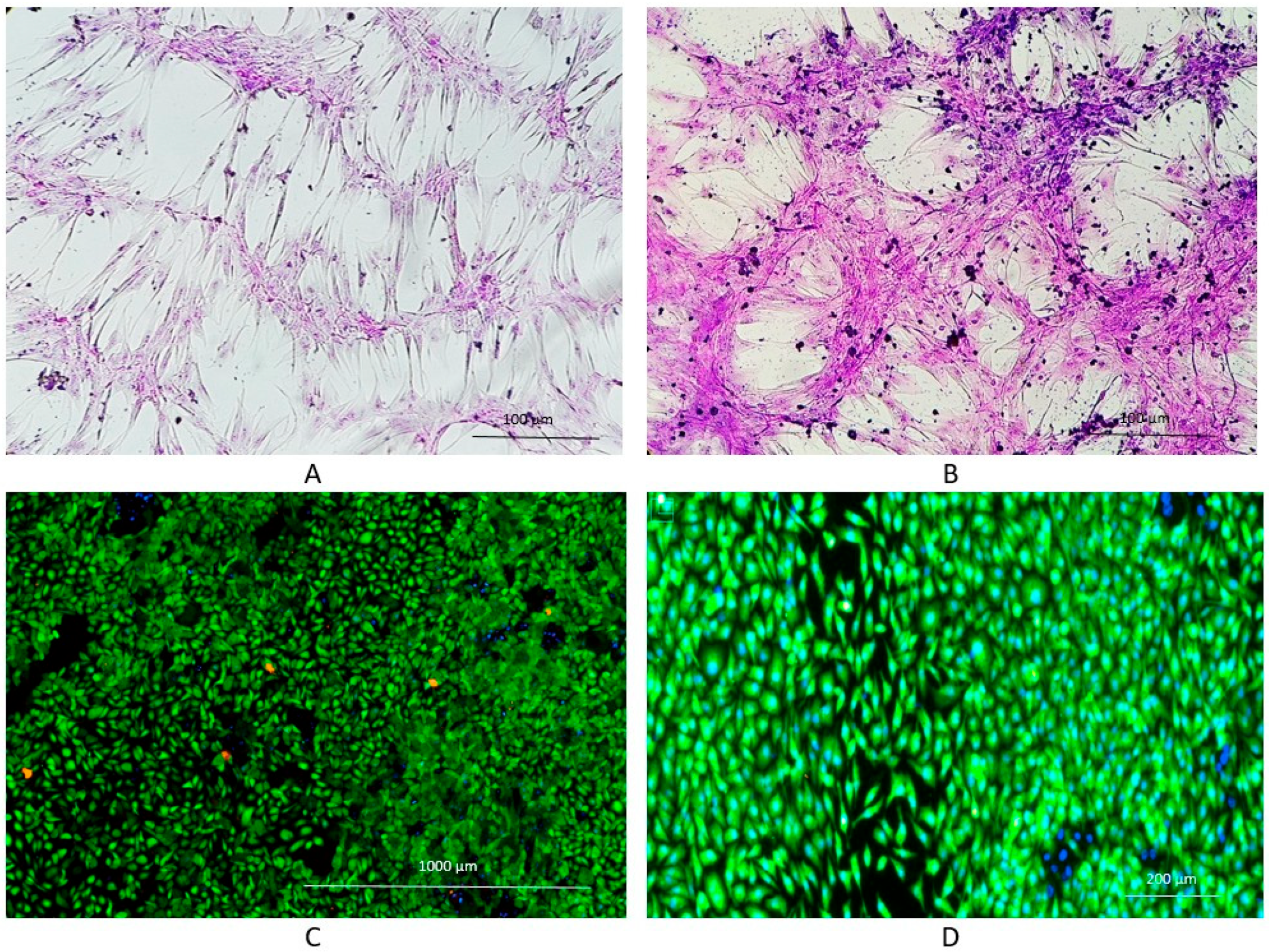
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted ti6al4v bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A Review. J. Alloys Compd. 2017, 717, 271–285. [Google Scholar] [CrossRef]
- Lv, X.; Wang, S.; Xu, Z.; Liu, X.; Liu, G.; Cao, F.; Ma, Y. Structural Mechanical Properties of 3D Printing Biomimetic Bone Replacement Materials. Biomimetics 2023, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.Y.; Tan, S.T. A simple and effective geometric representation for irregular porous structure modeling. Comput. -Aided Des. 2010, 42, 930–941. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, L.; Liu, Z.; Jiang, Z.; Liu, Y.; Wu, Y. Yield properties of closed-cell aluminum foam under triaxial loadings by a 3D Voronoi model. Mech. Mater. 2017, 104, 73–84. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, T. Cell models lead to understanding of multi-cellular morphogenesis consisting of successive self-construction of cells. J. Biochem. 2014, 157, 129–136. [Google Scholar] [CrossRef]
- Wang, G.; Shen, L.; Zhao, J.; Liang, H.; Xie, D.; Tian, Z.; Wang, C. Design and compressive behavior of controllable irregular porous scaffolds: Based on Voronoi-tessellation and for additive manufacturing. ACS Biomater. Sci. Eng. 2018, 4, 719–727. [Google Scholar] [CrossRef]
- Fantini, M.; Curto, M.; De Crescenzio, F. A method to design biomimetic scaffolds for bone tissue engineering based on Voronoi lattices. Virtual Phys. Prototyp. 2016, 11, 77–90. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Costa, A. Permeability-porosity relationship: A reexamination of the kozeny-carman equation based on a fractal pore-space geometry assumption. Geophys. Res. Lett. 2006, 33, L02318. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
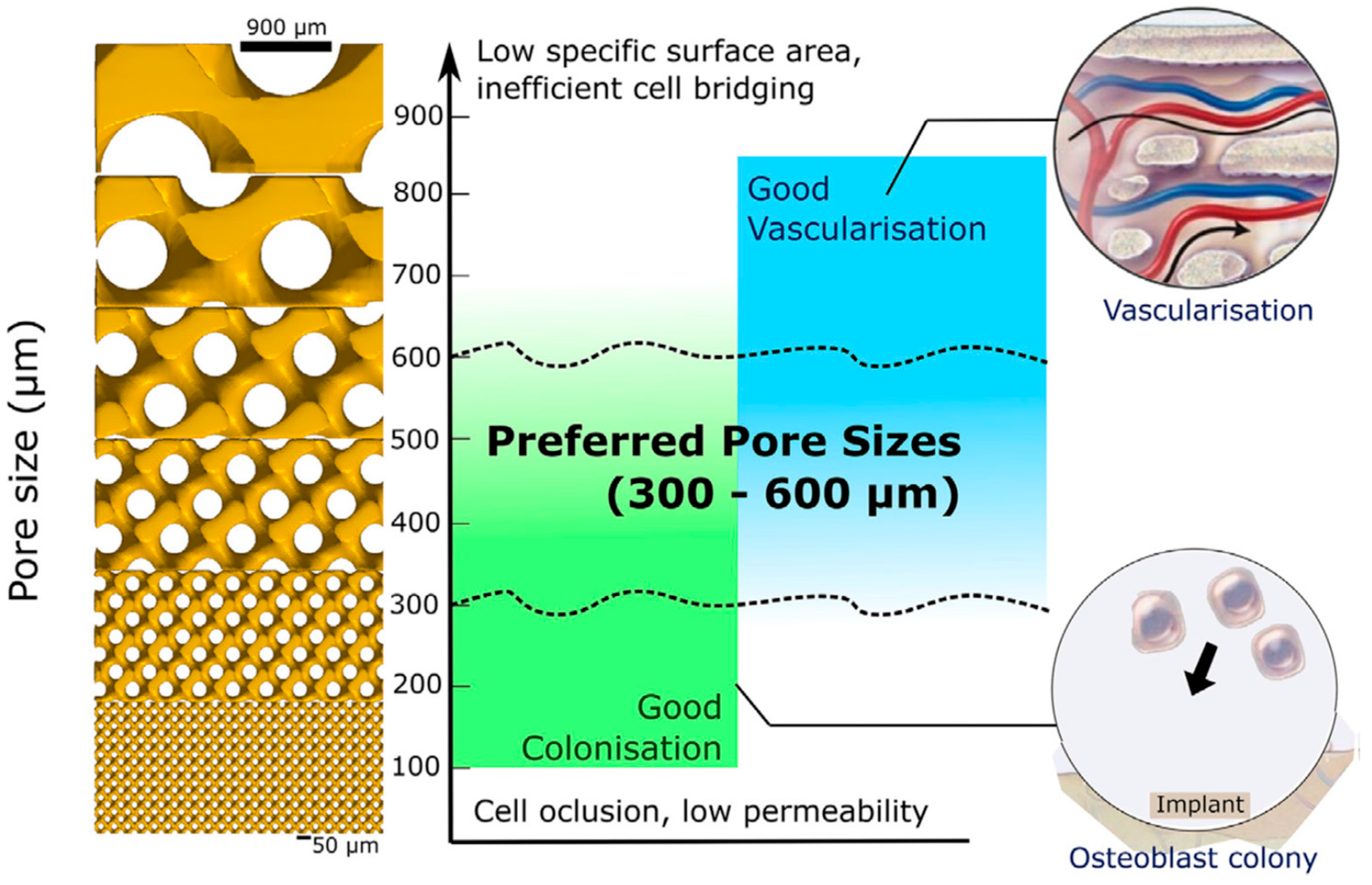
- Bobyn, J.D.; Pilliar, R.M.; Cameron, H.U.; Weatherly, G.C. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin. Orthop. Relat. Res. 1980, 150, 263–270. [Google Scholar] [CrossRef]
- Pilliar, R.M. Porous-surfaces metallic implants for orthopedic applications. J. Biomed. Mater. Res. 1987, 21 (Suppl. A1), 1–33. [Google Scholar] [PubMed]
- Lu, J.X.; Flautre, B.; Anselme, K.; Gallur, A.; Descamps, M.; Thierry, B.; Hardouin, P. Study of porous interconnections of bioceramic on cellular rehabilitation in vitro and in vivo. Bioceramics 1997, 10, 583–586. [Google Scholar]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in Rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and Bone Ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef]
- Knychala, J.; Bouropoulos, N.; Catt, C.J.; Katsamenis, O.L.; Please, C.P.; Sengers, B.G. Pore geometry regulates early stage human bone marrow cell tissue formation and organisation. Ann. Biomed. Eng. 2013, 41, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Jin, F. Influence of a non-biodegradable porous structure on Bone Repair. RSC Adv. 2016, 6, 80522–80528. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef]
- Dias, M.R.; Fernandes, P.R.; Guedes, J.M.; Hollister, S.J. Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 2012, 45, 938–944. [Google Scholar] [CrossRef]
- Porter, A.E.; Buckland, T.; Hing, K.; Best, S.M.; Bonfield, W. The structure of the bond between bone and porous silicon-substituted hydroxyapatite bioceramic implants. J. Biomed. Mater. Res. Part A 2006, 78A, 25–33. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Wauthle, R.; van der Stok, J.; Amin Yavari, S.; Van Humbeeck, J.; Kruth, J.-P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wally, Z.J.; Haque, A.M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Selective laser melting processed ti6al4v lattices with graded porosities for Dental Applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 20–29. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Gong, X.; Zhu, Y.; Shen, C.; Zhu, Z.; Li, Y.; Li, Z.; Ren, Z.; Chen, X.; et al. Ti6Al4V biomimetic scaffolds for bone tissue engineering: Fabrication, biomechanics and osseointegration. Mater. Des. 2023, 234, 112330. [Google Scholar] [CrossRef]
- Wieding, J.; Lindner, T.; Bergschmidt, P.; Bader, R. Biomechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep. Biomaterials 2015, 46, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Xu, G.; Liu, G.; Kou, H.; Zhou, L. Fabrication, pore structure and compressive behavior of anisotropic porous titanium for human trabecular bone implant applications. J. Mech. Behav. Biomed. Mater. 2015, 46, 104–114. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the pore size and porosity of selective laser melted ti6al4v Eli porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T.; Qiu, J.-X. The osteogenic effects of porous tantalum and titanium alloy scaffolds with different unit cell structure. Colloids Surf. B Biointerfaces 2022, 210, 112229. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, A.; Heo, S.-J.; Gweon, B.; Lim, D. Influences of surface topography of porous titanium scaffolds manufactured by powder bed fusion on osteogenesis. J. Mater. Res. Technol. 2023, 23, 2784–2797. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 30, 6014–6023. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Reactivity of Titanium Dental Implant Surfaces in Simulated Body Fluid. ACS Appl. Bio Mater. 2021, 4, 5575–5584. [Google Scholar] [CrossRef]
- Kon, M.; Hirakata, L.M.; Asaoka, K. Porous Ti-6Al-4 V alloy fabricated by spark plasma sintering for biomimetic surface modification. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 68, 88–93. [Google Scholar] [CrossRef]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36, S5–S7. [Google Scholar] [CrossRef]
- Ai-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Schmid, G.J.; Kobayashi, C.; Sandell, L.J.; Ornitz, D.M. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev. Dyn. 2009, 238, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Miclau, T.; Marcucio, R.S.; Colnot, C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone 2010, 46, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Gornet, M.F.; Dickman, C.A.; Zdeblick, T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. Clin. Spine Surg. 2002, 15, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, G.; Zilberman, Y.; Zhou, S.; Kelly, P.; Moutsatsos, I.K.; Kharode, Y.P.; Borella, L.E.; Bex, F.J.; Komm, B.S.; Bodine, P.V.; et al. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J. Cell. Biochem. 2002, 86, 461–474. [Google Scholar] [CrossRef]
- Dumic-Cule, I.; Brkljacic, J.; Rogic, D.; Bordukalo, N.T.; Tikvica, L.A.; Draca, N.; Kufner, V.; Trkulja, V.; Grgurevic, L.; Vukicevic, S. Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int. Orthop. 2014, 38, 1979–1985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor bmpria, leads to increased trabecular bone mass. J. Orthop. Res. 2014, 33, 208–215. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Decker, J.F.; Polimeni, G.; Cortella, C.A.; Rohrer, M.D.; Wozney, J.M.; Hall, J.; Susin, C.; Wikesjo, U.M. Evaluation of implants coated with rhbmp-2 using two different coating strategies: A critical-size supraalveolar peri-implant defect study in dogs. J. Clin. Periodontol. 2010, 37, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Jovanovic, J.; Horner, A.; Keel, M.J.; Lippuner, K.; Shintani, N. Optimisation of BMP-2 dosage for the osseointegration of porous titanium implants in an ovine model. Eur. Cells Mater. 2016, 32, 241–256. [Google Scholar] [CrossRef]
- Faundez, A.; Tournier, C.; Garcia, M.; Aunoble, S.; Le Huec, J.C. Bone morphogenetic protein use in spine surgery-complications and outcomes: A systematic review. Int. Orthop. 2016, 40, 1309–1319. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Hofer, A.S.; Levi, A.D. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J. Neurosurg. Spine 2016, 24, 457–475. [Google Scholar] [CrossRef]
- Vavken, J.; Mameghani, A.; Vavken, P.; Schaeren, S. Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2). Eur. Spine J. 2015, 25, 3979–3989. [Google Scholar] [CrossRef]
- Herberg, S.; Kondrikova, G.; Periyasamy-Thandavan, S.; Howie, R.N.; Elsalanty, M.E.; Weiss, L.; Campbell, P.; Hill, W.D.; Cray, J.J. Inkjet-based biopatterning of SDF-1β augments BMP-2-induced repair of critical size calvarial bone defects in mice. Bone 2014, 7, 95–103. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Duda, G.N.; Giesecke, M.T.; Dienelt, A.; Schwabe, P. High-dose recombinant human bone morphogenetic protein-2 impacts histological and biomechanical properties of a cervical spine fusion segment: Results from a sheep model. J. Tissue Eng. Regen. Med. 2017, 11, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Pang, W.P.; Yuan, H.; Kong, Y.Z.; Long, F.L.; Zhang, R.Z.; Yang, L.; Fang, Q.L.; Pan, A.P.; Fan, X.H.; et al. Molecular subtypes based on DNA sensors predict prognosis and tumor immunophenotype in hepatocellular carcinoma. Aging 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Aro, H.T.; Govender, S.; Patel, A.D.; Hernigou, P.; Perera de Gregorio, A.; Popescu, G.I.; Golden, J.D.; Christensen, J.; Valentin, A. Recombinant human bone morphogenetic protein-2: A randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Jt. Surg. Am. 2011, 93, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Wang, J.C.; Hashimoto, R.; Norvell, D.C. Complications related to osteobiologics use in spine surgery: A systematic review. Spine 2010, 35, S86–S104. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef]
- Baltzer, A.W.; Lattermann, C.; Whalen, J.D.; Ghivizzani, S.; Wooley, P.; Krauspe, R.; Robbins, P.D.; Evans, C.H. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clin. Orthop. Relat. Res. 2000, 379, S120–S125. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Z.; Koh, J.T.; Jin, T.; Franceschi, R.T. Combinatorial gene therapy for bone regeneration: Cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J. Cell. Biochem. 2005, 95, 1–16. [Google Scholar] [CrossRef]
- Lutz, R.; Park, J.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Schlegel, K.A. Bone regeneration after topical bmp-2-gene delivery in circumferential peri-implant bone defects. J. Clin. Oral Implant. Res. 2008, 19, 590–599. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen, Y.C.; Fu, Y.S.; Tsai, S.W.; Wu, P.K.; Chen, C.M.; Chang, M.C.; Chen, W.M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. [Google Scholar] [CrossRef]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef]
- Sakisaka, Y.; Ishihata, H.; Maruyama, K.; Nemoto, E.; Chiba, S.; Nagamine, M.; Hasegawa, H.; Hatsuzawa, T.; Yamada, S. Serial Cultivation of an MSC-Like Cell Line with Enzyme-Free Passaging Using a Microporous Titanium Scaffold. Materials 2023, 16, 1165. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, P.; Zhao, R.; Yang, X.; Xiao, Z.; Zhang, K.; Zhu, X.; Zhang, X. Functionalized 3D-printed porous titanium scaffold induces in situ vascularized bone regeneration by orchestrating bone microenvironment. J. Mater. Sci. Technol. 2023, 153, 92–105. [Google Scholar] [CrossRef]
- Steiner, D.; Reinhardt, L.; Fischer, L.; Popp, V.; Körner, C.; Geppert, C.I.; Bäuerle, T.; Horch, R.E.; Arkudas, A. Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds. Cells 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Henrich, D.; Kahling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto β-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Duffy, G.P.; Ahsan, T.; O’Brien, T.; Barry, F.; Nerem, R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng. Part A 2009, 15, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.; Torensma, B.; Wolfs, J. Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-year results of a prospective controlled trial. Spine J. 2020, 20, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Chai, X.; Zhuo, H. Application of three-dimensional printed porous titanium alloy cage and poly-ether-ether-ketone cage in posterior lumbar interbody fusion. Chin. J. Reparative Reconstr. Surg. 2022, 36, 1126–1131. [Google Scholar] [CrossRef]
- Salemyr, M.; Muren, O.; Eisler, T.; Bodén, H.; Chammout, G.; Stark, A.; Sköldenberg, O. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty. A randomised controlled trial. Int. Orthop. 2015, 39, 823–832. [Google Scholar] [CrossRef]
- Yoshioka, S.; Nakano, S.; Kinoshita, Y.; Nakamura, M.; Goto, T.; Hamada, D.; Sairyo, K. Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: A retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients. J. Orthop. Sci. 2018, 23, 967–972. [Google Scholar] [CrossRef]
- Kheder, W.; Al Kawas, S.; Khalaf, K.; Samsudin, A.R. Impact of tribocorrosion and titanium particles release on dental implant complications—A narrative review. Jpn. Dent. Sci. Rev. 2021, 57, 182–189. [Google Scholar] [CrossRef] [PubMed]

| Model | Expression |
|---|---|
| Diamond | cos X cos Y cos Z − sin X sin Y sin Z = c |
| IWP | 2 (cos X cos Y +cos Y cos Z + cos Z cos X) − (cos 2X +cos 2Y +cos 2Z) = 0 |
| Gyroid | sin Y cos X + sin Z cos Y + sin X cos Z = 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiselevskiy, M.V.; Anisimova, N.Y.; Kapustin, A.V.; Ryzhkin, A.A.; Kuznetsova, D.N.; Polyakova, V.V.; Enikeev, N.A. Development of Bioactive Scaffolds for Orthopedic Applications by Designing Additively Manufactured Titanium Porous Structures: A Critical Review. Biomimetics 2023, 8, 546. https://doi.org/10.3390/biomimetics8070546
Kiselevskiy MV, Anisimova NY, Kapustin AV, Ryzhkin AA, Kuznetsova DN, Polyakova VV, Enikeev NA. Development of Bioactive Scaffolds for Orthopedic Applications by Designing Additively Manufactured Titanium Porous Structures: A Critical Review. Biomimetics. 2023; 8(7):546. https://doi.org/10.3390/biomimetics8070546
Chicago/Turabian StyleKiselevskiy, Mikhail V., Natalia Yu. Anisimova, Alexei V. Kapustin, Alexander A. Ryzhkin, Daria N. Kuznetsova, Veronika V. Polyakova, and Nariman A. Enikeev. 2023. "Development of Bioactive Scaffolds for Orthopedic Applications by Designing Additively Manufactured Titanium Porous Structures: A Critical Review" Biomimetics 8, no. 7: 546. https://doi.org/10.3390/biomimetics8070546
APA StyleKiselevskiy, M. V., Anisimova, N. Y., Kapustin, A. V., Ryzhkin, A. A., Kuznetsova, D. N., Polyakova, V. V., & Enikeev, N. A. (2023). Development of Bioactive Scaffolds for Orthopedic Applications by Designing Additively Manufactured Titanium Porous Structures: A Critical Review. Biomimetics, 8(7), 546. https://doi.org/10.3390/biomimetics8070546









